-
PDF
- Split View
-
Views
-
Cite
Cite
Manuela Carugati, Sana Arif, Lindsay Y King, Matt Harris, Kayla B Evans, Andrew Barbas, Debra Sudan, Rachel Miller, Barbara D Alexander, 1463. Risk Factors for Primary Invasive Surgical Site Infections among Single Adult Liver Transplants at Duke University Hospital in the Period 2015-2019., Open Forum Infectious Diseases, Volume 10, Issue Supplement_2, December 2023, ofad500.1300, https://doi.org/10.1093/ofid/ofad500.1300
Close - Share Icon Share
Abstract
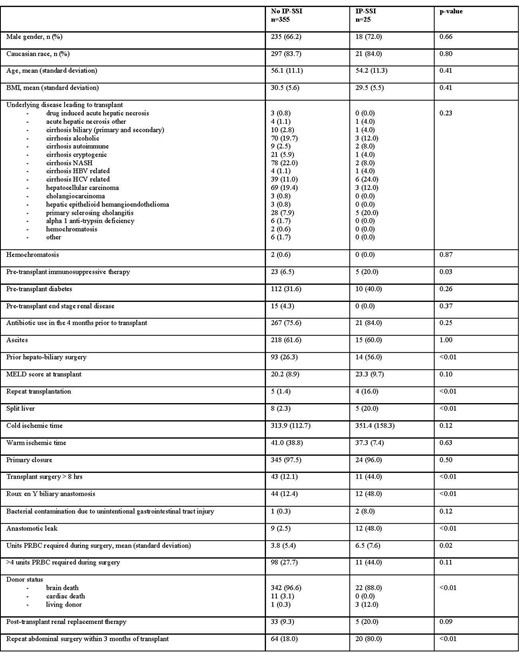
Baseline characteristics of adult patients who underwent a single liver transplant at Duke University Hospital in the period 1 Jan 2015 - 31 Dec 2019 stratified by the diagnosis of invasive primary surgical site infection (IP-SSI) within 90 days of transplant surgery. BMI, body mass index; HBV, hepatitis b virus; HCV, hepatitis c virus; MELD, model for end-stage liver disease; NASH, non-alcoholic steatohepatitis; PRBC, packed red blood cells
The overall IP-SSI rate in our cohort is low compared to published literature supporting effectiveness of current antimicrobial prophylaxis. In this setting, factors that portend risk for IP-SSI are related to the surgical procedures, thus are not easily modifiable. Patients with repeat transplantation, Roux-en-Y biliary anastomosis, anastomotic leak, and repeat abdominal surgery within 3 months of transplantation must be monitored closely for development of IP-SSI. However, the appropriateness of current prophylaxis regimens should be further evaluated given the high prevalence of drug-resistant pathogens causing breakthrough IP-SSI.
Matt Harris, PharmD, MHS, wolters kluer: Advisor/Consultant Barbara D. Alexander, MD, F2G Pharmaceuticals: Advisor/Consultant|HealthTrackRx: Advisor/Consultant|HealthTrackRx: Board Member|Leadiaint: Grant/Research Support|Merck: Advisor/Consultant|Scynexis: Grant/Research Support|Thermofisher: Advisor/Consultant
Author notes
Session: 145. HAI: Surgical Site Infections
Friday, October 13, 2023: 12:15 PM
- body mass index procedure
- antibiotic prophylaxis
- end stage liver disease
- adult
- roux-en-y anastomosis
- consultants
- disclosure
- fluconazole
- hospitals, university
- liver transplantation
- living donors
- surgical procedures, operative
- surgical wound infection
- therapeutic immunosuppression
- diagnosis
- hepatitis b virus
- liver
- microbiology
- transplantation
- steatohepatitis, non-alcoholic
- pathogenic organism
- echinocandins
- hepatitis c virus
- appropriateness
- abdominal surgery
- anastomotic leak
- gastric bypass, roux-en-y
- candida
- packed red blood cells
- prevention
- anastomosis of bile ducts
- doctor of pharmacy








Comments