-
PDF
- Split View
-
Views
-
Cite
Cite
Alina Adeel, Ming D Qu, Efaza Siddiqui, Stuart M Levitz, Richard T Ellison, Expanded Access Use of Rezafungin for Salvage Therapy of Invasive Candida glabrata Infection: A Case Report, Open Forum Infectious Diseases, Volume 8, Issue 11, November 2021, ofab431, https://doi.org/10.1093/ofid/ofab431
Close - Share Icon Share
Abstract
Rezafungin is a semisynthetic, long-acting echinocandin with broad-spectrum activity against many Candida species and Aspergillus species, including a subset of drug-resistant strains. It is currently in phase III trials and was found to be safe and effective for the treatment of candidemia and/or invasive Candida infections in a phase II trial. However, there are no long-term safety or efficacy data. We report on the successful ongoing compassionate use of rezafungin obtained through expanded access for over 1 year in a patient with a multidrug-resistant Candida glabrata mediastinal infection from a vascular graft infection and retained foreign material.
Invasive fungal infections are a significant cause of mortality and morbidity in humans despite the availability of several antifungal agents [1]. The rise in resistant isolates and species due to selective pressure from use as prophylaxis and treatment in various medical conditions has been particularly problematic, with some isolates being resistant to all currently available antifungals [2]. New antifungal agents have been slow to progress, with only a couple of new agents approved and marketed in the last decade [3]. One of the new antifungals in the pipeline, currently in phase III trials, is rezafungin [4, 5], a novel, long-acting, semisynthetic echinocandin that has broad-spectrum antifungal activity including against subsets of drug-resistant strains [6]. We report on the expanded-access successful use of rezafungin for >1 year for management of a case of recurrent mediastinitis due to a multidrug-resistant Candida glabrata isolate. The patient was initially treated with various antifungal agents with evolution of resistance to azoles and echinocandins, followed by rezafungin with good clinical response and significantly improved tolerability.
CASE REPORT
The patient is a 49-year-old man with no significant medical history, who initially presented to the hospital with a traumatic thoracic aortic tear due to a motor vehicle accident in 2000 requiring emergent thoracic aortic graft repair and splenectomy. In June 2017, he presented to the hospital with fever and chills. Workup revealed thoracic aortic graft infection (Figure 1) and an aorto-bronchial fistula involving the aortic graft; he then underwent resection of the left upper lobe of the lung, explantation of the infected aortic graft, and placement of a new thoracic aortic Dacron graft. Cultures from the explanted graft grew C. albicans, C. glabrata (Isolate 1, Table 1), and Lactobacillus paracasei. The postoperative course was complicated by chylothorax due to disruption of the thoracic duct, which required embolization with multiple platinum-fibered coils. Antimicrobial therapy with intravenous (IV) piperacillin-tazobactam, IV vancomycin, and IV micafungin was completed after 6 weeks, and suppressive therapy with oral fluconazole 800 mg daily was begun, given the concern that the new graft was in a contaminated field.
