-
PDF
- Split View
-
Views
-
Cite
Cite
Jonathan Underwood, Rowena Griffiths, David Gillespie, Ashley Akbari, Haroon Ahmed, All-cause and Infection-attributable Mortality Amongst Adults With Bloodstream Infection—a Population-based Study, Open Forum Infectious Diseases, Volume 11, Issue 5, May 2024, ofae126, https://doi.org/10.1093/ofid/ofae126
Close - Share Icon Share
Abstract
Bloodstream infections (BSIs) are common, life-threatening infections. However, it remains unclear whether deaths following BSIs are primarily from uncontrolled infection or underlying comorbidities. We aimed to determine the overall mortality, infection-attributable mortality, and causes of death for four leading BSI pathogens.
This retrospective cohort study was conducted within the Secure Anonymized Information Linkage Databank, containing anonymized population-scale electronic health record data for Wales, UK. We included adults with Escherichia coli, Klebsiella spp, Pseudomonas aeruginosa, and Staphylococcus aureus BSI between 2010 and 2022 using linked data from Public Health Wales and the Office for National Statistics. Thirty-day all-cause and sepsis-specific mortality, as a proxy for infection-attributable mortality, were compared using Cox proportional hazards and competing risk regression, respectively.
We identified 35 691 adults with BSI (59.6% E coli). Adjusted analyses revealed that all organisms had a higher 30-day mortality versus E coli with Pseudomonas aeruginosa the highest (hazard ratio, 1.96 [1.76–2.17], P < .001). Cancer was the leading cause of death following BSIs for all organisms, particularly deaths occurring between 30 and 90 days (35.9%). A total of 25.5% of deaths within 30 days involved sepsis. Methicillin-resistant Staphylococcus aureus was associated with the highest sepsis mortality versus E coli (hazard ratio, 2.56 [2.10–3.12], P < .001). Peak C-reactive protein was positively associated with increased sepsis mortality (P < .001).
This population-level study challenges the assumption that most deaths following BSIs are directly attributable to uncontrolled infection, particularly subacutely more than 30 days from BSI. Our findings underscore the need for reevaluating clinical trial design and developing better preventive strategies for BSIs.
Bacteremias, or bloodstream infections (BSIs), are common, life-threatening infections with high mortality rates worldwide [1]. Although the reported 30-day mortality for BSIs ranges from 15% to 30% [1–3], it is unclear whether these deaths are primarily from the infection itself or underlying morbidities. The implicit assumption with published BSI mortality figures is that most deaths are directly to the result of uncontrolled infection and sepsis. However, with modern healthcare and an aging multimorbid population, many patients with BSIs may die of their underlying morbidities, some of which may have precipitated the BSI, rather than of overwhelming infection. For example, in the largest published randomized controlled trial (RCT) of Staphylococcus aureus BSI (SAB), only half of deaths were attributed to SAB, with these deaths peaking in the first week [4]. Further details of the exact causes of death in the remaining 50% of people in this study were not reported, highlighting a wider problem of sparsity of data reporting cause of death after BSIs. A Danish population-based study [5] examined longer term (median, 1.2 years) causes of death following 25 855 SAB cases from 1992 to 2014. In total, 22% died of cardiovascular disease, 23% of cancer, and only 8% had a cause of death related to infection. Attributable mortality or sepsis-associated mortality, a reasonable proxy for attributable death from uncontrolled infection, was not reported. A more recent study [6] from Queensland, Australia, reported mortality and causes of death following 7061 cases of Gram-negative BSIs between 2005 and 2010. Gastrointestinal malignancy was the most common cause of death between 0 and 90 days and 90 and 365 days and up to 4 years after infection. This likely reflects many Gram-negative BSIs being a consequence of GI malignancy (eg, cholangitis from an obstructing tumor). Sepsis was reported as the fifth most common underlying cause of death between 0 and 90 days, accounting for roughly 20% of the total caused by gastrointestinal malignancy. Data were not reported as percentages of total deaths nor stratified by organism, preventing comparison between different organisms.
Detailed patient-level mortality data from RCTs are conflicting, reflect highly selected populations, and are rarely reported in the main trial publication. Large RCTs of people with drug-resistant Gram-negative BSI, many of whom had advanced cancer, have reported deaths attributable to BSIs varying between 0% and 45% [7, 8].
Understanding causes of death following BSIs is essential to determining infection attributable mortality and to successfully design clinical trials testing therapeutics. Here, we aimed to determine overall and infection attributable mortality and causes of death at different time points following BSI for the 4 leading BSI pathogens, Escherichia coli, Klebsiella spp, Pseudomonas aeruginosa (PsA), and S aureus using high-quality population level linked clinical and microbiological data.
METHODS
Study Design and Population
We performed a retrospective cohort study of adults (aged >18 years at the time of BSI) with E, Klebsiella spp, PsA, and S aureus BSIs. These organisms were selected because of their global burden and prevalence and to align with national reporting conventions. To be eligible, participants needed to have a BSI cultured from at least 1 bottle blood culture between April 2010 and 2022 collected in a Welsh National Health Service (NHS) hospital.
Data Sources
All microbiological samples taken from NHS primary and secondary care services in Wales are processed in United Kingdom Accreditation Services–accredited laboratories using standardized methodology. Blood cultures that grew E coli, Klebsiella spp, PsA, and S aureus from 1 April 2010 to 31 March 2022 were extracted from Public Health Wales’ data. These data were linked to anonymized individual-level, population-scale, routinely collected electronic health record data within the Secure Anonymized Information Linkage (SAIL) Databank [9]. The linked datasets included hospital episode data extracted from the Patient Episode Database for Wales. C-reactive protein (CRP) measurements were extracted from the Welsh Results Reporting Service, which contains data for all laboratory samples processed in NHS laboratories across Wales. Peak CRP, a proxy of the magnitude of the inflammatory response, was determined between the period starting 2 days before the BSI and ending 7 days after, to account for asynchronous blood draws because blood cultures are often taken separately when patients are febrile.
