-
PDF
- Split View
-
Views
-
Cite
Cite
Thomas Lemmet, Jean-Philippe Mazzucotelli, Olivier Collange, Léa Fath, Didier Mutter, Cécile Brigand, Pierre-Emmanuel Falcoz, François Danion, Nicolas Lefebvre, Morgane Bourne-Watrin, Victor Gerber, Baptiste Hoellinger, Thibaut Fabacher, Yves Hansmann, Yvon Ruch, Infectious Mediastinitis: A Retrospective Cohort Study, Open Forum Infectious Diseases, Volume 11, Issue 5, May 2024, ofae225, https://doi.org/10.1093/ofid/ofae225
Close - Share Icon Share
Abstract
This study aimed to characterize the demographics, microbiology, management and treatment outcomes of mediastinitis according to the origin of the infection.
This retrospective observational study enrolled patients who had mediastinitis diagnosed according to the criteria defined by the Centers for Disease Control and Prevention and were treated in Strasbourg University Hospital, France, between 1 January 2010 and 31 December 2020.
We investigated 151 cases, including 63 cases of poststernotomy mediastinitis (PSM), 60 cases of mediastinitis due to esophageal perforation (MEP) and 17 cases of descending necrotizing mediastinitis (DNM). The mean patient age (standard deviation) was 63 (14.5) years, and 109 of 151 patients were male. Microbiological documentation varied according to the origin of the infection. When documented, PSM cases were mostly monomicrobial (36 of 53 cases [67.9%]) and involved staphylococci (36 of 53 [67.9%]), whereas MEP and DNM cases were mostly plurimicrobial (38 of 48 [79.2%] and 8 of 12 [66.7%], respectively) and involved digestive or oral flora microorganisms, respectively. The median duration of anti-infective treatment was 41 days (interquartile range, 21–56 days), and 122 of 151 patients (80.8%) benefited from early surgical management. The overall 1-year survival rate was estimated to be 64.8% (95% confidence interval, 56.6%–74.3%), but varied from 80.1% for DNM to 61.5% for MEP.
Mediastinitis represents a rare yet deadly infection. The present cohort study exhibited the different patterns observed according to the origin of the infection. Greater insight and knowledge on these differences may help guide the management of these complex infections, especially with respect to empirical anti-infective treatments.
Mediastinitis is a rare and life-threatening infection of the mediastinum with high morbidity and mortality rates. Its accurate diagnosis relies on multiple arguments, including clinical and imaging data, perioperative evidence, microbiological documentation, and histopathological evidence from mediastinal samples [1]. The condition may be caused by any microbial contamination of the mediastinum. The 3 main causes of mediastinitis reported in the literature are poststernotomy wound infection, esophageal perforation, and oropharyngeal abscess.
Poststernotomy mediastinitis (PSM) is rare, with an incidence of 0.5%–2.5% [2–8]. Despite modern surgical techniques, widespread use of perioperative antibiotic prophylaxis, and development of recommendations for the prevention of poststernotomy infections [9, 10], its incidence has remained stable over the past decades. Mediastinitis due to esophageal perforation (MEP) can be observed regardless of the cause of the perforation, as the rupture leads to the spillage of gastric fluid, contaminating the mediastinum with digestive flora. It is mostly iatrogenic, in 50% of cases [11, 12], but it can also be spontaneous or traumatic. Finally, mediastinitis of oropharyngeal origin, also known as descending necrotizing mediastinitis (DNM), is due to the spread of an infection from the cervical sphere along the fascia to the mediastinum. It occurs in about 2%–5% of deep neck infections [13–15].
Regardless of its origin, the management of a mediastinitis almost always requires a surgical approach that depends on the anatomic origin of the infection combined with antimicrobial therapy [16–18]. However, the optimal regimen and duration of the antimicrobial therapy still remain unclear. The objective of the current study was to investigate cases of mediastinitis managed in the Strasbourg University Hospital between 2010 and 2020, with a particular focus on the origin of the infection, microbiological documentation, disease management, and prognosis.
METHODS
Study Design
This monocentric observational study used data collected retrospectively from patients with a diagnosis of mediastinitis who were managed at the Strasbourg University Hospital between 1 January 2010 and 31 December 2020. Medical files were selected by querying the Program of Medicalization of Information Systems (PMSI). Patients were included if the diagnosis of mediastinitis was retained. Those aged <18 years were excluded.
The diagnosis of mediastinitis was based on the definition of the Centers for Disease Control and Prevention as follows: patients had to meet either the microbiological or histological criteria (ie, microbiological documentation or histological proof of infection on a sample of mediastinal origin), have perioperative evidence of mediastinal infection, or present compatible clinical and radiological data (ie, evocative clinical symptoms such as fever >38°C, chest pain, or sternal instability, associated with purulent mediastinal drainage or suggestive imaging features) [1].
We considered the date of diagnosis as the day we initiated the high-dosage intravenous antimicrobial therapy. Data were collected from any surgical procedures that were performed within 1 year before inclusion and that were held accountable for the mediastinitis. Any surgical interventions for mediastinitis following the initial surgical management were defined as revisions surgery. Of note, the local preoperative antibiotic prophylaxis protocol recommends the use of cefuroxime before thoracic or cardiac surgery and cefazolin before most esophageal surgery.
Data Collection
Baseline data were collected, including demographics, comorbid conditions, clinical and radiological data. Mediastinitis was classified according to its origin as follows: PSM, mediastinitis associated with esophageal perforation (MEP), or DNM of dental or oropharyngeal origin.
Collected data related to mediastinitis management included the duration of antimicrobial therapy (intravenous and total length) and the recourse to surgery. Drainage of oropharyngeal collections and endoscopic treatment of esophageal perforation were also accounted as surgical management when linked to mediastinitis.
Microbiological documentation was considered if retained from blood cultures or deep samples, such as mediastinal fluid or abscess puncture. Microorganisms known to be potential contaminants (ie, coagulase-negative staphylococci or Cutibacterium acnes) were not considered in the present study if isolated on a single sample of several. Outcome measures included hospital length of stay, intensive care unit length of stay, all-cause mortality, and in-hospital mortality.
Statistical Analysis
Quantitative variables were expressed as mean values with standard deviation or median with interquartile range (IQR). Qualitative variables were expressed as percentages. Comparisons of qualitative data were assessed using Student t test (Gaussian distribution of the variable) or a nonparametric test in the opposite case (Mann-Whitney U test). Qualitative data were compared using Pearson's χ2 or Fisher exact tests when the theoretical number was ≤5. A 1-way between-groups analysis of variance was conducted to compare cases of mediastinitis according to their origins using the Fisher F test while assuming variance homogeneity (verified with a Levene test) or using the Welch W test in the opposite case. For all analyses, the alpha risk was set to 5% with a confidence interval (CI) of 95%. Survival times were assessed from the date of inclusion to the date of all-cause death or last available news. Survival curves were performed using the Kaplan-Meier method. All analyses were performed using R software, version 3.1.2.
Patient Consent Statement, Ethical Statement
This study does not include factors necessitating patient written consent. However, each eligible patient was sent a nonobjection form together with an information leaflet. Consent was assumed for all eligible patients as no objections were raised. The study was approved by the Ethics Committee of Strasbourg University Hospital (reference 2021-65) and was registered on ClinicalTrials.gov (NCT05001308). The processing of personal data has been declared to the French data protection authority (Commission Nationale de l'IInformatique et des Libertés [CNIL]; 2208067v0).
RESULTS
Study Population
During the study period, 372 files were extracted, of which 151 were included as they fulfilled the Centers for Disease Control and Prevention criteria for mediastinitis. Population characteristics are provided in Table 1, and a flow chart is presented in Figure 1. Three causes accounted for the majority of the cases included, namely, PSM (n = 63 [41.7%]), MEP (n = 60 [39.7%]), and DNM (n = 17 [11.3%]). The remaining 11 cases were of diverse origins and were not pooled. We recorded 10 323 sternotomy procedures during the study period, suggesting that the incidence rate of PSM was 0.6%.