Minimum Inhibitory Concentration Values for Candida glabrata Isolates for Different Antifungal Agents With Clinical and Laboratory Standards Institute Interpretations
| Drug . | Susceptibility (MIC, µg/mL) and Clinical Interpretation . | . | . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| . | Isolate 1 . | . | Isolate 2 . | . | Isolate 3 . | . | Isolate 4 . | . |
| . | MIC . | Int. . | MIC . | Int. . | MIC . | Int. . | MIC . | Int. . |
| Caspofungin | ≤0.25 | S | 0.25 | I | 0.5 | R | 0.5 | I |
| Fluconazole | 4 | S | 256 | R | >256 | R | 64 | R |
| Voriconazole | 0.25 | NA | 4 | NA | >8 | NA | ||
| Anidulafungin | 0.12 | S | 0.5 | R | 1 | R | ||
| Rezafungin | 2 | NA | ||||||
| Micafungin | 0.03 | S | 0.12 | I | 0.5 | R | ||
| 5-flucytosine | ≤0.06 | NA | ≤0.06 | NA | ||||
| Posaconazole | 1 | NA | >8 | NA | ||||
| Itraconazole | 1 | NA | >16 | NA | ||||
| Amphotericin B | ≤0.12 | NA | 1 | NA | 0.5 | NA |
| Drug . | Susceptibility (MIC, µg/mL) and Clinical Interpretation . | . | . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| . | Isolate 1 . | . | Isolate 2 . | . | Isolate 3 . | . | Isolate 4 . | . |
| . | MIC . | Int. . | MIC . | Int. . | MIC . | Int. . | MIC . | Int. . |
| Caspofungin | ≤0.25 | S | 0.25 | I | 0.5 | R | 0.5 | I |
| Fluconazole | 4 | S | 256 | R | >256 | R | 64 | R |
| Voriconazole | 0.25 | NA | 4 | NA | >8 | NA | ||
| Anidulafungin | 0.12 | S | 0.5 | R | 1 | R | ||
| Rezafungin | 2 | NA | ||||||
| Micafungin | 0.03 | S | 0.12 | I | 0.5 | R | ||
| 5-flucytosine | ≤0.06 | NA | ≤0.06 | NA | ||||
| Posaconazole | 1 | NA | >8 | NA | ||||
| Itraconazole | 1 | NA | >16 | NA | ||||
| Amphotericin B | ≤0.12 | NA | 1 | NA | 0.5 | NA |
Isolate 1: aortic graft explant in 2017; Isolate 2: thoracic duct embolization coil in 2019; Isolate 3: first aortic Dacron graft explant tissue isolate; Isolate 4: second aortic Dacron graft explant tissue isolate in 2020 (48-hour MIC data are reported due to insufficient growth at 24 hours; isolate possesses FKS2 D666Y mutation).
Abbreviations: I, intermediate; MIC, minimum inhibitory concentration; NA, not applicable; R, resistant; S, susceptible.
Minimum Inhibitory Concentration Values for Candida glabrata Isolates for Different Antifungal Agents With Clinical and Laboratory Standards Institute Interpretations
| Drug . | Susceptibility (MIC, µg/mL) and Clinical Interpretation . | . | . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| . | Isolate 1 . | . | Isolate 2 . | . | Isolate 3 . | . | Isolate 4 . | . |
| . | MIC . | Int. . | MIC . | Int. . | MIC . | Int. . | MIC . | Int. . |
| Caspofungin | ≤0.25 | S | 0.25 | I | 0.5 | R | 0.5 | I |
| Fluconazole | 4 | S | 256 | R | >256 | R | 64 | R |
| Voriconazole | 0.25 | NA | 4 | NA | >8 | NA | ||
| Anidulafungin | 0.12 | S | 0.5 | R | 1 | R | ||
| Rezafungin | 2 | NA | ||||||
| Micafungin | 0.03 | S | 0.12 | I | 0.5 | R | ||
| 5-flucytosine | ≤0.06 | NA | ≤0.06 | NA | ||||
| Posaconazole | 1 | NA | >8 | NA | ||||
| Itraconazole | 1 | NA | >16 | NA | ||||
| Amphotericin B | ≤0.12 | NA | 1 | NA | 0.5 | NA |
| Drug . | Susceptibility (MIC, µg/mL) and Clinical Interpretation . | . | . | . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| . | Isolate 1 . | . | Isolate 2 . | . | Isolate 3 . | . | Isolate 4 . | . |
| . | MIC . | Int. . | MIC . | Int. . | MIC . | Int. . | MIC . | Int. . |
| Caspofungin | ≤0.25 | S | 0.25 | I | 0.5 | R | 0.5 | I |
| Fluconazole | 4 | S | 256 | R | >256 | R | 64 | R |
| Voriconazole | 0.25 | NA | 4 | NA | >8 | NA | ||
| Anidulafungin | 0.12 | S | 0.5 | R | 1 | R | ||
| Rezafungin | 2 | NA | ||||||
| Micafungin | 0.03 | S | 0.12 | I | 0.5 | R | ||
| 5-flucytosine | ≤0.06 | NA | ≤0.06 | NA | ||||
| Posaconazole | 1 | NA | >8 | NA | ||||
| Itraconazole | 1 | NA | >16 | NA | ||||
| Amphotericin B | ≤0.12 | NA | 1 | NA | 0.5 | NA |
Isolate 1: aortic graft explant in 2017; Isolate 2: thoracic duct embolization coil in 2019; Isolate 3: first aortic Dacron graft explant tissue isolate; Isolate 4: second aortic Dacron graft explant tissue isolate in 2020 (48-hour MIC data are reported due to insufficient growth at 24 hours; isolate possesses FKS2 D666Y mutation).