Positive cultures with the same organism within 14 days were considered to be the same BSI. S aureus BSIs were subdivided by methicillin sensitivity because of national reporting conventions. More than 1 organism isolated from the same sample was regarded as “polymicrobial” and considered separately. In the case of multiple positive blood cultures, only the most recent was considered for analysis.
Causes of death were determined from linked Office for National Statistics (ONS) records which are based on the death certificate written by the attending doctor. Underlying cause of death was determined using standard ONS methodology based on International Classification of Diseases 10th Revision (ICD-10) and World Health Organization definitions [10]. Underlying cause of death, defined as the disease or injury that initiated the train of events directly leading to death, was chosen as the primary outcome to align with ONS and World Health Organization recommendations and to allow comparison across geographical regions. We also looked for cases with any mention of sepsis listed as a cause of death as another method of quantifying BSI-attributable deaths. This framework is similar to the ONS method for official reporting of deaths involving COVID-19 [11]. Deaths involving sepsis, hereafter sepsis deaths, were defined using already established ICD-10 code lists (Supplementary Table m1) [12]. Deaths were grouped according to standard ONS methodology (eg, malignant neoplasms [cancer], dementia). Code lists are in Supplementary Table m2.
Exposure
The exposure was most recent monomicrobial blood culture that grew E coli, Klebsiella spp, PsA, or S aureus. Polymicrobial infections including these organisms were considered separately.
Outcomes
The primary outcomes were 30-day all-cause mortality and 30- and 90-day cause-specific mortality following BSIs. Secondary outcomes included mortality associated with sepsis at 30 days following BSIs.
Covariates
Baseline covariates included age, sex, number of comorbidities, hospital/community onset, Charlson comorbidity index (CCI) score, Welsh Index of Multiple Deprivation (WIMD) version 2019 as quintiles mapped from Lower-level Super Output Area of residence (administrative authority locality) and electronic frailty index (EFI) [13]. Chosen covariates could all confound the relationship between BSI organism and mortality and were available in a standardized format at the population level.
Statistical Analysis
There was no imputation for missing data. Demographic characteristics were compared by organism with Kruskal-Wallis rank sum and Pearson’s chi-squared tests as appropriate. Kaplan-Meier graphs were used to show mortality following BSI stratified by BSI organism. The log-rank test was used to assess differences in mortality by organism.
In the mortality analyses, associations between BSI organism and 30-day mortality were determined using a Cox proportional hazards model adjusting for age, sex, WIMD, CCI, and EFI. Associations between BSI organism and 30-day sepsis death was determined using competing risks regression, a proportional subdistribution hazards regression model (with death from a nonsepsis cause as a competing risk) adjusting for age, sex, WIMD, CCI, and EFI.
To assess how the magnitude of the inflammatory response affected infection-attributable mortality the association between peak CRP and 30-day sepsis death was determined using competing risks regression (with death from a nonsepsis cause as a competing risk) adjusting for organism, age, sex, WIMD, CCI, and EFI. Because there are no accepted thresholds for CRP, it was arbitrarily split into 5 groups: <100; 100–199; 200–299; 300–399; and 400+ mg/L.
All statistical analyses were conducted using R v4.1.3 using survival and cmprsk packages [14].
Preplanned Sensitivity Analyses
We also considered mortality in patients who only ever had 1 BSI during the study period. As a sensitivity analysis for sepsis recording, we analyzed whether patients with E coli and methicillin-sensitive S aureus (MSSA) BSI (the 2 most common pathogens) with a clear infection syndrome as the underlying cause of death also had sepsis listed as a cause of death anywhere on the death certificate.
RESULTS
A total of 35 691 adults with BSIs were identified, of whom 21 270 (59.6%) were E coli; 3488 (9.8%) Klebsiella spp; 6558 (18.4%) MSSA; 957 (2.7%) methicillin-resistant S aureus (MRSA); 1177 (3.3%) PsA BSIs; and 2241 (6.3%) were polymicrobial. Median age ranged from 69 years for MSSA to 77 years for MRSA (Table 1). A greater proportion of females were observed in the E coli BSI group (11 345 [53%]), compared with the other BSI groups that contained >60% males. Patients with MRSA BSIs tended to have more comorbidities. Peak CRP level was relatively consistent across the different BSIs.