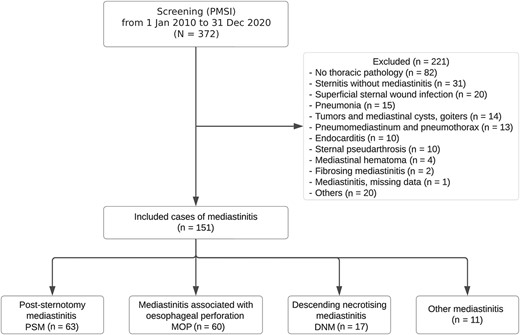
Study flow chart. Abbreviations: DNM, descending necrotizing mediastinitis; MEP, mediastinitis due to esophageal perforation; PMSI, Program of Medicalization of Information Systems; PSM, poststernotomy mediastinitis. Other mediastinitis included broncho-mediastinal fistulas (2/11), necrotic pulmonary infarction complicated with mediastinitis (1/11), mediastinitis in a heart transplant patient (1/11), infection of a mediastinal hematoma of undetermined origin (1/11), mediastinitis due to a perforation by port-a-cath (1/11) and by an extracorporeal circulatory assistance device (1/11), and mediastinitis of unknown origin (4/11).
| Characteristic . | Patients, No. (%)a . | P Value . | |||
|---|---|---|---|---|---|
| All Mediastinitis (N = 151) . | PSM (n = 63 [41.7%]) . | MEP (n = 60 [39.7%]) . | DNM (n = 17 [11.3%]) . | ||
| Demographics | |||||
| Age, mean (SD), y | 63.3 (14.5) | 64.9 (11.8) | 65.6 (12.6) | 46.3 (18) | .002b |
| Male sex | 109 (72.2) | 45 (71.4) | 40 (66.7) | 14 (82.4) | .33 |
| BMI, mean (SD)c | 27 (5.7) | 29.6 (5.9) | 24.4 (3.7) | 26.2 (6) | <.001b |
| Obesity (BMI >30)c | 31 (20.5) | 24 (38.1) | 4 (6.7) | 3 (17.6) | |
| Chronic renal failure | 19 (12.6) | 14 (22.2) | 5 (8.3) | 0 (0) | .02b |
| Cardiovascular comorbid conditions | |||||
| Total no., mean (SD) | 2.4 (2) | 3.8 (1.9) | 1.5 (1.5) | 1 (1.5) | <.001b |
| High blood pressure | 78 (51.7) | 46 (73.0) | 25 (41.7) | 3 (17.6) | <.001b |
| Diabetes | 56 (37.1) | 39 (61.9) | 12 (20.0) | 3 (17.6) | <.001b |
| Dyslipidemia | 62 (41.1) | 42 (66.7) | 14 (23.3) | 4 (23.5) | <.001b |
| Coronary heart disease | 51 (33.8) | 45 (71.4) | 5 (8.3) | 1 (5.9) | <.001b |
| Lower-extremity artery disease | 22 (14.6) | 15 (23.8) | 6 (10.0) | 1 (5.9) | .06 |
| Valvular disease | 22 (14.6) | 18 (28.6) | 4 (6.7) | 0 (0) | <.001b |
| Valvular prosthesis | 16 (10.6) | 14 (22.2) | 1 (1.7) | 0 (0) | <.001b |
| Cardiac implantable device | 7 (4.6) | 6 (9.5) | 0 (0) | 0 (0) | .04b |
| History of neoplasia | 46 (30.4) | 9 (14.3) | 33 (55.0) | 1 (5.9) | <.001b |
| Immunosuppression | 29 (19.2) | 8 (12.7) | 18 (30.0) | 0 (0) | .008b |
| Immunosuppressive therapy | 9 (6.0) | 6 (9.5) | 1 (1.7) | … | … |
| Recent chemotherapy | 19 (12.6) | 2 (3.2) | 16 (26.7) | … | … |
| Solid organ transplantation | 7 (4.6) | 5 (7.9) | 0 (0) | … | … |
| No known comorbid conditions | 19 (12.6) | 3 (4.8) | 7 (11.7) | 9 (52.9) | <.001b |
| Surgical history before mediastinitis | |||||
| Surgery within 1 y | 105 (69.5) | 61 (96.8) | 38 (63.3) | 1 (5.9) | <.001b |
| Cardiovascular surgery | 60 (57.1) | 58 (95.1) | 0 (0) | 0 (0) | … |
| Coronary graft | 29 (48.3) | 29 (50.0) | … | … | … |
| Valvular surgery | 19 (31.7) | 18 (31.0) | … | … | … |
| Otherd | 12 (20.0) | 11 (19.1) | … | … | … |
| Gastroesophageal surgery/procedure | 36 (34.3) | 0 (0) | 36 (94.7) | 0 (0) | … |
| Lewis-Santy procedure | 17 (47.2) | … | 17 (47.2) | … | … |
| Esophageal dilation | 7 (19.4) | … | 7 (19.4) | … | … |
| Othere | 12 (33.3) | … | 12 (33.3) | … | … |
| Surgical revision before mediastinitis | 17 (16.2) | 6 (9.8) | 9 (23.7) | 0 (0) | .10 |
| Time between initial surgery and mediastinitis, median, d | 11 (5–23) | 16 (10–28) | 5 (1–11) | 1 | … |
| Clinical and radiological data | |||||
| Fever | 110 (72.8) | 45 (71.4) | 35 (63.3) | 17 (100) | .005b |
| Chest pain | 55 (38.7) | 23 (38.3) | 22 (40.0) | 7 (41.2) | .94 |
| Sternal scar abnormalities | 48 (31.8) | 46 (73.0) | 0 (0) | 0 (0) | <.001b |
| Septic shock | 68 (45.0) | 21 (33.3) | 40 (66.7) | 3 (17.6) | <.001b |
| Chest CT performed | 138 (91.4) | 52 (82.5) | 59 (98.3) | 17 (100) | .007b |
| Chest CT contributory to diagnosis | 132/138 (95.7) | 47/52 (90.4) | 58/59 (98.3) | 17/17 (100) | .20 |
| Management | |||||
| Surgery for mediastinitis | 122 (80.8) | 58 (92.1) | 45 (75.0) | 13 (76.5) | .006b |
| Time between diagnosis and surgery, median, d | 1 (0–5) | 2 (0–8) | 0 (0–3) | 3 (0–4) | .80 |
| Perioperative evidence of mediastinitis | 99 (81.1) | 52 (89.7) | 31 (68.9) | 11 (84.6) | .052 |
| Surgical revision for mediastinitis | 38 (31.1) | 20 (34.5) | 9 (20.0) | 7 (53.4) | .10 |
| Duration of intravenous antimicrobial therapy, median, d | 32 (15–50) | 42 (20–52) | 28 (14–47) | 22 (17–34) | .42 |
| Switch to oral antimicrobial therapy | 28 (20.1) | 16 (25.4) | 5 (9.8) | 5 (33.3) | .08 |
| Total duration of antimicrobial therapy, median, d | 41 (21–56) | 49 (40–63) | 28 (15–49) | 30 (20–38) | .30 |
| Prognosis | |||||
| Length of hospital stay, median, d | 32 (21–59) | 34 (21–53) | 33 (25–64) | 25 (12–35) | .71 |
| ICU hospitalization | 129 (85.4) | 52 (82.5) | 55 (91.7) | 13 (76.5) | .24 |
| Length of ICU stay, median, d | 6 (2–18) | 4 (1–9) | 8 (4–24) | 13 (5–23) | .42 |
| All-cause mortality | 50 (33.1) | 21 (33.3) | 19 (31.7) | 2 (11.8) | .01b |
| In-hospital mortality | 36 (23.8) | 14 (22.2) | 14 (23.3) | 1 (5.9) | .01b |
| Time between inclusion and death, median, d | 32 (21–59) | 49 (19–201) | 28 (9–84) | 41 (38–43) | .28 |
| Characteristic . | Patients, No. (%)a . | P Value . | |||
|---|---|---|---|---|---|