Abbreviations: I, intermediate; MIC, minimum inhibitory concentration; NA, not applicable; R, resistant; S, susceptible.
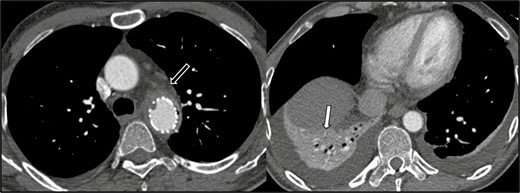
Axial CT angiogram sections below the level of the aortic arch, performed on June 11, 2017. Soft tissue thickening and stranding surrounding the descending thoracic aortic stent graft concerning for graft infection (black arrow). There is no mediastinal fluid collection. There is right lower lobe consolidation and/or collapse (white arrow) and small bilateral pleural effusions. Abbreviation: CT, computed tomography.
In October 2019, the patient had recurrent fever and chest pain; a repeat chest computed tomography (CT) scan found increased mediastinal fluid, pneumomediastinum, a potential thrombus in the graft, and a suspected aorto-esophageal fistula. Esophageal endoscopy indicated migration of 1 of the platinum coils from the thoracic duct embolization protruding through the esophageal wall, along with purulent secretions, but no evidence of aorto-esophageal fistula. The coil was removed endoscopically and grew C. glabrata on culture that demonstrated intermediate susceptibility to caspofungin (Isolate 2, Table 1). Antimicrobial therapy including IV micafungin was started and was later changed to IV liposomal amphotericin B after a chest CT scan showed increased gas in the mediastinum as well as narrowing and irregularity of the aortic graft (Figure 2). IV liposomal amphotericin was ultimately changed to oral posaconazole after 6 weeks of therapy.
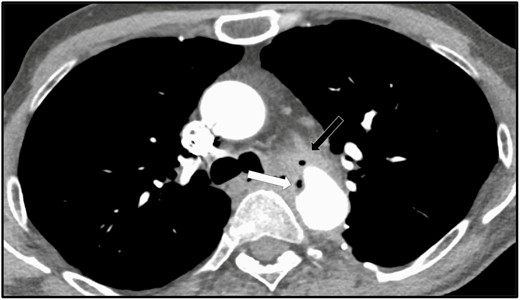
Axial contrast-enhanced CT section at the level of the aortic arch, performed on October 9, 2019 (32 months post–thoracic aortic Dacron graft placement). Unchanged Dacron interposition graft replacement of the descending thoracic aorta, with persistent mediastinal soft tissue formation anterior to the graft at the level of the distal anastomosis (black arrow), more air within the abnormal soft tissue formation, as well as more irregularity and mild narrowing of the anterior aspect of the distal anastomosis (white arrow), consistent with ongoing infectious process. Abbreviation: CT, computed tomography.