| . | Organism . | ||||||
|---|---|---|---|---|---|---|---|
| Variable . | E coli, N = 21 270a . | Klebsiella, N = 3488a . | MRSA, N = 957a . | MSSA, N = 6558a . | Polymicrobial, N = 2241a . | PsA, N = 1177a . | P Valueb . |
| Age (y) | 76 (66–84) | 74 (64–82) | 77 (66–85) | 69 (54–80) | 74 (63–83) | 75 (66–83) | <.001 |
| Onset | … | … | … | … | … | … | <.001 |
| Community | 12 444 (59) | 1603 (47) | 298 (31) | 3163 (48) | 1070 (48) | 544 (53) | |
| Hospital | 8517 (41) | 1824 (53) | 658 (69) | 3363 (52) | 1140 (52) | 610 (53) | |
| Missing | 309 | 61 | 1 | 32 | 31 | 23 | |
| Sex | … | … | … | … | … | … | <.001 |
| Female | 11 345 (53) | 1331 (38) | 311 (32) | 2449 (37) | 879 (39) | 451 (38) | |
| Male | 9925 (47) | 2157 (62) | 646 (68) | 4109 (63) | 1362 (61) | 726 (62) | |
| Frailty rating | … | … | … | … | … | … | <.001 |
| Fit | 7346 (35) | 1201 (34) | 268 (28) | 2759 (42) | 734 (33) | 409 (35) | |
| Mild | 6676 (31) | 1171 (34) | 272 (28) | 2021 (31) | 773 (34) | 402 (34) | |
| Moderate | 4993 (23) | 786 (23) | 266 (28) | 1286 (20) | 529 (24) | 274 (23) | |
| Severe | 2255 (11) | 330 (9.5) | 151 (16) | 492 (7.5) | 205 (9.1) | 92 (7.8) | |
| Charlson Index | 8 (0–18) | 10 (2–22) | 14 (5–25) | 8 (0–19) | 9 (1–19) | 11 (4–21) | <.001 |
| Welsh Index of Multiple Deprivation | … | … | … | … | … | … | <.001 |
| 1 | 4298 (21) | 723 (22) | 193 (21) | 1569 (25) | 468 (22) | 249 (22) | |
| 2 | 4392 (22) | 690 (21) | 193 (21) | 1373 (22) | 471 (22) | 219 (20) | |
| 3 | 4471 (22) | 641 (19) | 195 (22) | 1247 (20) | 444 (21) | 216 (19) | |
| 4 | 3642 (18) | 608 (18) | 171 (19) | 1019 (16) | 382 (18) | 192 (17) | |
| 5 | 3389 (17) | 642 (19) | 146 (16) | 1002 (16) | 383 (18) | 245 (22) | |
| Missing | 1078 | 184 | 59 | 348 | 93 | 56 | |
| Peak CRP (mg/L) | 202 (122–290) | 199 (119–292) | 206 (124–295) | 213 (121–311) | 200 (119–285) | 214 (134–314) | <.001 |
| Missing | 1858 | 367 | 167 | 687 | 209 | 185 | |
| . | Organism . | ||||||
|---|---|---|---|---|---|---|---|
| Variable . | E coli, N = 21 270a . | Klebsiella, N = 3488a . | MRSA, N = 957a . | MSSA, N = 6558a . | Polymicrobial, N = 2241a . | PsA, N = 1177a . | P Valueb . |
| Age (y) | 76 (66–84) | 74 (64–82) | 77 (66–85) | 69 (54–80) | 74 (63–83) | 75 (66–83) | <.001 |
| Onset | … | … | … | … | … | … | <.001 |
| Community | 12 444 (59) | 1603 (47) | 298 (31) | 3163 (48) | 1070 (48) | 544 (53) | |
| Hospital | 8517 (41) | 1824 (53) | 658 (69) | 3363 (52) | 1140 (52) | 610 (53) | |
| Missing | 309 | 61 | 1 | 32 | 31 | 23 | |
| Sex | … | … | … | … | … | … | <.001 |
| Female | 11 345 (53) | 1331 (38) | 311 (32) | 2449 (37) | 879 (39) | 451 (38) | |
| Male | 9925 (47) | 2157 (62) | 646 (68) | 4109 (63) | 1362 (61) | 726 (62) | |
| Frailty rating | … | … | … | … | … | … | <.001 |
| Fit | 7346 (35) | 1201 (34) | 268 (28) | 2759 (42) | 734 (33) | 409 (35) | |
| Mild | 6676 (31) | 1171 (34) | 272 (28) | 2021 (31) | 773 (34) | 402 (34) | |
| Moderate | 4993 (23) | 786 (23) | 266 (28) | 1286 (20) | 529 (24) | 274 (23) | |
| Severe | 2255 (11) | 330 (9.5) | 151 (16) | 492 (7.5) | 205 (9.1) | 92 (7.8) | |
| Charlson Index | 8 (0–18) | 10 (2–22) | 14 (5–25) | 8 (0–19) | 9 (1–19) | 11 (4–21) | <.001 |
| Welsh Index of Multiple Deprivation | … | … | … | … | … | … | <.001 |
| 1 | 4298 (21) | 723 (22) | 193 (21) | 1569 (25) | 468 (22) | 249 (22) | |
| 2 | 4392 (22) | 690 (21) | 193 (21) | 1373 (22) | 471 (22) | 219 (20) | |
| 3 | 4471 (22) | 641 (19) | 195 (22) | 1247 (20) | 444 (21) | 216 (19) | |
| 4 | 3642 (18) | 608 (18) | 171 (19) | 1019 (16) | 382 (18) | 192 (17) | |
| 5 | 3389 (17) | 642 (19) | 146 (16) | 1002 (16) | 383 (18) | 245 (22) | |
| Missing | 1078 | 184 | 59 | 348 | 93 | 56 | |
| Peak CRP (mg/L) | 202 (122–290) | 199 (119–292) | 206 (124–295) | 213 (121–311) | 200 (119–285) | 214 (134–314) | <.001 |
| Missing | 1858 | 367 | 167 | 687 | 209 | 185 | |
Abbreviations: CRP, C-reactive protein; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; PsA, Pseudomonas aeruginosa.
aMedian (interquartile range); n (%).
bKruskal-Wallis rank sum test; Pearson's chi-squared test.