| All Mediastinitis (N = 151) . | PSM (n = 63 [41.7%]) . | MEP (n = 60 [39.7%]) . | DNM (n = 17 [11.3%]) . | ||
| Demographics | |||||
| Age, mean (SD), y | 63.3 (14.5) | 64.9 (11.8) | 65.6 (12.6) | 46.3 (18) | .002b |
| Male sex | 109 (72.2) | 45 (71.4) | 40 (66.7) | 14 (82.4) | .33 |
| BMI, mean (SD)c | 27 (5.7) | 29.6 (5.9) | 24.4 (3.7) | 26.2 (6) | <.001b |
| Obesity (BMI >30)c | 31 (20.5) | 24 (38.1) | 4 (6.7) | 3 (17.6) | |
| Chronic renal failure | 19 (12.6) | 14 (22.2) | 5 (8.3) | 0 (0) | .02b |
| Cardiovascular comorbid conditions | |||||
| Total no., mean (SD) | 2.4 (2) | 3.8 (1.9) | 1.5 (1.5) | 1 (1.5) | <.001b |
| High blood pressure | 78 (51.7) | 46 (73.0) | 25 (41.7) | 3 (17.6) | <.001b |
| Diabetes | 56 (37.1) | 39 (61.9) | 12 (20.0) | 3 (17.6) | <.001b |
| Dyslipidemia | 62 (41.1) | 42 (66.7) | 14 (23.3) | 4 (23.5) | <.001b |
| Coronary heart disease | 51 (33.8) | 45 (71.4) | 5 (8.3) | 1 (5.9) | <.001b |
| Lower-extremity artery disease | 22 (14.6) | 15 (23.8) | 6 (10.0) | 1 (5.9) | .06 |
| Valvular disease | 22 (14.6) | 18 (28.6) | 4 (6.7) | 0 (0) | <.001b |
| Valvular prosthesis | 16 (10.6) | 14 (22.2) | 1 (1.7) | 0 (0) | <.001b |
| Cardiac implantable device | 7 (4.6) | 6 (9.5) | 0 (0) | 0 (0) | .04b |
| History of neoplasia | 46 (30.4) | 9 (14.3) | 33 (55.0) | 1 (5.9) | <.001b |
| Immunosuppression | 29 (19.2) | 8 (12.7) | 18 (30.0) | 0 (0) | .008b |
| Immunosuppressive therapy | 9 (6.0) | 6 (9.5) | 1 (1.7) | … | … |
| Recent chemotherapy | 19 (12.6) | 2 (3.2) | 16 (26.7) | … | … |
| Solid organ transplantation | 7 (4.6) | 5 (7.9) | 0 (0) | … | … |
| No known comorbid conditions | 19 (12.6) | 3 (4.8) | 7 (11.7) | 9 (52.9) | <.001b |
| Surgical history before mediastinitis | |||||
| Surgery within 1 y | 105 (69.5) | 61 (96.8) | 38 (63.3) | 1 (5.9) | <.001b |
| Cardiovascular surgery | 60 (57.1) | 58 (95.1) | 0 (0) | 0 (0) | … |
| Coronary graft | 29 (48.3) | 29 (50.0) | … | … | … |
| Valvular surgery | 19 (31.7) | 18 (31.0) | … | … | … |
| Otherd | 12 (20.0) | 11 (19.1) | … | … | … |
| Gastroesophageal surgery/procedure | 36 (34.3) | 0 (0) | 36 (94.7) | 0 (0) | … |
| Lewis-Santy procedure | 17 (47.2) | … | 17 (47.2) | … | … |
| Esophageal dilation | 7 (19.4) | … | 7 (19.4) | … | … |
| Othere | 12 (33.3) | … | 12 (33.3) | … | … |
| Surgical revision before mediastinitis | 17 (16.2) | 6 (9.8) | 9 (23.7) | 0 (0) | .10 |
| Time between initial surgery and mediastinitis, median, d | 11 (5–23) | 16 (10–28) | 5 (1–11) | 1 | … |
| Clinical and radiological data | |||||
| Fever | 110 (72.8) | 45 (71.4) | 35 (63.3) | 17 (100) | .005b |
| Chest pain | 55 (38.7) | 23 (38.3) | 22 (40.0) | 7 (41.2) | .94 |
| Sternal scar abnormalities | 48 (31.8) | 46 (73.0) | 0 (0) | 0 (0) | <.001b |
| Septic shock | 68 (45.0) | 21 (33.3) | 40 (66.7) | 3 (17.6) | <.001b |
| Chest CT performed | 138 (91.4) | 52 (82.5) | 59 (98.3) | 17 (100) | .007b |
| Chest CT contributory to diagnosis | 132/138 (95.7) | 47/52 (90.4) | 58/59 (98.3) | 17/17 (100) | .20 |
| Management | |||||
| Surgery for mediastinitis | 122 (80.8) | 58 (92.1) | 45 (75.0) | 13 (76.5) | .006b |
| Time between diagnosis and surgery, median, d | 1 (0–5) | 2 (0–8) | 0 (0–3) | 3 (0–4) | .80 |
| Perioperative evidence of mediastinitis | 99 (81.1) | 52 (89.7) | 31 (68.9) | 11 (84.6) | .052 |
| Surgical revision for mediastinitis | 38 (31.1) | 20 (34.5) | 9 (20.0) | 7 (53.4) | .10 |
| Duration of intravenous antimicrobial therapy, median, d | 32 (15–50) | 42 (20–52) | 28 (14–47) | 22 (17–34) | .42 |
| Switch to oral antimicrobial therapy | 28 (20.1) | 16 (25.4) | 5 (9.8) | 5 (33.3) | .08 |
| Total duration of antimicrobial therapy, median, d | 41 (21–56) | 49 (40–63) | 28 (15–49) | 30 (20–38) | .30 |
| Prognosis | |||||
| Length of hospital stay, median, d | 32 (21–59) | 34 (21–53) | 33 (25–64) | 25 (12–35) | .71 |
| ICU hospitalization | 129 (85.4) | 52 (82.5) | 55 (91.7) | 13 (76.5) | .24 |
| Length of ICU stay, median, d | 6 (2–18) | 4 (1–9) | 8 (4–24) | 13 (5–23) | .42 |
| All-cause mortality | 50 (33.1) | 21 (33.3) | 19 (31.7) | 2 (11.8) | .01b |
| In-hospital mortality | 36 (23.8) | 14 (22.2) | 14 (23.3) | 1 (5.9) | .01b |
| Time between inclusion and death, median, d | 32 (21–59) | 49 (19–201) | 28 (9–84) | 41 (38–43) | .28 |
Abbreviations: BMI, body mass index; CT, computed tomography; DNM, descending necrotizing mediastinitis; ICU, intensive care unit; MEP, mediastinitis due to esophageal perforation; PSM, poststernotomy mediastinitis; SD, standard deviation.
aData represent no. (%) of patients unless otherwise specified.
bSignificant at P < .05.
cBMI calculated as weight in kilograms divided by height in meters squared.
dOther cardiovascular surgery included aortic surgery (8 of 60 patients), cardiac graft (3 of 60), and electronic cardiac device implantation (1 of 60);
eOther gastroesophageal procedures included hiatal hernia repair (4 of 36 patients); esophageal diverticulum surgery (3 of 36); and stomach ulcer suture, complicated ultrasonography-endoscopy, complicated transesophageal echocardiography, surgery for spontaneous rupture of the esophagus, and cervical corpectomy complicated by an esophageal fistula (each 1 of 36).