In February 2020, due to the recurrent mediastinitis while on antifungal therapy with oral posaconazole, the patient underwent a 2-stage procedure with placement of an extra anatomic ascending aorta to supraceliac abdominal aorta bypass graft, followed by excision of the infected thoracic aortic Dacron graft that was originally placed in June 2017. However, the remaining thoracic duct platinum coils were not amenable to safe extraction and removal. Explanted thoracic graft tissue cultures grew 2 different isolates of C. glabrata (Isolates 3, 4, Table 1). The postoperative course was complicated by esophageal perforation requiring a change in antifungal therapy to IV micafungin due to risk of exacerbating esophageal perforation with oral administration. Based on the susceptibility results for both C. glabrata isolates—one (Isolate 3) that demonstrated intermediate susceptibility and another (Isolate 4) that demonstrated resistance to micafungin—the antifungal therapy was changed to IV liposomal amphotericin B and oral flucytosine, given with a concurrent 3-week course of antibiotic therapy. Both antifungals were discontinued after 6 weeks of therapy due to renal toxicity.
During outpatient follow-up in April 2020, several long-term suppressive options were discussed due to the retained platinum coils and concern of ongoing chronic infection with multidrug-resistant C. glabrata. Antifungal susceptibility testing to rezafungin was performed on an isolate (Isolate 4) from the aortic graft material using the Clinical and Laboratory Standards Institute (CLSI) reference method for broth microdilution antifungal susceptibility testing of yeasts [7]. Sanger sequencing [8] was performed on polymerase chain reaction–amplified “hot spot” (HS) HS1- and HS2-encoding regions of FKS1 and FKS2 genes, which showed a D666Y mutation in the FKS2 gene HS1 [9]. Informed consent and institutional review board approval were obtained through expanded access to starting rezafungin on a compassionate use basis. IV rezafungin was started in May 2020 with a loading dose of 400 mg, followed by maintenance dosing of 200 mg weekly, which remains ongoing as of this writing. The patient has not exhibited laboratory abnormalities and has only had a transient rash with no evidence of clinical toxicities related to use of rezafungin (Table 2). Serial serum β-D-glucan assays remained negative from May 2020 through June 2021, and a repeat chest CT angiogram in October 2020 did not show any findings suggestive of ongoing infection. A timeline of the patient’s clinical course and antifungal therapy is presented in Figure 3.
Laboratory Results Including Comprehensive Metabolic Panel, Serum B-D-Glucan, and Complete Blood Count Over a Period of 1 Year While on Therapy With Rezafungin
| Test . | 2020 . | . | . | . | . | . | . | . | 2021 . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | May . | June . | July . | Aug . | Sept . | Oct . | Nov . | Dec . | Jan . | Feb . | Mar . | Apr . | May . |
| Sodium, mmol/L | 141 | 141 | 140 | 140 | 140 | 141 | 140 | 140 | 141 | 141 | 140 | 140 | 139 |
| Potassium, mmol/L | 3.7 | 3.7 | 3.7 | 4.5 | 4.1 | 3.9 | 4.1 | 4.3 | 4.1 | 3.9 | 3.7 | 4.1 | 3.9 |