| . | Organism . | ||||||
|---|---|---|---|---|---|---|---|
| Variable . | E coli, N = 21 270a . | Klebsiella, N = 3488a . | MRSA, N = 957a . | MSSA, N = 6558a . | Polymicrobial, N = 2241a . | PsA, N = 1177a . | P Valueb . |
| Age (y) | 76 (66–84) | 74 (64–82) | 77 (66–85) | 69 (54–80) | 74 (63–83) | 75 (66–83) | <.001 |
| Onset | … | … | … | … | … | … | <.001 |
| Community | 12 444 (59) | 1603 (47) | 298 (31) | 3163 (48) | 1070 (48) | 544 (53) | |
| Hospital | 8517 (41) | 1824 (53) | 658 (69) | 3363 (52) | 1140 (52) | 610 (53) | |
| Missing | 309 | 61 | 1 | 32 | 31 | 23 | |
| Sex | … | … | … | … | … | … | <.001 |
| Female | 11 345 (53) | 1331 (38) | 311 (32) | 2449 (37) | 879 (39) | 451 (38) | |
| Male | 9925 (47) | 2157 (62) | 646 (68) | 4109 (63) | 1362 (61) | 726 (62) | |
| Frailty rating | … | … | … | … | … | … | <.001 |
| Fit | 7346 (35) | 1201 (34) | 268 (28) | 2759 (42) | 734 (33) | 409 (35) | |
| Mild | 6676 (31) | 1171 (34) | 272 (28) | 2021 (31) | 773 (34) | 402 (34) | |
| Moderate | 4993 (23) | 786 (23) | 266 (28) | 1286 (20) | 529 (24) | 274 (23) | |
| Severe | 2255 (11) | 330 (9.5) | 151 (16) | 492 (7.5) | 205 (9.1) | 92 (7.8) | |
| Charlson Index | 8 (0–18) | 10 (2–22) | 14 (5–25) | 8 (0–19) | 9 (1–19) | 11 (4–21) | <.001 |
| Welsh Index of Multiple Deprivation | … | … | … | … | … | … | <.001 |
| 1 | 4298 (21) | 723 (22) | 193 (21) | 1569 (25) | 468 (22) | 249 (22) | |
| 2 | 4392 (22) | 690 (21) | 193 (21) | 1373 (22) | 471 (22) | 219 (20) | |
| 3 | 4471 (22) | 641 (19) | 195 (22) | 1247 (20) | 444 (21) | 216 (19) | |
| 4 | 3642 (18) | 608 (18) | 171 (19) | 1019 (16) | 382 (18) | 192 (17) | |
| 5 | 3389 (17) | 642 (19) | 146 (16) | 1002 (16) | 383 (18) | 245 (22) | |
| Missing | 1078 | 184 | 59 | 348 | 93 | 56 | |
| Peak CRP (mg/L) | 202 (122–290) | 199 (119–292) | 206 (124–295) | 213 (121–311) | 200 (119–285) | 214 (134–314) | <.001 |
| Missing | 1858 | 367 | 167 | 687 | 209 | 185 | |
| . | Organism . | ||||||
|---|---|---|---|---|---|---|---|
| Variable . | E coli, N = 21 270a . | Klebsiella, N = 3488a . | MRSA, N = 957a . | MSSA, N = 6558a . | Polymicrobial, N = 2241a . | PsA, N = 1177a . | P Valueb . |
| Age (y) | 76 (66–84) | 74 (64–82) | 77 (66–85) | 69 (54–80) | 74 (63–83) | 75 (66–83) | <.001 |
| Onset | … | … | … | … | … | … | <.001 |
| Community | 12 444 (59) | 1603 (47) | 298 (31) | 3163 (48) | 1070 (48) | 544 (53) | |
| Hospital | 8517 (41) | 1824 (53) | 658 (69) | 3363 (52) | 1140 (52) | 610 (53) | |
| Missing | 309 | 61 | 1 | 32 | 31 | 23 | |
| Sex | … | … | … | … | … | … | <.001 |
| Female | 11 345 (53) | 1331 (38) | 311 (32) | 2449 (37) | 879 (39) | 451 (38) | |
| Male | 9925 (47) | 2157 (62) | 646 (68) | 4109 (63) | 1362 (61) | 726 (62) | |
| Frailty rating | … | … | … | … | … | … | <.001 |
| Fit | 7346 (35) | 1201 (34) | 268 (28) | 2759 (42) | 734 (33) | 409 (35) | |
| Mild | 6676 (31) | 1171 (34) | 272 (28) | 2021 (31) | 773 (34) | 402 (34) | |
| Moderate | 4993 (23) | 786 (23) | 266 (28) | 1286 (20) | 529 (24) | 274 (23) | |
| Severe | 2255 (11) | 330 (9.5) | 151 (16) | 492 (7.5) | 205 (9.1) | 92 (7.8) | |
| Charlson Index | 8 (0–18) | 10 (2–22) | 14 (5–25) | 8 (0–19) | 9 (1–19) | 11 (4–21) | <.001 |
| Welsh Index of Multiple Deprivation | … | … | … | … | … | … | <.001 |
| 1 | 4298 (21) | 723 (22) | 193 (21) | 1569 (25) | 468 (22) | 249 (22) | |
| 2 | 4392 (22) | 690 (21) | 193 (21) | 1373 (22) | 471 (22) | 219 (20) | |
| 3 | 4471 (22) | 641 (19) | 195 (22) | 1247 (20) | 444 (21) | 216 (19) | |
| 4 | 3642 (18) | 608 (18) | 171 (19) | 1019 (16) | 382 (18) | 192 (17) | |
| 5 | 3389 (17) | 642 (19) | 146 (16) | 1002 (16) | 383 (18) | 245 (22) | |
| Missing | 1078 | 184 | 59 | 348 | 93 | 56 | |
| Peak CRP (mg/L) | 202 (122–290) | 199 (119–292) | 206 (124–295) | 213 (121–311) | 200 (119–285) | 214 (134–314) | <.001 |
| Missing | 1858 | 367 | 167 | 687 | 209 | 185 | |
Abbreviations: CRP, C-reactive protein; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; PsA, Pseudomonas aeruginosa.
aMedian (interquartile range); n (%).
bKruskal-Wallis rank sum test; Pearson's chi-squared test.