| Characteristic . | Patients, No. (%)a . | P Value . | |||
|---|---|---|---|---|---|
| All Mediastinitis (N = 151) . | PSM (n = 63 [41.7%]) . | MEP (n = 60 [39.7%]) . | DNM (n = 17 [11.3%]) . | ||
| Demographics | |||||
| Age, mean (SD), y | 63.3 (14.5) | 64.9 (11.8) | 65.6 (12.6) | 46.3 (18) | .002b |
| Male sex | 109 (72.2) | 45 (71.4) | 40 (66.7) | 14 (82.4) | .33 |
| BMI, mean (SD)c | 27 (5.7) | 29.6 (5.9) | 24.4 (3.7) | 26.2 (6) | <.001b |
| Obesity (BMI >30)c | 31 (20.5) | 24 (38.1) | 4 (6.7) | 3 (17.6) | |
| Chronic renal failure | 19 (12.6) | 14 (22.2) | 5 (8.3) | 0 (0) | .02b |
| Cardiovascular comorbid conditions | |||||
| Total no., mean (SD) | 2.4 (2) | 3.8 (1.9) | 1.5 (1.5) | 1 (1.5) | <.001b |
| High blood pressure | 78 (51.7) | 46 (73.0) | 25 (41.7) | 3 (17.6) | <.001b |
| Diabetes | 56 (37.1) | 39 (61.9) | 12 (20.0) | 3 (17.6) | <.001b |
| Dyslipidemia | 62 (41.1) | 42 (66.7) | 14 (23.3) | 4 (23.5) | <.001b |
| Coronary heart disease | 51 (33.8) | 45 (71.4) | 5 (8.3) | 1 (5.9) | <.001b |
| Lower-extremity artery disease | 22 (14.6) | 15 (23.8) | 6 (10.0) | 1 (5.9) | .06 |
| Valvular disease | 22 (14.6) | 18 (28.6) | 4 (6.7) | 0 (0) | <.001b |
| Valvular prosthesis | 16 (10.6) | 14 (22.2) | 1 (1.7) | 0 (0) | <.001b |
| Cardiac implantable device | 7 (4.6) | 6 (9.5) | 0 (0) | 0 (0) | .04b |
| History of neoplasia | 46 (30.4) | 9 (14.3) | 33 (55.0) | 1 (5.9) | <.001b |
| Immunosuppression | 29 (19.2) | 8 (12.7) | 18 (30.0) | 0 (0) | .008b |
| Immunosuppressive therapy | 9 (6.0) | 6 (9.5) | 1 (1.7) | … | … |
| Recent chemotherapy | 19 (12.6) | 2 (3.2) | 16 (26.7) | … | … |
| Solid organ transplantation | 7 (4.6) | 5 (7.9) | 0 (0) | … | … |
| No known comorbid conditions | 19 (12.6) | 3 (4.8) | 7 (11.7) | 9 (52.9) | <.001b |
| Surgical history before mediastinitis | |||||
| Surgery within 1 y | 105 (69.5) | 61 (96.8) | 38 (63.3) | 1 (5.9) | <.001b |
| Cardiovascular surgery | 60 (57.1) | 58 (95.1) | 0 (0) | 0 (0) | … |
| Coronary graft | 29 (48.3) | 29 (50.0) | … | … | … |
| Valvular surgery | 19 (31.7) | 18 (31.0) | … | … | … |
| Otherd | 12 (20.0) | 11 (19.1) | … | … | … |
| Gastroesophageal surgery/procedure | 36 (34.3) | 0 (0) | 36 (94.7) | 0 (0) | … |
| Lewis-Santy procedure | 17 (47.2) | … | 17 (47.2) | … | … |
| Esophageal dilation | 7 (19.4) | … | 7 (19.4) | … | … |
| Othere | 12 (33.3) | … | 12 (33.3) | … | … |
| Surgical revision before mediastinitis | 17 (16.2) | 6 (9.8) | 9 (23.7) | 0 (0) | .10 |
| Time between initial surgery and mediastinitis, median, d | 11 (5–23) | 16 (10–28) | 5 (1–11) | 1 | … |
| Clinical and radiological data | |||||
| Fever | 110 (72.8) | 45 (71.4) | 35 (63.3) | 17 (100) | .005b |
| Chest pain | 55 (38.7) | 23 (38.3) | 22 (40.0) | 7 (41.2) | .94 |
| Sternal scar abnormalities | 48 (31.8) | 46 (73.0) | 0 (0) | 0 (0) | <.001b |
| Septic shock | 68 (45.0) | 21 (33.3) | 40 (66.7) | 3 (17.6) | <.001b |
| Chest CT performed | 138 (91.4) | 52 (82.5) | 59 (98.3) | 17 (100) | .007b |
| Chest CT contributory to diagnosis | 132/138 (95.7) | 47/52 (90.4) | 58/59 (98.3) | 17/17 (100) | .20 |
| Management | |||||
| Surgery for mediastinitis | 122 (80.8) | 58 (92.1) | 45 (75.0) | 13 (76.5) | .006b |
| Time between diagnosis and surgery, median, d | 1 (0–5) | 2 (0–8) | 0 (0–3) | 3 (0–4) | .80 |
| Perioperative evidence of mediastinitis | 99 (81.1) | 52 (89.7) | 31 (68.9) | 11 (84.6) | .052 |
| Surgical revision for mediastinitis | 38 (31.1) | 20 (34.5) | 9 (20.0) | 7 (53.4) | .10 |
| Duration of intravenous antimicrobial therapy, median, d | 32 (15–50) | 42 (20–52) | 28 (14–47) | 22 (17–34) | .42 |
| Switch to oral antimicrobial therapy | 28 (20.1) | 16 (25.4) | 5 (9.8) | 5 (33.3) | .08 |
| Total duration of antimicrobial therapy, median, d | 41 (21–56) | 49 (40–63) | 28 (15–49) | 30 (20–38) | .30 |
| Prognosis | |||||
| Length of hospital stay, median, d | 32 (21–59) | 34 (21–53) | 33 (25–64) | 25 (12–35) | .71 |
| ICU hospitalization | 129 (85.4) | 52 (82.5) | 55 (91.7) | 13 (76.5) | .24 |
| Length of ICU stay, median, d | 6 (2–18) | 4 (1–9) | 8 (4–24) | 13 (5–23) | .42 |
| All-cause mortality | 50 (33.1) | 21 (33.3) | 19 (31.7) | 2 (11.8) | .01b |
| In-hospital mortality | 36 (23.8) | 14 (22.2) | 14 (23.3) | 1 (5.9) | .01b |
| Time between inclusion and death, median, d | 32 (21–59) | 49 (19–201) | 28 (9–84) | 41 (38–43) | .28 |
| Characteristic . | Patients, No. (%)a . | P Value . | |||
|---|---|---|---|---|---|
| All Mediastinitis (N = 151) . | PSM (n = 63 [41.7%]) . | MEP (n = 60 [39.7%]) . | DNM (n = 17 [11.3%]) . | ||
| Demographics | |||||
| Age, mean (SD), y | 63.3 (14.5) | 64.9 (11.8) | 65.6 (12.6) | 46.3 (18) | .002b |
| Male sex | 109 (72.2) | 45 (71.4) | 40 (66.7) | 14 (82.4) | .33 |
| BMI, mean (SD)c | 27 (5.7) | 29.6 (5.9) | 24.4 (3.7) | 26.2 (6) | <.001b |
| Obesity (BMI >30)c | 31 (20.5) | 24 (38.1) | 4 (6.7) | 3 (17.6) | |
| Chronic renal failure | 19 (12.6) | 14 (22.2) | 5 (8.3) | 0 (0) | .02b |
| Cardiovascular comorbid conditions | |||||
| Total no., mean (SD) | 2.4 (2) | 3.8 (1.9) | 1.5 (1.5) | 1 (1.5) | <.001b |
| High blood pressure | 78 (51.7) | 46 (73.0) | 25 (41.7) | 3 (17.6) | <.001b |
| Diabetes | 56 (37.1) | 39 (61.9) | 12 (20.0) | 3 (17.6) | <.001b |
| Dyslipidemia | 62 (41.1) | 42 (66.7) | 14 (23.3) | 4 (23.5) | <.001b |
| Coronary heart disease | 51 (33.8) | 45 (71.4) | 5 (8.3) | 1 (5.9) | <.001b |
| Lower-extremity artery disease | 22 (14.6) | 15 (23.8) | 6 (10.0) | 1 (5.9) | .06 |
| Valvular disease | 22 (14.6) | 18 (28.6) | 4 (6.7) | 0 (0) | <.001b |
| Valvular prosthesis | 16 (10.6) | 14 (22.2) | 1 (1.7) | 0 (0) | <.001b |
| Cardiac implantable device | 7 (4.6) | 6 (9.5) | 0 (0) | 0 (0) | .04b |
| History of neoplasia | 46 (30.4) | 9 (14.3) | 33 (55.0) | 1 (5.9) | <.001b |
| Immunosuppression | 29 (19.2) | 8 (12.7) | 18 (30.0) | 0 (0) | .008b |
