-
PDF
- Split View
-
Views
-
Cite
Cite
Benjamin Lindsey, Jagne Jankey, Edwin Armitage, David Jeffries, Nuredin Mohammed, Sainabou Drammeh, Elina Senghore, Hadi Sallah, John Tregoning, Katja Hoschler, Tao Dong, Ed Clarke, Beate Kampmann, Thushan De Silva, 1663. Marked Improvement in Pandemic H1N1 Component Shedding and Immunogenicity in 2017–2018 Russian-Backbone Live Attenuated Influenza Vaccine (LAIV) in Gambian Children, Open Forum Infectious Diseases, Volume 5, Issue suppl_1, November 2018, Page S50, https://doi.org/10.1093/ofid/ofy209.120
Close - Share Icon Share
Abstract
Recent observational studies in the United States have reported reduced effectiveness of the Ann Arbor-backbone live attenuated influenza vaccine (LAIV), coinciding with emergence of 2009 pandemic H1N1 (pH1N1). A recent RCT in Senegal of the Russian-backbone LAIV also showed no efficacy, with pH1N1 the predominant vaccine-matched strain circulating during the study. The reasons for this reduced effectiveness and efficacy are unclear but may involve pre-existing immunity or pH1N1 virus-specific factors. We explore these underlying reasons through an LAIV immunogenicity study in Gambian children across 2 influenza seasons.
Gambian children aged 24–59 months (n = 118) were given 2016–17 northern hemisphere Russian-backbone trivalent LAIV. Vaccine shedding, haemagglutinin inhibition (HAI) titre, influenza-specific T-cell responses, and mucosal IgA were measured using RT-PCR, HAI assay, flow cytometry, and ELISA, respectively. The following year, a further 127 children were given 2017–2018 formulation LAIV, where the pH1N1 strain was updated.
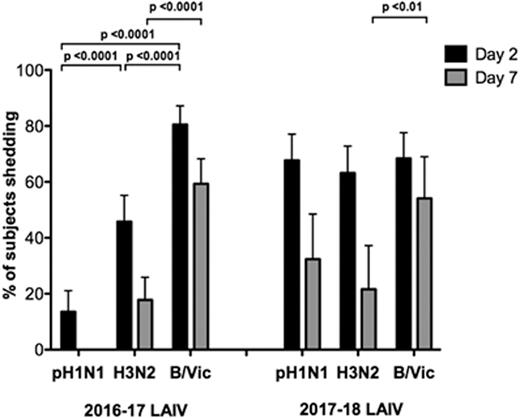
In 2016–2017, significantly less pH1N1 shedding (13.6% children) was seen compared with H3N2 (45.8%) and B/Victoria (80.5%). Similarly, poor pH1N1-specific HAI (5.1% seroconversion), mucosal IgA (18.6% responders) and T-cell responses (<10% responses to pH1N1 HA) were seen, whereas significantly greater responses in ≥1 immune compartments were seen to H3N2 and B/Victoria. pH1N1 shedding was not related to pre-existing immunity in 2016–2017. Vaccination with 2017–2018 LAIV showed improvement in pH1N1 shedding with no significant difference between strains: 67.7%, 63.2%, and 68.4% children shedding pH1N1, H3N2, and B/Victoria at day 2 post-LAIV (see Figure 1). This was matched by enhanced pH1N1 HA-specific T-cell responses, with 47.1% children showing a CD4+IFNg+ and 54.4% a CD4+IL2+ response (see Figure 2). HAI and mucosal IgA data for 2017–2018 are currently being generated and will be presented, as well as key interactions between the parameters measured.
Our data suggest that poor pH1N1 A/California strain replication in vivo may explain recent suboptimal LAIV performance and suggest that an improvement can be expected with new pH1N1 strains included in current LAIV formulations.

All authors: No reported disclosures.
Session: 178. PIDS Featured Oral Abstract
Friday, October 5, 2018: 3:30 PM
- influenza
- enzyme-linked immunosorbent assay
- flow cytometry
- child
- disclosure
- hemagglutinins
- immunity
- interferon type ii
- reverse transcriptase polymerase chain reaction
- senegal
- t-lymphocytes
- vaccination
- vaccines
- immunoglobulin a
- mucous membrane
- viruses
- influenza vaccine, trivalent live attenuated
- pandemics
- influenza a virus, h1n1 subtype
- influenza a virus, h3n2 subtype
- swine influenza
- swine-origin influenza virus
- seroconversion
- immunogenicity





Comments