Overall 30-day mortality was highest in patients with MRSA BSIs (38.7%; 95% confidence interval, 35.7–41.9) and lowest for patients with E coli BSIs (18.2%; 17.7–18.8) (Figure 1). A total of 2523 patients (32.2% of deaths by 30 days) died in 2 or fewer days after collection of the blood culture that identified BSI (ie, before identification using traditional culture-based methods). Baseline characteristics by timing of death in the first 30 days are shown in Supplementary Table 2. Death within 2 days of BSI was highest for patients with PsA BSIs (45.3%; 40.3–50.3) and lowest for patients with MSSA (24.8%; 22.8–26.9). For patients who only ever had 1 recorded BSI during the study period (n = 31,259, 45% E coli, Supplementary Table 1) mortality was very similar to the whole cohort (Supplementary Table 3).
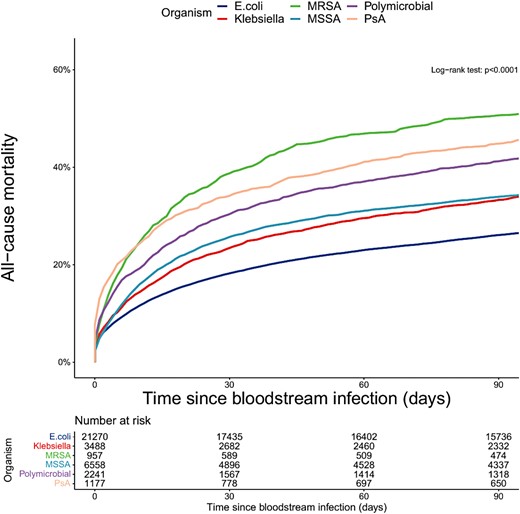
Mortality following bloodstream infection—stratified by organism. Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; PsA, Pseudomonas aeruginosa.
After adjusting for age, sex, onset, WIMD, CCI, and frailty, all other organisms were associated with greater 30-day mortality compared with E coli BSIs (Table 2), with PsA BSIs having the highest mortality (hazard ratio [HR], 1.96; 95% confidence intervals, 1.76–2.17; P < .001 for all).
Cox Proportional Hazards Model for 30-day Mortality Following BSI Stratified by Organism Adjusted for age, sex, Onset, WIMD, Charlson Comorbidity Index, and Frailty
| . | N . | HR . | 95% CI . | P Value . |
|---|---|---|---|---|
| Organism . | 35 264 . | . | . | . |
| E coli | … | … | … | |
| Klebsiella | … | 1.25 | 1.16–1.35 | <.001 |
| MRSA | … | 1.93 | 1.73–2.15 | <.001 |
| MSSA | … | 1.59 | 1.50–1.69 | <.001 |
| Polymicrobial | … | 1.81 | 1.66–1.96 | <.001 |
| PsA | … | 1.96 | 1.76–2.17 | <.001 |
| . | N . | HR . | 95% CI . | P Value . |
|---|---|---|---|---|
| Organism . | 35 264 . | . | . | . |
| E coli | … | … | … | |
| Klebsiella | … | 1.25 | 1.16–1.35 | <.001 |
| MRSA | … | 1.93 | 1.73–2.15 | <.001 |
| MSSA | … | 1.59 | 1.50–1.69 | <.001 |
| Polymicrobial | … | 1.81 | 1.66–1.96 | <.001 |
| PsA | … | 1.96 | 1.76–2.17 | <.001 |
Abbreviations: BSI, bloodstream infection; CI, confidence interval; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; PsA, Pseudomonas aeruginosa; WIMD, Welsh Index of Multiple Deprivation.
Cox Proportional Hazards Model for 30-day Mortality Following BSI Stratified by Organism Adjusted for age, sex, Onset, WIMD, Charlson Comorbidity Index, and Frailty
| . | N . | HR . | 95% CI . | P Value . |
|---|---|---|---|---|
| Organism . | 35 264 . | . | . | . |
| E coli | … | … | … | |
| Klebsiella | … | 1.25 | 1.16–1.35 | <.001 |
| MRSA | … | 1.93 | 1.73–2.15 | <.001 |
| MSSA | … | 1.59 | 1.50–1.69 | <.001 |
| Polymicrobial | … | 1.81 | 1.66–1.96 | <.001 |
| PsA | … | 1.96 | 1.76–2.17 | <.001 |
| . | N . | HR . | 95% CI . | P Value . |
|---|---|---|---|---|
| Organism . | 35 264 . | . | . | . |
| E coli | … | … | … | |
| Klebsiella | … | 1.25 | 1.16–1.35 | <.001 |
| MRSA | … | 1.93 | 1.73–2.15 | <.001 |
| MSSA | … | 1.59 | 1.50–1.69 | <.001 |
| Polymicrobial | … | 1.81 | 1.66–1.96 | <.001 |
| PsA | … | 1.96 | 1.76–2.17 | <.001 |
Abbreviations: BSI, bloodstream infection; CI, confidence interval; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; PsA, Pseudomonas aeruginosa; WIMD, Welsh Index of Multiple Deprivation.
Causes of Death
For all organisms, cancer was the leading underlying cause of death following BSI (25.2% of deaths by 30 days and 35.9% occurring between 30–90 days; Figure 2). For E coli BSIs, cancer was the underlying cause of death for 26.9% and 38.8% of deaths occurring up to 30 days and between 30–90 days, respectively. The leading specific underlying causes of deaths by 30 days were mainly from infective causes with urinary tract infection the most common diagnosis. However, after 30 days many deaths were caused by dementia and other noncommunicable diseases. For Klebsiella spp BSIs, pancreatic cancer was the leading specific cause of death between 30 and 90 days with malignancy the underlying cause of death in 29.8% and 42.7% occurring up to 30 days and 30 to 90 days, respectively. There were fewer cases of PsA BSIs and therefore fewer deaths. However, underlying causes of death followed a similar pattern whereby deaths occurring up to 30 days after BSIs were predominantly of infective causes, whereas those occurring 30 to 90 days after BSI were dominated by noncommunicable diseases—particularly cancer (31.3% by 30 days and 35.0% by 30–90 days). For MSSA BSI, ischemic heart disease was the second most common specific underlying cause of death by 30 days and most common between 30 and 90 days. Cancer was the underlying cause of death in 17.6% by 30 days and 23.6% between 30 and 90 days. For MRSA, sepsis was the leading specific underlying cause of death at both 30 and 30 to 90 days. Malignancy was responsible for relatively fewer deaths (16.2% up to 30 days, 20.2% between 30 and 90 days). Overall, dementia and cancer were leading causes of death between 30 and 90 days, accounting for 6.9% and 35.9%, respectively. Cancer was also the leading underlying cause of very early (within 2 days) deaths after BSIs (Supplementary Figure 1).