| Immunosuppressive therapy | 9 (6.0) | 6 (9.5) | 1 (1.7) | … | … |
| Recent chemotherapy | 19 (12.6) | 2 (3.2) | 16 (26.7) | … | … |
| Solid organ transplantation | 7 (4.6) | 5 (7.9) | 0 (0) | … | … |
| No known comorbid conditions | 19 (12.6) | 3 (4.8) | 7 (11.7) | 9 (52.9) | <.001b |
| Surgical history before mediastinitis | |||||
| Surgery within 1 y | 105 (69.5) | 61 (96.8) | 38 (63.3) | 1 (5.9) | <.001b |
| Cardiovascular surgery | 60 (57.1) | 58 (95.1) | 0 (0) | 0 (0) | … |
| Coronary graft | 29 (48.3) | 29 (50.0) | … | … | … |
| Valvular surgery | 19 (31.7) | 18 (31.0) | … | … | … |
| Otherd | 12 (20.0) | 11 (19.1) | … | … | … |
| Gastroesophageal surgery/procedure | 36 (34.3) | 0 (0) | 36 (94.7) | 0 (0) | … |
| Lewis-Santy procedure | 17 (47.2) | … | 17 (47.2) | … | … |
| Esophageal dilation | 7 (19.4) | … | 7 (19.4) | … | … |
| Othere | 12 (33.3) | … | 12 (33.3) | … | … |
| Surgical revision before mediastinitis | 17 (16.2) | 6 (9.8) | 9 (23.7) | 0 (0) | .10 |
| Time between initial surgery and mediastinitis, median, d | 11 (5–23) | 16 (10–28) | 5 (1–11) | 1 | … |
| Clinical and radiological data | |||||
| Fever | 110 (72.8) | 45 (71.4) | 35 (63.3) | 17 (100) | .005b |
| Chest pain | 55 (38.7) | 23 (38.3) | 22 (40.0) | 7 (41.2) | .94 |
| Sternal scar abnormalities | 48 (31.8) | 46 (73.0) | 0 (0) | 0 (0) | <.001b |
| Septic shock | 68 (45.0) | 21 (33.3) | 40 (66.7) | 3 (17.6) | <.001b |
| Chest CT performed | 138 (91.4) | 52 (82.5) | 59 (98.3) | 17 (100) | .007b |
| Chest CT contributory to diagnosis | 132/138 (95.7) | 47/52 (90.4) | 58/59 (98.3) | 17/17 (100) | .20 |
| Management | |||||
| Surgery for mediastinitis | 122 (80.8) | 58 (92.1) | 45 (75.0) | 13 (76.5) | .006b |
| Time between diagnosis and surgery, median, d | 1 (0–5) | 2 (0–8) | 0 (0–3) | 3 (0–4) | .80 |
| Perioperative evidence of mediastinitis | 99 (81.1) | 52 (89.7) | 31 (68.9) | 11 (84.6) | .052 |
| Surgical revision for mediastinitis | 38 (31.1) | 20 (34.5) | 9 (20.0) | 7 (53.4) | .10 |
| Duration of intravenous antimicrobial therapy, median, d | 32 (15–50) | 42 (20–52) | 28 (14–47) | 22 (17–34) | .42 |
| Switch to oral antimicrobial therapy | 28 (20.1) | 16 (25.4) | 5 (9.8) | 5 (33.3) | .08 |
| Total duration of antimicrobial therapy, median, d | 41 (21–56) | 49 (40–63) | 28 (15–49) | 30 (20–38) | .30 |
| Prognosis | |||||
| Length of hospital stay, median, d | 32 (21–59) | 34 (21–53) | 33 (25–64) | 25 (12–35) | .71 |
| ICU hospitalization | 129 (85.4) | 52 (82.5) | 55 (91.7) | 13 (76.5) | .24 |
| Length of ICU stay, median, d | 6 (2–18) | 4 (1–9) | 8 (4–24) | 13 (5–23) | .42 |
| All-cause mortality | 50 (33.1) | 21 (33.3) | 19 (31.7) | 2 (11.8) | .01b |
| In-hospital mortality | 36 (23.8) | 14 (22.2) | 14 (23.3) | 1 (5.9) | .01b |
| Time between inclusion and death, median, d | 32 (21–59) | 49 (19–201) | 28 (9–84) | 41 (38–43) | .28 |
Abbreviations: BMI, body mass index; CT, computed tomography; DNM, descending necrotizing mediastinitis; ICU, intensive care unit; MEP, mediastinitis due to esophageal perforation; PSM, poststernotomy mediastinitis; SD, standard deviation.
aData represent no. (%) of patients unless otherwise specified.
bSignificant at P < .05.
cBMI calculated as weight in kilograms divided by height in meters squared.
dOther cardiovascular surgery included aortic surgery (8 of 60 patients), cardiac graft (3 of 60), and electronic cardiac device implantation (1 of 60);
eOther gastroesophageal procedures included hiatal hernia repair (4 of 36 patients); esophageal diverticulum surgery (3 of 36); and stomach ulcer suture, complicated ultrasonography-endoscopy, complicated transesophageal echocardiography, surgery for spontaneous rupture of the esophagus, and cervical corpectomy complicated by an esophageal fistula (each 1 of 36).
Demographic, Clinical, and Radiological Features
The mean patent age (standard deviation) was 63.3 (14.5) years. Patients with DNM were younger than those with PSM or MEP (46.3 vs 64.9 and 65.6 years, respectively; P = .002). Most patients were male (109 of 151 [72.2%]), regardless of the etiology (P = .33).
Patients with PSM had more cardiovascular comorbid conditions than those with MEP or DNM (3.8 vs 1.5 and 1.0, respectively; P < .001). Cardiovascular comorbid conditions were noted more frequently in the PSM group. A personal history of neoplasia was more common in patients with MEP than in those with PSM or DNM (33 of 60 [55.0%] vs 9 of 63 [14.3%] and 1 of 17 [5.9%], respectively; P < .001), as was immunosuppression (18 of 60 [30.0%] vs 8 of 63 [12.7%] and 0 of 17 [0%], respectively; P = .008), typically resulting from recent chemotherapy (16 of 60 [26.7%]). Notably, 9 of 17 patients (52.9%) with DNM had no known comorbid conditions.
Sixty-five percent of patients (105 of 151) had a history of surgery in the preceding year. Among patients with PSM, the main surgical intervention was cardiac surgery (92%), consisting mostly of coronary graft procedures (29 of 61 [47.5%]) and valvular surgeries (18 of 61 [29.5%]). The median time for PSM to occur was 16 days after surgery (95% CI, 10–28 days). Among patients with MEP, 60% (36/60) had undergone a recent esophageal surgery. MEP occurred a median of 4.5 days after the procedure (95% CI, 1–10.8). NDM after a surgical procedure was noted in only 1 (5.9%) of the 17 cases included (arytenoidectomy).
Initial symptoms included fever (72.8% [110 of 151 patients]) and chest pain (8.7% [55 of 151]). Sternal scar abnormalities were noticed in 73.0% of patients with PSM (46 of 63). Furthermore, septic shock was observed in 45.0% of all patients (68 of 151), a percentage that increased up to 66.7% (40 of 60) for MEP. Most patients benefited from chest computed tomography (138 of 151 [91.4%]), which contributed to the diagnosis in 95.7% (132 of 138).