| Chloride, mmol/L | 105 | 107 | 106 | 105 | 106 | 106 | 107 | 106 | 105 | 107 | 106 | 107 | 104 |
| CO2, mmol/L | 28 | 25 | 24 | 26 | 26 | 27 | 26 | 24 | 26 | 26 | 26 | 25 | 25 |
| Glucose, mg/dL | 103 | 85 | 89 | 91 | 93 | 89 | 90 | 108 | 93 | 99 | 109 | 86 | 106 |
| BUN, mg/dL | 28 | 14 | 17 | 17 | 14 | 18 | 17 | 14 | 21 | 17 | 23 | 19 | 15 |
| Creatinine, mg/dL | 0.91 | 0.90 | 1.03 | 1.07 | 0.83 | 0.92 | 0.90 | 1.08 | 0.89 | 1.08 | 1.06 | 0.93 | 1.05 |
| T. bili, mg/dL | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.30 | 0.3 | 0.4 | 0.3 | 0.4 | 0.3 |
| Alk phos, U/L | 129 | 115 | 113 | 114 | 114 | 105 | 116 | 100 | 99 | 90 | 97 | 89 | 96 |
| AST, U/L | 15 | 11 | 10 | 13 | 15 | 16 | 15 | 20 | 17 | 15 | 15 | 13 | 20 |
| ALT, U/L | 11 | 9 | 7 | 9 | 11 | 13 | 15 | 16 | 15 | 12 | 14 | 11 | 14 |
| Hemoglobin, g/dL | 10.8 | 10.4 | 10.9 | 11.1 | 11.2 | 11.3 | 11.3 | 12.0 | 11.5 | 11.8 | 12.1 | 12.4 | 13.7 |
| Hematocrit, % | 33.0 | 32.9 | 34.7 | 34.0 | 34.9 | 35.1 | 36.0 | 38.5 | 35.9 | 37.0 | 37.4 | 38.2 | 41.8 |
| WBC, 103/µL | 6.8 | 6.4 | 6.8 | 5.7 | 6.3 | 6.0 | 7.1 | 6.8 | 6.3 | 7.2 | 9.3 | 7.3 | 5.9 |
| Platelet, 103/µL | 274 | 295 | 280 | 293 | 248 | 227 | 235 | 256 | 252 | 290 | 291 | 272 | 212 |
| β-D-glucan | 49 | 53 | 33 | <31 | <31 | 40 | 56 | 32 | 55 | 52 | 56 | <31 | 39 |
| Test . | 2020 . | . | . | . | . | . | . | . | 2021 . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | May . | June . | July . | Aug . | Sept . | Oct . | Nov . | Dec . | Jan . | Feb . | Mar . | Apr . | May . |
| Sodium, mmol/L | 141 | 141 | 140 | 140 | 140 | 141 | 140 | 140 | 141 | 141 | 140 | 140 | 139 |
| Potassium, mmol/L | 3.7 | 3.7 | 3.7 | 4.5 | 4.1 | 3.9 | 4.1 | 4.3 | 4.1 | 3.9 | 3.7 | 4.1 | 3.9 |
| Chloride, mmol/L | 105 | 107 | 106 | 105 | 106 | 106 | 107 | 106 | 105 | 107 | 106 | 107 | 104 |
| CO2, mmol/L | 28 | 25 | 24 | 26 | 26 | 27 | 26 | 24 | 26 | 26 | 26 | 25 | 25 |
| Glucose, mg/dL | 103 | 85 | 89 | 91 | 93 | 89 | 90 | 108 | 93 | 99 | 109 | 86 | 106 |
| BUN, mg/dL | 28 | 14 | 17 | 17 | 14 | 18 | 17 | 14 | 21 | 17 | 23 | 19 | 15 |
| Creatinine, mg/dL | 0.91 | 0.90 | 1.03 | 1.07 | 0.83 | 0.92 | 0.90 | 1.08 | 0.89 | 1.08 | 1.06 | 0.93 | 1.05 |
| T. bili, mg/dL | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.30 | 0.3 | 0.4 | 0.3 | 0.4 | 0.3 |
| Alk phos, U/L | 129 | 115 | 113 | 114 | 114 | 105 | 116 | 100 | 99 | 90 | 97 | 89 | 96 |
| AST, U/L | 15 | 11 | 10 | 13 | 15 | 16 | 15 | 20 | 17 | 15 | 15 | 13 | 20 |
| ALT, U/L | 11 | 9 | 7 | 9 | 11 | 13 | 15 | 16 | 15 | 12 | 14 | 11 | 14 |
| Hemoglobin, g/dL | 10.8 | 10.4 | 10.9 | 11.1 | 11.2 | 11.3 | 11.3 | 12.0 | 11.5 | 11.8 | 12.1 | 12.4 | 13.7 |
| Hematocrit, % | 33.0 | 32.9 | 34.7 | 34.0 | 34.9 | 35.1 | 36.0 | 38.5 | 35.9 | 37.0 | 37.4 | 38.2 | 41.8 |
| WBC, 103/µL | 6.8 | 6.4 | 6.8 | 5.7 | 6.3 | 6.0 | 7.1 | 6.8 | 6.3 | 7.2 | 9.3 | 7.3 | 5.9 |
| Platelet, 103/µL | 274 | 295 | 280 | 293 | 248 | 227 | 235 | 256 | 252 | 290 | 291 | 272 | 212 |
| β-D-glucan | 49 | 53 | 33 | <31 | <31 | 40 | 56 | 32 | 55 | 52 | 56 | <31 | 39 |
Abbreviations: ALT, alanine transaminase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; WBC, white blood cell.