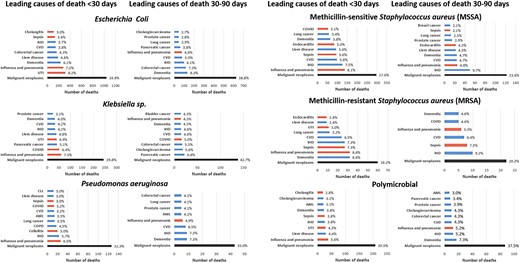
Underlying cause of death by organism. Red bars indicate infective causes. Causes with fewer than 5 deaths not displayed for SAIL information governance reasons.
Deaths Involving Sepsis
Of those who died within 30 days of BSIs, 1505 (18.7%) had sepsis listed as the primary cause of death and 2054 (25.5%) had sepsis listed as a primary or secondary cause of death. The proportion of deaths from sepsis reduced over time (Supplementary Figure 2). Median survival was shorter for sepsis versus nonsepsis deaths (7 [7–8] days vs 45 [43–47] days) and a greater proportion of sepsis deaths occurred within 2 days of BSI (38% vs 29%, P < .001). Deaths from sepsis as a proportion of deaths by 30 days following BSI varied by organism and were highest for MRSA (31.3% [26.7–36.3]%) and lowest for Klebsiella spp BSI (20.7% [17.9–23.6]%). Using a competing risk analysis, 30-day sepsis mortality was significantly greater for MSSA, MRSA, PsA, and polymicrobial versus E coli BSI (all P < .001, Table 3) with MRSA associated with the highest mortality (HR, 2.56 [2.10–3.12]; P < .001). In an exploratory analysis, the magnitude of the inflammatory response following BSI, measured by peak CRP, was associated with 30-day sepsis mortality. Compared with those with a peak CRP of <100 mg/L, having a peak CRP >400 mg/L was associated with a higher mortality (HR, 2.98 [2.46–3.60]; P < .001; Table 3).
Competing Risk Regression for 30-day Sepsis Mortality Following BSI Stratified by Organism and Peak CRP
| . | N . | HR . | 95% CI . | P Value . | . | N . | HR . | (95% CI) . | P Value . |
|---|---|---|---|---|---|---|---|---|---|
| Organisma | 33 437 | Peak CRP (mg/L)b | 30 137 | ||||||
| E coli | … | — | … | <100 | … | … | |||
| Klebsiella | … | 1.06 | .89– 1.25 | .52 | 100–200 | 1.09 | .93–1.29 | .29 | |
| MRSA | … | 2.56 | 2.10– 3.12 | <.001 | 200–300 | 1.49 | 1.27–1.75 | <.001 | |
| MSSA | … | 1.72 | 1.52– 1.94 | <.001 | 300–400 | 2.26 | 1.92–2.65 | <.001 | |
| Polymicrobial | … | 2.05 | 1.75– 2.40 | <.001 | 400+ | 2.98 | 2.46–3.60 | <.001 | |
| E coli | … | 2.37 | 1.94–2.88 | <.001 | … | … |
| . | N . | HR . | 95% CI . | P Value . | . | N . | HR . | (95% CI) . | P Value . |
|---|---|---|---|---|---|---|---|---|---|
| Organisma | 33 437 | Peak CRP (mg/L)b | 30 137 | ||||||
| E coli | … | — | … | <100 | … | … | |||
| Klebsiella | … | 1.06 | .89– 1.25 | .52 | 100–200 | 1.09 | .93–1.29 | .29 | |
| MRSA | … | 2.56 | 2.10– 3.12 | <.001 | 200–300 | 1.49 | 1.27–1.75 | <.001 | |
| MSSA | … | 1.72 | 1.52– 1.94 | <.001 | 300–400 | 2.26 | 1.92–2.65 | <.001 | |
| Polymicrobial | … | 2.05 | 1.75– 2.40 | <.001 | 400+ | 2.98 | 2.46–3.60 | <.001 | |
| E coli | … | 2.37 | 1.94–2.88 | <.001 | … | … |
Abbreviations: BSI, bloodstream infection; CI, confidence interval; CRP, C-reactive protein; HR, hazard ratio; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; PsA, Pseudomonas aeruginosa; WIMD, Welsh Index of Multiple Deprivation.
aAdjusted for age, sex, onset, WIMD, Charlson comorbidity index, and frailty.
bAdjusted for organism, age, sex, onset, WIMD, Charlson comorbidity index, and frailty.