Microbiology
Microbiological documentation was available in 80.8% of cases (122 of 151), as shown in Table 2. When documented, PSM was monomicrobial in 67.9% of patients (36 of 53), whereas MEP and DNM were bimicrobial or plurimicrobial in 79.2% (38 of 48) and 66.6% (8 of 12), respectively (P < .001).
| Microbial Documentation . | Patients, No. (%) . | P Value . | |||
|---|---|---|---|---|---|
| All Mediastinitis (N = 151) . | PSM (n = 63 [41.7%]) . | MEP (n = 60 [39.7%]) . | DNM (n = 17 [11.3%]) . | ||
| No documentation | 29 (19.2) | 10 (15.9) | 12 (20) | 5 (29.4) | .84 |
| Documented | 122 (80.8) | 53 (84.1) | 48 (80.0) | 12 (70.6) | .84 |
| Monomicrobial | 53 (44.4) | 36 (67.9) | 10 (20.8) | 4 (33.3) | <.001a |
| Bimicrobial or plurimicrobial | 69 (56.6) | 17 (32.1) | 38 (79.2) | 8 (66.6) | <.001a |
| Bacteremia | 60 (39.7) | 34 (54) | 20 (33.3) | 3 (17.6) | .02a |
| Microorganism | |||||
| Staphylococcib | 60 (49.2) | 36 (67.9) | 13 (27.1) | 4 (33.3) | <.001a |
| MSSA | 25 (20.5) | 14 (26.4) | 5 (10.4) | 4 (33.3) | .12 |
| MRSA | 1 (<1) | 1 (1.9) | 0 (0) | 0 (0) | >.99 |
| Coagulase-negative staphylococci | 36 (29.5) | 21 (39.6) | 9 (18.8) | 1 (8.3) | .007a |
| Streptococci | 28 (23.0) | 0 (0) | 20 (41.7) | 8 (66.6) | <.001a |
| Enterococci | 21 (17.2) | 8 (15.1) | 12 (25.0) | 1 (8.3) | .10 |
| Enterobacteriaceae | 48 (39.3) | 20 (37.7) | 23 (47.9) | 0 (0) | .02a |
| Nonfermenting gram-negative bacilli | 7 (5.7) | 1 (1.9) | 5 (10.4) | 1 (8.3) | .18 |
| Anaerobes | 31 (25.4) | 4 (7.5) | 20 (41.7) | 6 (50.0) | <.001a |
| Fungi | 41 (33.6) | 6 (11.3) | 28 (58.3) | 3 (25) | <.001a |
| Other | 6 (4.9) | 0 (0) | 6 (12.5) | 0 (0) | .03 |
| Microbial Documentation . | Patients, No. (%) . | P Value . | |||
|---|---|---|---|---|---|
| All Mediastinitis (N = 151) . | PSM (n = 63 [41.7%]) . | MEP (n = 60 [39.7%]) . | DNM (n = 17 [11.3%]) . | ||
| No documentation | 29 (19.2) | 10 (15.9) | 12 (20) | 5 (29.4) | .84 |
| Documented | 122 (80.8) | 53 (84.1) | 48 (80.0) | 12 (70.6) | .84 |
| Monomicrobial | 53 (44.4) | 36 (67.9) | 10 (20.8) | 4 (33.3) | <.001a |
| Bimicrobial or plurimicrobial | 69 (56.6) | 17 (32.1) | 38 (79.2) | 8 (66.6) | <.001a |
| Bacteremia | 60 (39.7) | 34 (54) | 20 (33.3) | 3 (17.6) | .02a |
| Microorganism | |||||
| Staphylococcib | 60 (49.2) | 36 (67.9) | 13 (27.1) | 4 (33.3) | <.001a |
| MSSA | 25 (20.5) | 14 (26.4) | 5 (10.4) | 4 (33.3) | .12 |
| MRSA | 1 (<1) | 1 (1.9) | 0 (0) | 0 (0) | >.99 |
| Coagulase-negative staphylococci | 36 (29.5) | 21 (39.6) | 9 (18.8) | 1 (8.3) | .007a |
| Streptococci | 28 (23.0) | 0 (0) | 20 (41.7) | 8 (66.6) | <.001a |
| Enterococci | 21 (17.2) | 8 (15.1) | 12 (25.0) | 1 (8.3) | .10 |
| Enterobacteriaceae | 48 (39.3) | 20 (37.7) | 23 (47.9) | 0 (0) | .02a |
| Nonfermenting gram-negative bacilli | 7 (5.7) | 1 (1.9) | 5 (10.4) | 1 (8.3) | .18 |
| Anaerobes | 31 (25.4) | 4 (7.5) | 20 (41.7) | 6 (50.0) | <.001a |
| Fungi | 41 (33.6) | 6 (11.3) | 28 (58.3) | 3 (25) | <.001a |
| Other | 6 (4.9) | 0 (0) | 6 (12.5) | 0 (0) | .03 |
Abbreviations: DNM, descending necrotizing mediastinitis; MEP, mediastinitis due to esophageal perforation; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; PSM, poststernotomy mediastinitis.
aSignificant at P < .05.
bSeveral species of staphylococci may have been isolated in the same patient, so the sum of subcategories may exceed the total.
| Microbial Documentation . | Patients, No. (%) . | P Value . | |||
|---|---|---|---|---|---|
| All Mediastinitis (N = 151) . | PSM (n = 63 [41.7%]) . | MEP (n = 60 [39.7%]) . | DNM (n = 17 [11.3%]) . | ||
| No documentation | 29 (19.2) | 10 (15.9) | 12 (20) | 5 (29.4) | .84 |
| Documented | 122 (80.8) | 53 (84.1) | 48 (80.0) | 12 (70.6) | .84 |
| Monomicrobial | 53 (44.4) | 36 (67.9) | 10 (20.8) | 4 (33.3) | <.001a |
| Bimicrobial or plurimicrobial | 69 (56.6) | 17 (32.1) | 38 (79.2) | 8 (66.6) | <.001a |
| Bacteremia | 60 (39.7) | 34 (54) | 20 (33.3) | 3 (17.6) | .02a |
| Microorganism | |||||
| Staphylococcib | 60 (49.2) | 36 (67.9) | 13 (27.1) | 4 (33.3) | <.001a |
| MSSA | 25 (20.5) | 14 (26.4) | 5 (10.4) | 4 (33.3) | .12 |
| MRSA | 1 (<1) | 1 (1.9) | 0 (0) | 0 (0) | >.99 |
| Coagulase-negative staphylococci | 36 (29.5) | 21 (39.6) | 9 (18.8) | 1 (8.3) | .007a |
| Streptococci | 28 (23.0) | 0 (0) | 20 (41.7) | 8 (66.6) | <.001a |
| Enterococci | 21 (17.2) | 8 (15.1) | 12 (25.0) | 1 (8.3) | .10 |
| Enterobacteriaceae | 48 (39.3) | 20 (37.7) | 23 (47.9) | 0 (0) | .02a |
| Nonfermenting gram-negative bacilli | 7 (5.7) | 1 (1.9) | 5 (10.4) | 1 (8.3) | .18 |
| Anaerobes | 31 (25.4) | 4 (7.5) | 20 (41.7) | 6 (50.0) | <.001a |
| Fungi | 41 (33.6) | 6 (11.3) | 28 (58.3) | 3 (25) | <.001a |
| Other | 6 (4.9) | 0 (0) | 6 (12.5) | 0 (0) | .03 |
| Microbial Documentation . | Patients, No. (%) . | P Value . | |||
|---|---|---|---|---|---|
| All Mediastinitis (N = 151) . | PSM (n = 63 [41.7%]) . | MEP (n = 60 [39.7%]) . | DNM (n = 17 [11.3%]) . | ||
| No documentation | 29 (19.2) | 10 (15.9) | 12 (20) | 5 (29.4) | .84 |
| Documented | 122 (80.8) | 53 (84.1) | 48 (80.0) | 12 (70.6) | .84 |
| Monomicrobial | 53 (44.4) | 36 (67.9) | 10 (20.8) | 4 (33.3) | <.001a |
| Bimicrobial or plurimicrobial | 69 (56.6) | 17 (32.1) | 38 (79.2) | 8 (66.6) | <.001a |
| Bacteremia | 60 (39.7) | 34 (54) | 20 (33.3) | 3 (17.6) | .02a |
| Microorganism | |||||
| Staphylococcib | 60 (49.2) | 36 (67.9) | 13 (27.1) | 4 (33.3) | <.001a |
| MSSA | 25 (20.5) | 14 (26.4) | 5 (10.4) | 4 (33.3) | .12 |
| MRSA | 1 (<1) | 1 (1.9) | 0 (0) | 0 (0) | >.99 |
| Coagulase-negative staphylococci | 36 (29.5) | 21 (39.6) | 9 (18.8) | 1 (8.3) | .007a |
| Streptococci | 28 (23.0) | 0 (0) | 20 (41.7) | 8 (66.6) | <.001a |
| Enterococci | 21 (17.2) | 8 (15.1) | 12 (25.0) | 1 (8.3) | .10 |
| Enterobacteriaceae | 48 (39.3) | 20 (37.7) | 23 (47.9) | 0 (0) | .02a |
| Nonfermenting gram-negative bacilli | 7 (5.7) | 1 (1.9) | 5 (10.4) | 1 (8.3) | .18 |
| Anaerobes | 31 (25.4) | 4 (7.5) | 20 (41.7) | 6 (50.0) | <.001a |
| Fungi | 41 (33.6) | 6 (11.3) | 28 (58.3) | 3 (25) | <.001a |
| Other | 6 (4.9) | 0 (0) | 6 (12.5) | 0 (0) | .03 |
Abbreviations: DNM, descending necrotizing mediastinitis; MEP, mediastinitis due to esophageal perforation; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; PSM, poststernotomy mediastinitis.