Laboratory Results Including Comprehensive Metabolic Panel, Serum B-D-Glucan, and Complete Blood Count Over a Period of 1 Year While on Therapy With Rezafungin
| Test . | 2020 . | . | . | . | . | . | . | . | 2021 . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | May . | June . | July . | Aug . | Sept . | Oct . | Nov . | Dec . | Jan . | Feb . | Mar . | Apr . | May . |
| Sodium, mmol/L | 141 | 141 | 140 | 140 | 140 | 141 | 140 | 140 | 141 | 141 | 140 | 140 | 139 |
| Potassium, mmol/L | 3.7 | 3.7 | 3.7 | 4.5 | 4.1 | 3.9 | 4.1 | 4.3 | 4.1 | 3.9 | 3.7 | 4.1 | 3.9 |
| Chloride, mmol/L | 105 | 107 | 106 | 105 | 106 | 106 | 107 | 106 | 105 | 107 | 106 | 107 | 104 |
| CO2, mmol/L | 28 | 25 | 24 | 26 | 26 | 27 | 26 | 24 | 26 | 26 | 26 | 25 | 25 |
| Glucose, mg/dL | 103 | 85 | 89 | 91 | 93 | 89 | 90 | 108 | 93 | 99 | 109 | 86 | 106 |
| BUN, mg/dL | 28 | 14 | 17 | 17 | 14 | 18 | 17 | 14 | 21 | 17 | 23 | 19 | 15 |
| Creatinine, mg/dL | 0.91 | 0.90 | 1.03 | 1.07 | 0.83 | 0.92 | 0.90 | 1.08 | 0.89 | 1.08 | 1.06 | 0.93 | 1.05 |
| T. bili, mg/dL | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.30 | 0.3 | 0.4 | 0.3 | 0.4 | 0.3 |
| Alk phos, U/L | 129 | 115 | 113 | 114 | 114 | 105 | 116 | 100 | 99 | 90 | 97 | 89 | 96 |
| AST, U/L | 15 | 11 | 10 | 13 | 15 | 16 | 15 | 20 | 17 | 15 | 15 | 13 | 20 |
| ALT, U/L | 11 | 9 | 7 | 9 | 11 | 13 | 15 | 16 | 15 | 12 | 14 | 11 | 14 |
| Hemoglobin, g/dL | 10.8 | 10.4 | 10.9 | 11.1 | 11.2 | 11.3 | 11.3 | 12.0 | 11.5 | 11.8 | 12.1 | 12.4 | 13.7 |
| Hematocrit, % | 33.0 | 32.9 | 34.7 | 34.0 | 34.9 | 35.1 | 36.0 | 38.5 | 35.9 | 37.0 | 37.4 | 38.2 | 41.8 |
| WBC, 103/µL | 6.8 | 6.4 | 6.8 | 5.7 | 6.3 | 6.0 | 7.1 | 6.8 | 6.3 | 7.2 | 9.3 | 7.3 | 5.9 |
| Platelet, 103/µL | 274 | 295 | 280 | 293 | 248 | 227 | 235 | 256 | 252 | 290 | 291 | 272 | 212 |
| β-D-glucan | 49 | 53 | 33 | <31 | <31 | 40 | 56 | 32 | 55 | 52 | 56 | <31 | 39 |
| Test . | 2020 . | . | . | . | . | . | . | . | 2021 . | . | . | . | . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | May . | June . | July . | Aug . | Sept . | Oct . | Nov . | Dec . | Jan . | Feb . | Mar . | Apr . | May . |
| Sodium, mmol/L | 141 | 141 | 140 | 140 | 140 | 141 | 140 | 140 | 141 | 141 | 140 | 140 | 139 |
| Potassium, mmol/L | 3.7 | 3.7 | 3.7 | 4.5 | 4.1 | 3.9 | 4.1 | 4.3 | 4.1 | 3.9 | 3.7 | 4.1 | 3.9 |
| Chloride, mmol/L | 105 | 107 | 106 | 105 | 106 | 106 | 107 | 106 | 105 | 107 | 106 | 107 | 104 |
| CO2, mmol/L | 28 | 25 | 24 | 26 | 26 | 27 | 26 | 24 | 26 | 26 | 26 | 25 | 25 |
| Glucose, mg/dL | 103 | 85 | 89 | 91 | 93 | 89 | 90 | 108 | 93 | 99 | 109 | 86 | 106 |
| BUN, mg/dL | 28 | 14 | 17 | 17 | 14 | 18 | 17 | 14 | 21 | 17 | 23 | 19 | 15 |