Competing Risk Regression for 30-day Sepsis Mortality Following BSI Stratified by Organism and Peak CRP
| . | N . | HR . | 95% CI . | P Value . | . | N . | HR . | (95% CI) . | P Value . |
|---|---|---|---|---|---|---|---|---|---|
| Organisma | 33 437 | Peak CRP (mg/L)b | 30 137 | ||||||
| E coli | … | — | … | <100 | … | … | |||
| Klebsiella | … | 1.06 | .89– 1.25 | .52 | 100–200 | 1.09 | .93–1.29 | .29 | |
| MRSA | … | 2.56 | 2.10– 3.12 | <.001 | 200–300 | 1.49 | 1.27–1.75 | <.001 | |
| MSSA | … | 1.72 | 1.52– 1.94 | <.001 | 300–400 | 2.26 | 1.92–2.65 | <.001 | |
| Polymicrobial | … | 2.05 | 1.75– 2.40 | <.001 | 400+ | 2.98 | 2.46–3.60 | <.001 | |
| E coli | … | 2.37 | 1.94–2.88 | <.001 | … | … |
| . | N . | HR . | 95% CI . | P Value . | . | N . | HR . | (95% CI) . | P Value . |
|---|---|---|---|---|---|---|---|---|---|
| Organisma | 33 437 | Peak CRP (mg/L)b | 30 137 | ||||||
| E coli | … | — | … | <100 | … | … | |||
| Klebsiella | … | 1.06 | .89– 1.25 | .52 | 100–200 | 1.09 | .93–1.29 | .29 | |
| MRSA | … | 2.56 | 2.10– 3.12 | <.001 | 200–300 | 1.49 | 1.27–1.75 | <.001 | |
| MSSA | … | 1.72 | 1.52– 1.94 | <.001 | 300–400 | 2.26 | 1.92–2.65 | <.001 | |
| Polymicrobial | … | 2.05 | 1.75– 2.40 | <.001 | 400+ | 2.98 | 2.46–3.60 | <.001 | |
| E coli | … | 2.37 | 1.94–2.88 | <.001 | … | … |
Abbreviations: BSI, bloodstream infection; CI, confidence interval; CRP, C-reactive protein; HR, hazard ratio; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; PsA, Pseudomonas aeruginosa; WIMD, Welsh Index of Multiple Deprivation.
aAdjusted for age, sex, onset, WIMD, Charlson comorbidity index, and frailty.
bAdjusted for organism, age, sex, onset, WIMD, Charlson comorbidity index, and frailty.
Sensitivity Analyses
For patients with E coli BSI urinary tract infection was the most common underlying infective cause of death within 30 days. Sepsis was reportedly involved in 48.3% of these cases. There were no differences in potentially confounding factors, peak CRP, or days survived between those involving versus those not involving sepsis (Supplementary Table s4). Similarly for patients with MSSA BSIs and endocarditis as the underlying cause of death within 30 days, 23.8% reportedly involved sepsis. Again, there were no differences in potentially confounding factors, peak CRP, or days survived between those involving versus those not involving sepsis (Supplementary Table s5).
DISCUSSION
This national cohort study provides a comprehensive description of mortality, timing, and causes of death following common BSIs. We found that many deaths after BSIs are not from uncontrolled infection but from underlying conditions like cancer, especially in the subacute phase beyond 30 days after BSIs.
To our knowledge, this is the largest study detailing causes of death following BSIs. These data support earlier observational work suggesting that noninfective causes, and in particular cancer, are responsible for most deaths following BSIs, particularly for Gram-negative pathogens [5, 6]. Pancreatic cancer and cholangiocarcinoma were common underlying causes of death in patients with E coli, Klebsiella, and polymicrobial BSIs. This is unsurprising given these cancers can cause biliary obstruction leading to biliary stasis, cholangitis, and BSIs with enteric organisms. The BSI is amenable to curative treatment with antibiotics and supportive care, but the underlying malignancy is not.
Our finding that about one quarter of deaths are directly attributable to BSIs is a little higher than previous, smaller observational studies in which infection was the reported cause of death in 8% to 20% [5, 6]. RCTs may provide more detailed information on a smaller, selected population, but selection bias may limit their generalization. In the largest published RCT of SAB, ARREST, half of all deaths were attributed to SAB [4]. This contrasts sharply with the largest published RCT of ceftriaxone-resistant E coli and Klebsiella pneumoniae BSI, MERINO [15], in which no participants died of their index infection. We believe our findings, based on comprehensive, unselected population level data, are robust and provide a reliable, contemporaneous estimate of the infection-attributable mortality of BSIs for these common organisms in high-income settings where the prevalence of antimicrobial resistance is relatively low.
We found differential mortality by organism, with MRSA and PsA BSI associated with higher mortality, replicating earlier work [16–19]. Given differences in underlying causes of death, these differences are likely multifactorial and due to patient factors such as underlying disease (eg, acute myeloid leukemia being more common in patients with PsA BSI) and not just intrinsic pathogenicity and antimicrobial resistance. Nonetheless, our competing risks analysis with deaths involving sepsis as the primary outcome suggests that infection-attributable mortality is genuinely higher with these pathogens, urging the need for more trials of novel and combination antibiotics in patients with these pathogens.
We found the magnitude of the inflammatory response, quantified with peak CRP within 7 days of BSI, was associated with sepsis mortality. Elevated CRP has been associated with overall mortality in BSI previously, with Gram-negative infections reportedly having higher initial concentrations [16, 20, 21]. Our robust analyses confirm these earlier findings. Is the magnitude of the inflammatory response causally related to sepsis death? These data cannot answer that question, especially given we do not have treatment data, and the exploratory nature of these analyses. Peak CRP is at the mercy of comorbidities, treatment, timing of testing, as well as time spent alive. However, it is plausible that infection-triggered inflammation has a destabilizing effect on underlying morbidities. However, given the magnitude of the HR comparing patients with higher versus lower peak CRP, understanding immunopathogenesis is essential to further reduce BSI mortality. Current BSI trials almost exclusively focus on pathogen-directed therapy. Immunomodulation has been shown to be successful in other life-threatening infectious diseases, most notably COVID [22, 23]. Addressing the host response to BSI in addition to pathogen-directed therapy may reduce mortality and morbidity and should be explored further.