aSignificant at P < .05.
bSeveral species of staphylococci may have been isolated in the same patient, so the sum of subcategories may exceed the total.
The main microorganisms isolated were as follows: in PSM, coagulase-negative staphylococci (21 of 53 [39.6%]), Enterobacteriaceae (20 of 53 [37.7%]), and Staphylococcus aureus (15 of 53 [28.3%]); in MEP, fungi (28 of 48 [58.3%], all corresponding to yeasts, with a clear predominance of Candida albicans, followed by Candida glabrata), Enterobacteriaceae (23 of 48 [47.9%]), and streptococci and anaerobes (both 20 of 48 [41.7%]); in DNM, streptococci (8 of 12 [66.6%]), anaerobes (6 of 12 [50.0%]), and S aureus (4 of 12 [33.3%]). Bacteremia was identified in 34 of 63 patients with PSM (54.0%), in 20 of 60 (33.3%) with MEP, and in 3 of 17 (17.6%) with DNM (P = .02).
Mediastinitis Management
Debridement surgery was carried out in 122 of 151 patients (80.8%) and was more common in those with PSM (58 of 63 [92.1%] vs 45 of 60 [75.0%] with MEP and 13 of 17, [76.5%] with DNM; P = .006). The median time (IQR) between diagnosis and surgery was 1 (0–5) day, without significant differences among the groups. Surgery found perioperative evidence of infection in 81.1% of patients (99 of 122), and surgical revision was deemed necessary for 31.1% (38 of 122). Of 151 patients, 129 (85.4%) required intensive care, with a median (IQR) intensive care unit stay of 6 (2–18) days. The median (IQR) hospital stay was 32 (21–59) days.
The median (IQR) total duration of antimicrobial therapy was 41 (21–56) days. It was longer in patients with PSM, although the difference was not significant (48 days vs 28 for MEP and 30 for DNM; P = .30). The median (IQR) total duration of intravenous antimicrobial therapy was 32 (15–50) days, and an oral switch was carried out in 28 of 151 patients (18.5%).
Outcomes
Fifty patients (33.1%) died during follow-up, within a median (IQR) of 32 (21–59) days after inclusion (49 days for PSM, 28 for MEP, and 41 for DNM; P = .28). The mortality rate was significantly lower in patients with DNM (11.8% [2 of 17]) than in those with PSM (33.3% [21 of 63]) or MEP (31.7% [19 of 60]) (P = .01). The in-hospital mortality rate was 23.8% (36 of 151), ranging from 5.9% for patients with DNM (1 of 17) to 23.3% for patients with MEP (14 of 60) (P = .01).
Survival analysis showed that the 1-year estimated survival was 64.8% (95% CI, 56.6%–74.3%). However, we observed disparities depending on the origin: 1-year estimated survival was 80% for patients DNM, 71% for those with PSM, and 61% for those with MEP. Survival curves are presented in Figure 2.
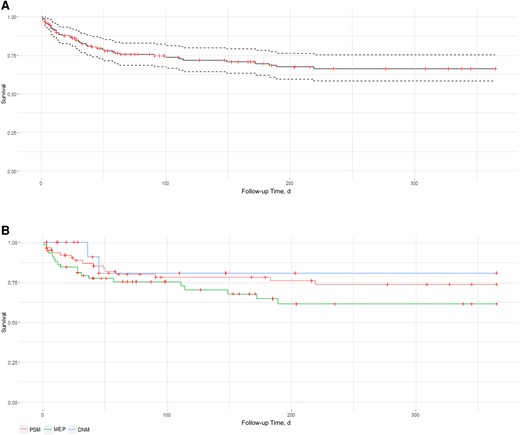
Survival curves: all-cause mortality at one-year follow-up. Abbreviations: DNM, descending necrotizing mediastinitis; MEP, mediastinitis due to esophageal perforation; PSM, poststernotomy mediastinitis. A: All mediastinitis. B: For the main aetiologies: PSM, mediastinitis associated with oesophageal perforation (MOP), DNM.
DISCUSSION
Mediastinitis Origins
Our findings revealed that PSM was the main cause of mediastinitis in our center, with 63 registered cases (42%) over a period of 10 years, mostly involving male patients aged 60–70 years, with increased body mass index and multiple cardiovascular comorbid conditions, as previously reported [10, 19, 20]. The incidence of PSM in this study was 0.6%, which is in the low range compared with current literature [2, 3, 5, 7]. Mediastinitis usually occurred within a month after sternotomy, which is consistent with the results of previous studies reporting delays of 2 weeks [21, 22]. However, we noted that 25% of our cases occurred after a period of 28 days, highlighting the possibility of a subacute course. Remarkably, 54% of patients with PSM had bacteremia. A study performed by Cobo et al [23] found similar results and evaluated the specificity of positive blood cultures for the diagnosis of mediastinitis at >90%. It has even been proposed that the incidence of any bacteremia within 90 days from sternotomy should suggest the development of mediastinitis, especially if S aureus is involved [16, 24].
MEP was the second most frequent etiology of mediastinitis, with 60 cases (40%). Most patients had a diagnosis of esophageal or gastric neoplasia, for which they benefited from a surgery or an endoscopic procedure. The time between this procedure and the onset of mediastinitis was shorter than that for PSM. One hypothesis could be a greater bacterial inoculum from a massive contamination by the digestive flora as a result of the perforation. This may also help explain the significantly more frequent cases of septic shock reported at onset in this group of patients. Finally, DNM was the third cause of mediastinitis, with 17 cases (11%), mostly involving younger patients that were often free of any comorbid conditions, as already presented [25].
Microbiology
Regarding PSM, the predominance of staphylococci and Enterobacteriaceae has been described elsewhere [3, 5]. Of all staphylococci species, we noted that coagulase-negative staphylococci were noticeably involved in our findings despite excluding contamination. MEP and DNM were both principally plurimicrobial and involved various aero-anaerobic microorganisms, reflecting the involvement of digestive and oral flora, respectively [15, 18, 25].