| Creatinine, mg/dL | 0.91 | 0.90 | 1.03 | 1.07 | 0.83 | 0.92 | 0.90 | 1.08 | 0.89 | 1.08 | 1.06 | 0.93 | 1.05 |
| T. bili, mg/dL | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.30 | 0.3 | 0.4 | 0.3 | 0.4 | 0.3 |
| Alk phos, U/L | 129 | 115 | 113 | 114 | 114 | 105 | 116 | 100 | 99 | 90 | 97 | 89 | 96 |
| AST, U/L | 15 | 11 | 10 | 13 | 15 | 16 | 15 | 20 | 17 | 15 | 15 | 13 | 20 |
| ALT, U/L | 11 | 9 | 7 | 9 | 11 | 13 | 15 | 16 | 15 | 12 | 14 | 11 | 14 |
| Hemoglobin, g/dL | 10.8 | 10.4 | 10.9 | 11.1 | 11.2 | 11.3 | 11.3 | 12.0 | 11.5 | 11.8 | 12.1 | 12.4 | 13.7 |
| Hematocrit, % | 33.0 | 32.9 | 34.7 | 34.0 | 34.9 | 35.1 | 36.0 | 38.5 | 35.9 | 37.0 | 37.4 | 38.2 | 41.8 |
| WBC, 103/µL | 6.8 | 6.4 | 6.8 | 5.7 | 6.3 | 6.0 | 7.1 | 6.8 | 6.3 | 7.2 | 9.3 | 7.3 | 5.9 |
| Platelet, 103/µL | 274 | 295 | 280 | 293 | 248 | 227 | 235 | 256 | 252 | 290 | 291 | 272 | 212 |
| β-D-glucan | 49 | 53 | 33 | <31 | <31 | 40 | 56 | 32 | 55 | 52 | 56 | <31 | 39 |
Abbreviations: ALT, alanine transaminase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; WBC, white blood cell.
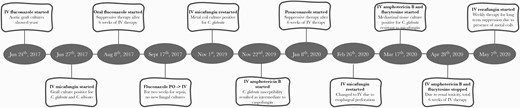
Timeline representing initiation of systemic antifungal therapy and changes occurring over the course of 3 years until the present time. Abbreviations: IV, intravenous; PO, per os (oral).
DISCUSSION
Treatment of invasive fungal infections remains challenging due to limited options, toxicities, and emergence of resistance in many Candida spp. Multiple novel antifungal agents are in various phases of development including rezafungin, fosmanogepix, and VT-1161 [6]. Recent Food and Drug Administration approval of oral ibrexafungerp for the treatment of vaginal yeast infection is a step forward in expansion of antifungal options [10]. This case report demonstrates the therapeutic challenges encountered while treating a recurrent invasive fungal infection with resistant C. glabrata that necessitated the use of a novel agent due to toxicity with other agents and issues related to the practicability of indefinite use. Our case reports the first known use of rezafungin for an extended duration of >1 year. This patient has demonstrated the safety and tolerability of prolonged use of rezafungin, albeit with the caveat that clinical experience with additional patients is needed. Notably, no significant adverse effects were noted on close serial monitoring, and the patient had a good clinical response with no signs of recurrent infection. However, given the retained platinum coils, clinical cure is not expected and ongoing treatment for an indefinite time is planned.