Strengths and Weaknesses
The main strengths of our study are the size of the study population, completeness of data in SAIL, and historical microbiological data, allowing an unselected cohort of all adults in Wales with BSIs. The main limitation of this study is reliability of death certification for accurately identifying cause of death and lack of postmortem examinations in current UK healthcare. To mitigate this, we determined deaths involving sepsis as a proxy for deaths directly attributable to BSIs. This also relies on accurate death certification. However, usual practice in the United Kingdom is for significant microbiological results, such as BSIs, to be telephoned promptly to the attending clinical team. As such, BSIs would have been identified in almost every patient by the time of death certification. Therefore, it is reasonable to assume that the treating clinician would put this, sepsis, or a specific infection syndrome, such as endocarditis, as the cause of death if it had contributed to death. This contrasts with other potential infection syndromes, such as pneumonia, where the pathogen is often unidentified and there is more scope for misdiagnosis (eg, cardiac failure, pulmonary embolus), particularly if death occurs shortly after presentation. Our sensitivity analyses show the limitations of using deaths involving sepsis as a proxy for BSI attributable mortality. Approximately half of patients with an underlying cause of death of urinary tract infection did not have a sepsis code recorded on the death certificate. This observation is hard to reconcile without accepting that death certification ascertainment of sepsis is incomplete (ie, urinary tract infection is the sole listed cause of death without further detail) and therefore deaths involving sepsis recorded here should be considered a reasonable lower bound of the true number of deaths attributable to uncontrolled infection. A further complication is the changing definition of sepsis over the study period as Sepsis-3 definitions were published in 2016 [24].
Other significant limitations include lack of data regarding the infection syndrome associated with BSIs, physiological, and other data to confirm the presence of sepsis. Other limitations include lack of detailed antimicrobial resistance data, treatment, residual confounding in adjusted analyses, and that causal inference with respect to mortality can only be inferred from an observational study with caution. However, given the large effect sizes observed after adjusting for potential confounders, any bias would have to be large to completely account for our findings. Additionally, missing data were limited to 5% or fewer of the cohort and are unlikely to influence associations.
Implications for Future Study
These data have potentially significant implications for clinical trials of BSI. Our data suggest that pathogen directed clinical trials relying on organism identification before enrollment will not recruit a significant proportion of people who die of BSI because at least one third are dead or moribund by the time of organism identification. Therefore, these trials will not be representative of all patients with BSIs and may be underpowered to detect mortality differences. In the largest published RCT of SAB (6% of whom had MRSA), ARREST, mortality at 12 weeks was 15% [25]. Half of deaths were attributed to SAB, with the majority occurring within the first 2 weeks [4]. Using our national sample (14% of whom had MRSA), 30-day overall mortality was nearly double this at 28%. Underestimated mortality post-SAB is not limited to RCTs. In pooled observational data of SAB (21% MRSA), 90-day mortality was 29% compared with 38% here [19]. Similarly, in a large trial of drug-resistant E coli and K pneumoniae BSI, MERINO, where mortality may be expected to be higher than usual, overall 30-day mortality was 8% [15]. This is much lower than the overall 30-day mortality we observed of 20%, again suggesting selection bias. Of the 30 patients who died in this study, 14 (47%) died of malignancy, with the remainder dying of other morbidities, particularly liver disease, with none dying of the index BSI [26]. These data suggest that many people who die of their BSIs are not enrolled in clinical trials.
What should we do given these results? Overall mortality as the primary outcome in clinical trials of BSI has the advantage of being unbiased compared with BSI-attributed mortality, although this can be mitigated with blinding and independent panels of experts to attribute cause of death. Analogous to progression-free survival in cancer trials in BSIs, a composite endpoint of clinical/microbiological recurrence or death, similar to that used in ARREST, may be advantageous. However, given BSI-attributable death rates decay significantly over the first 2 weeks following BSIs, we advocate for 30- rather than 90-day composite endpoints be used.
Earlier identification of culprit pathogens in patients presenting with infection syndromes, using novel technologies, will be key to facilitate earlier enrollment in pathogen-specific clinical trials. Alternatives to this approach are novel trial designs that enroll patients with defined life-threatening infection syndromes (eg, suspected sepsis, urinary tract infection with sepsis) at initial presentation and initially test different empirical therapies with subsequent stratification by organism/resistance mechanism. Key to this would be using a different model of consent such as deferred consent to allow early, less-biased recruitment of patients with life-threatening infections. This model been used successfully used in clinical trials of out-of-hospital cardiac arrest and in patients presenting with suspected sepsis [27, 28]. Given early sepsis mortality and comorbid disease accounting for most later deaths, preventive strategies including vaccination are likely to be important for reducing the overall disease burden associated with BSIs.
CONCLUSION
BSIs are common and herald death in a significant proportion. However, sepsis is the direct cause of death in only one quarter of patients, usually occurring in the first few days of infection. Preventive approaches, rapid diagnostics, and novel trial design are needed to significantly impact mortality in the future.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
For the purpose of open access, the author has applied a CC BY public copyright license (where permitted by UKRI, “Open Government Licence” or “CC BY-ND public copyright licence” may be stated instead) to any Author Accepted Manuscript version arising.
Ethics and Patient Consent Statement. This project uses anonymized individual-level data sources held within the Trusted Research Environment provided by the SAIL Databank at Swansea University, Swansea, UK. As such, individual written patient consent was not needed. All proposals to use SAIL data are subject to review by the independent Information Governance Review Panel (IGRP). This work was approved under proposal number 0923 after careful considerations by IGRP Panel.
R code is available at written request.
Financial support. This work was supported by the Medical Research Council [grant number MR/T023791/1].
References
Deaths involving COVID-19 by religious group and ethnic group, England: methodology—Office for National Statistics. Accessed 20 July 2023. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/methodologies/deathsinvolvingcovid19byreligiousgroupandethnicgroupenglandmethodology
Author notes
Potential conflicts of interest. The authors nor their institutions have not received any payments or services in the past 36 months from a third party that could be perceived to influence, or give the appearance of potentially influencing, the submitted work. All authors: No reported conflicts.





Comments