Mediastinitis Management
Data on optimal antimicrobial therapy are scarce. Improved knowledge of the microbiology associated with mediastinitis may help clinicians optimize their decisions for empirical regimen. According to our findings, a combination therapy of a broad-spectrum β-lactam (piperacillin-tazobactam or cefepime) and daptomycin or vancomycin could be appropriate for PSM. In cases of MEP or DNM, empirical therapy should aim at the mixed aero-anaerobic flora, where resistance to β-lactams is frequent due to the production of β-lactamase [26]. A broad-spectrum β-lactam plus an antibiotic that is effective against most anaerobes could be appropriate, such as a third-generation cephalosporin plus metronidazole for DNM, whereas piperacillin-tazobactam or cefepime plus metronidazole may be more appropriate for MEP to cover a wide range of Enterobacteriaceae. The isolation of fungal species in almost half of the cases of MEP raises questions regarding the relevance of an empirical antifungal treatment. Above all, it may be judicious in cases of septic shock, immunosuppression, or prior antibiotic therapy within 48 hours, similar to what has been proposed for intra-abdominal infections [27].
Table 3 demonstrates our propositions for empirical antimicrobial therapy. These propositions are only indicative, as the local epidemiology should also be considered when prescribing. Therapy is usually prolonged, ranging from weeks to months [16, 28]. However, there is a lack of data to define the optimal duration. In our cohort, antimicrobial therapy duration in PSM was consistent with previous studies [29, 30] and expert opinions [16]. The common involvement of sternal bone or foreign bodies, such as sternal wires, may justify a treatment duration of ≥6 weeks in most cases. The removal of foreign material should always be discussed, as it may facilitate the persistence of a biofilm and foster infection relapse [31].
| Etiology . | Suggested Empiric Therapya . |
|---|---|
| PSM | Piperacillin-tazobactam or cefepime plus metronidazole and vancomycin or daptomycin; if sign of gravityb, consider adjunctive use of an aminoglycoside |
| MEP | Piperacillin-tazobactam or cefepim plus metronidazole; consider adjunctive caspofungin; if sign of gravityb, consider adjunctive use of an aminoglycoside |
| DNM | Third-generation cephalosporin plus metronidazole; if sign of gravityb, consider adjunctive use of an aminoglycoside |
| Etiology . | Suggested Empiric Therapya . |
|---|---|
| PSM | Piperacillin-tazobactam or cefepime plus metronidazole and vancomycin or daptomycin; if sign of gravityb, consider adjunctive use of an aminoglycoside |
| MEP | Piperacillin-tazobactam or cefepim plus metronidazole; consider adjunctive caspofungin; if sign of gravityb, consider adjunctive use of an aminoglycoside |
| DNM | Third-generation cephalosporin plus metronidazole; if sign of gravityb, consider adjunctive use of an aminoglycoside |
Abbreviations: DMN, descending necrotizing mediastinitis; MEP, mediastinitis due to esophageal perforation; PSM, poststernotomy mediastinitis.
aThese suggestions are based on observational data. Local epidemiology regarding antimicrobial susceptibility should always be considered when prescribing treatment. bSign of gravity: sepsis with widespread repercussion or septick shock.
| Etiology . | Suggested Empiric Therapya . |
|---|---|
| PSM | Piperacillin-tazobactam or cefepime plus metronidazole and vancomycin or daptomycin; if sign of gravityb, consider adjunctive use of an aminoglycoside |
| MEP | Piperacillin-tazobactam or cefepim plus metronidazole; consider adjunctive caspofungin; if sign of gravityb, consider adjunctive use of an aminoglycoside |
| DNM | Third-generation cephalosporin plus metronidazole; if sign of gravityb, consider adjunctive use of an aminoglycoside |
| Etiology . | Suggested Empiric Therapya . |
|---|---|
| PSM | Piperacillin-tazobactam or cefepime plus metronidazole and vancomycin or daptomycin; if sign of gravityb, consider adjunctive use of an aminoglycoside |
| MEP | Piperacillin-tazobactam or cefepim plus metronidazole; consider adjunctive caspofungin; if sign of gravityb, consider adjunctive use of an aminoglycoside |
| DNM | Third-generation cephalosporin plus metronidazole; if sign of gravityb, consider adjunctive use of an aminoglycoside |
Abbreviations: DMN, descending necrotizing mediastinitis; MEP, mediastinitis due to esophageal perforation; PSM, poststernotomy mediastinitis.
aThese suggestions are based on observational data. Local epidemiology regarding antimicrobial susceptibility should always be considered when prescribing treatment. bSign of gravity: sepsis with widespread repercussion or septick shock.
In the current study, treatment of DNM and MEP was shorter, with a median duration of 4 weeks. Most previous studies support that the antimicrobial therapy should last for at least 2 weeks after surgery [25, 28, 32, 33]. However, certain situations should be treated cautiously, such as the persistence of a breach or fistula, particularly in patients with MEP. Importantly treatment of mediastinitis also relies on surgical debridement, which should be considered promptly, as its precocity is one of the main prognostic factors at the acute phase of the infection [34].
Outcome
The mortality rate among patients with PSM was higher than reported previously [2, 7], as it was for MEP [35, 36]. We believe that difference this could be explained by a selection bias, because all included patients in the present study had an established diagnosis of mediastinitis, whereas other studies included either patients with any type of deep sternal wound infection or any patient with esophageal perforation.
The mortality rate for DNM was the lowest, although several patients were lost to follow-up. We may assume that the patients lost to follow-up were in good health condition, given their excellent evolution reported at the early stages of disease management and the lack of any contact with our hospital settings. Moreover, this rate was consistent with those previously reported, such as by Palma et al [25], who reported a mortality rate of 12% in 2016.
Study Strengths and Limitations
The main strength of the current study is the relatively large population sample, which is an asset, considering the rarity of this disease. To the best of our knowledge, this study is the first to analyze and compare various cases of mediastinitis according to the origin of the infection and also propose an in-depth analysis of the respective microbiological documentation. However, its single-center nature limits the generalizability of our results, especially pertaining to our microbiological findings, and the empirical antibiotic regimens we proposed are based on observational data only. Furthermore, confounding biases are expected when comparing mediastinitis cases according their origins, so comparisons should be made with caution.
In conclusion, the current study highlighted three underlying causes of mediastinitis: esophageal perforation, poststernotomy complication, and DNM. This study emphasizes the peculiarities of each of these situations in terms of demographics, microbiology, and prognosis. Empirical antimicrobial therapy regimens and treatment durations could therefore be considered, depending on the origin of this infection. However, larger randomized trials are necessary to clarify the optimal management of these complex infections.
Acknowledgments
The authors thank the following colleagues from Strasbourg University Hospital for their collaboration: Patrick Ohlmann (Cardiology Department), Nabil Chafke (Vascular Surgery Department), Fehrat Meziani (Medical Intensive Care), Francis Schneider (Intensive Care), Julian Pottecher (Surgical Intensive Care), and Paul-Michel Mertes (Cardiovascular Intensive Care).
Author contributions. Conceptualization and methodology: T. L. and Y. R. Validation: N. L., Y. H., and Y.R. Formal analysis: T. L. and T. F. Investigation: T. L. and Y. R. Data curation: T. L. and Y. R. Writing—original draft: T. L. Writing—review and editing: T. L., J. P. M., O. C., L. F., D. M., C. B., P. E. F., F. D., N. L., M. B. W., V. G., B. H., Y. H., and Y. R. All authors revised and approved the final report.
Financial support. The authors received no external funding for this work.
References
Author notes
Potential conflicts of interest. F. D. declares personal fees from Gilead, Pfizer, and MSD, outside the submitted work. V. G. declares honoraria from Pfizer, outside the submitted work. Y. H. declares receiving support for attending meetings and/or travel from Pfizer, outside the submitted work. All other authors report no potential conflicts.





Comments