Rezafungin is a novel intravenous, semisynthetic echinocandin with an extended half-life [11]. It is synthesized from a fermentation product of Aspergillus nidulans. Rezafungin, like other echinocandins, inhibits the 1,3-β-D-glucan synthase enzyme complex, making it intrinsically fungicidal against Candida spp. and fungistatic against Aspergillus spp. It has been shown to deplete the cyst (asci) form of Pneumocystis spp. and inhibit the organisms from completing sexual reproduction and proliferation, thereby allowing a broader spectrum of activity against multiple fungal species. As with other echinocandins, rezafungin has poor activity against Cryptococcus neoformans and some rare molds, for example, Mucorales, Fusarium spp., and Scedosporium spp. [12]. The in vitro activity of rezafungin was found to be similar in activity to that of anidulafungin when tested against a collection of clinical isolates of Candida spp. enriched with echinocandin- and/or azole-resistant strains. The potency of rezafungin against strains with documented FKS mutations was 2- to 8-fold greater than that of caspofungin and similar to that of anidulafungin [13]. The FKS genes encode the catalytic subunit of the 1,3-β-D-glucan synthase enzyme complex targeted by echinocandins. Mutations in the FKS genes within the 2 specific HS regions (HS1 and HS2, encoding 9 and 8 amino acids, respectively, in C. glabrata) are associated with higher minimum inhibitory concentrations (MICs), reduced susceptibility, and increased clinical failure rates [14]. The rezafungin weekly dosing regimen is shown to achieve high plasma concentrations, which might be able to overcome some FKS mutations in echinocandin-resistant Candida spp. strains [15]. In mouse infection models, rezafungin has demonstrated activity against C. glabrata strains possessing FKS mutations [16] and has shown a high predicted target attainment for this species [17]. Chronic biofilm studies as well as serial passaging studies show that chronic exposure to rezafungin does not result in the accumulation of FKS mutations as with other echinocandins, demonstrating a high barrier to resistance [18].
In a phase 2 double-blind clinical trial, patients with candidemia and/or invasive candidiasis were treated with either once-weekly rezafungin or intravenous caspofungin, with the option for oral fluconazole step-down therapy for up to 4 weeks. Rezafungin was determined to be safe and efficacious [18]. No notable differences were found in treatment-emergent adverse events across different study groups (rezafungin 400 mg/400 mg, rezafungin 400 mg/200 mg, and caspofungin). There was a lower incidence of treatment-emergent adverse events leading to study drug discontinuation and of serious adverse events leading to death in the rezafungin 400 mg/200 mg group [18]. Two phase 3, multicenter, double-blind, randomized clinical trials are currently underway, one to evaluate the efficacy and safety of rezafungin vs the standard antimicrobial regimen to prevent invasive fungal disease in adults undergoing allogeneic hematopoietic stem cell transplant, and the other to evaluate rezafungin vs caspofungin followed by optional oral fluconazole step-down therapy in subjects with candidemia and/or invasive candidiasis [4, 5]. The Food and Drug Administration (FDA) has granted Qualified Infectious Disease Product, Fast track, and Orphan Drug designation to rezafungin, but rezafungin has not yet been approved by the FDA for use in humans. In our case, weekly infusions of rezafungin obtained through expanded access were safe and efficacious over an extended period of >1 year.
Acknowledgments
The authors would like to acknowledge Cidara Therapeutics for providing rezafungin through their expanded access program and for assistance with susceptibility and genetic testing on 1 of the C. glabrata isolates.
Financial support. The authors received no financial support for the research, authorship, or publication of this article.
Potential conflicts of interest. The authors of this manuscript have no conflicts of interest to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.





Comments