-
PDF
- Split View
-
Views
-
Cite
Cite
Jessica K DeMartino, Marie-Hélène Lafeuille, Bruno Emond, Carmine Rossi, Jingru Wang, Stephanie Liu, Patrick Lefebvre, Girishanthy Krishnarajah, Respiratory Syncytial Virus–Related Complications and Healthcare Costs Among a Medicare-Insured Population in the United States, Open Forum Infectious Diseases, Volume 10, Issue 5, May 2023, ofad203, https://doi.org/10.1093/ofid/ofad203
Close - Share Icon Share
Abstract
Literature describing respiratory syncytial virus (RSV)–related complications in older adults in the United States is scarce. This study described risk factors of RSV-related complications and healthcare costs of Medicare-insured patients aged ≥60 years with medically attended RSV.
100% Medicare Research Identifiable Files (1 January 2007–31 December 2019) were used to identify adults aged ≥60 years with RSV (index: first diagnosis date). Predictors of ≥1 RSV-related complication (ie, pneumonia, acute respiratory failure, congestive heart failure, hypoxia/dyspnea, non-RSV lower/upper respiratory tract infections, or chronic respiratory disease) during the up to 6-month post–RSV diagnosis period were identified. Patients with all aforementioned diagnoses during the 6 months pre-index could not be evaluated for a complication and were therefore ineligible for analyses. Differences between 6-month pre- and post-index total all-cause and respiratory/infection-related healthcare costs were assessed.
Overall, 175 392 patients with RSV were identified. Post–RSV diagnosis, 47.9% had ≥1 RSV-related complication, with mean time-to-event of 1.0 month. The most common complications were pneumonia (24.0%), chronic respiratory disease (23.6%), and hypoxia or dyspnea (22.0%). Baseline predictors of ≥1 RSV-related complication included having previous diagnoses for complication/comorbidity listed in the Methods, hypoxemia, chemotherapy, chest radiograph, stem cell transplant, and anti-asthmatic and bronchodilator use. Total all-cause and respiratory/infection-related healthcare costs were $7797 and $8863 higher, respectively, post-index versus pre-index (both P < .001).
In this real-world study, almost half of patients with medically attended RSV experienced an RSV-related complication within 1 month post–RSV diagnosis, and costs significantly increased post-diagnosis. Having a complication/comorbidity pre-RSV predicted a higher risk of developing a different complication post–RSV infection.
Respiratory syncytial virus (RSV) infection has historically been recognized as a disease that affects infants and young children. However, it also causes considerable morbidity and mortality in adults [1]. The incidence of medically attended RSV ranges from 8 to 178 per 100 000 adults in hospital settings, 74 to 133 per 100 000 adults in emergency department (ED) settings, and 934 to 1519 per 100 000 adults in outpatient settings in the United States (US), with rates generally higher among adults ≥65 years of age [2]. Older adults are at particularly high risk of experiencing severe RSV infection, with an estimated 177 000 hospitalized each year and 14 000 dying from the infection [3]. A real-world study found that older patients aged ≥65 years with RSV had more than twice as many inpatient and ED visits over 12 months than patients aged 18–64 years [4].
The clinical presentation of RSV in adults can range from cold-like symptoms to full respiratory distress [1]. RSV in older adults is associated with a number of potential clinically serious complications, including non-RSV lower and upper respiratory tract infections (eg, influenza, adenovirus, rhinovirus), pneumonia, hypoxia, and exacerbation of existing chronic obstructive pulmonary disease (COPD) and congestive heart failure [5, 6]. Many of these RSV-related complications have been shown to be associated with severe RSV or hospitalization [6–8]. Additionally, increasing age was also associated with higher odds of hospitalization immediately following RSV diagnosis [6].
Despite the large clinical burden associated with RSV, there are currently no prophylactic options or treatments for adults, although 2 RSV vaccines now await approval by the Food and Drug Administration (FDA) among patients ≥60 years old [9]. Palivizumab and nirsevimab may be used to prevent serious lower respiratory tract disease caused by RSV in high-risk pediatric patients [10, 11]. Aerosolized ribavirin may be used to treat hospitalized children with severe lower respiratory tract infection due to RSV [12], and aerosolized/oral ribavirin may be used to treat lung transplant recipients with upper or lower respiratory tract infection due to RSV [13]. However, neither of these medications is approved for use in adults and the effectiveness of oral ribavirin in immunocompromised adults has only been assessed in observational settings [10–13]. Therefore, there is a substantial need for prophylactic options for patients with RSV, especially older adults who are already at higher risk of experiencing severe outcomes [3].
While the general clinical burden of medically attended RSV has previously been described [14, 15], little is known about the frequency and factors associated with severe RSV-related complications in older adults. In addition, there is limited literature regarding the incremental cost of RSV by type of healthcare service among older adults. To this end, the current study was conducted to fill this gap in the literature by describing the proportion of patients experiencing RSV-related complications, evaluating risk factors for developing these complications, and comparing the healthcare costs incurred 6 months before and up to 6 months after RSV diagnosis among Medicare beneficiaries aged ≥60 years in the US.
METHODS
Data Source
Data from 1 January 2007 to 31 December 2019 from the Centers for Medicare and Medicaid Services (CMS) 100% Medicare Research Identifiable Files (RIF) with Part D linkage were used for this study. CMS 100% Medicare data contains information collected by Medicare to pay for healthcare services or drug events of Medicare beneficiaries. Fee-for-service data are available for each institutional (Part A), noninstitutional (Part B), and drug event (Part D) claim type. RIF datasets include information on durable medical equipment, outpatient, home health agency, hospice, inpatient, skilled nursing facility, carrier, and Part D claims, along with beneficiary demographics (including date of death).
Data were accessed through the Research Data Assistance Center’s Virtual Research Data Center. Since RIF data contain actual beneficiary-specific and physician-specific information, an institutional review board exemption was obtained from Western Copernicus Group Institutional Review Board. Data were de-identified and comply with the patient requirements of the Health Insurance Portability and Accountability Act. Per data user agreement, due to the cell suppression policy of the CMS, cell values of <11 cannot be displayed for results from RIF data.
Study Design and Sample Selection
A retrospective, longitudinal cohort study design was used. The index date was defined as the date of the first claim with a diagnosis code for RSV (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM]: 079.6, 466.11, 480.1; International Classification of Diseases, 10th Revision, Clinical Modification [ICD-10-CM]: B97.4, J12.1, J20.5, J21.0). The baseline period was defined as the 6-month period preceding the index date, while the observation period spanned from the index date through to the earliest of 6 months following the index date, the end of continuous health plan coverage, or the end of data availability. No minimum follow-up time was required to avoid survival bias. To avoid capturing costs associated with symptoms and testing before RSV diagnosis, the 30 most recent days of the baseline period were not considered as part of the analysis of costs (see Study Outcomes).
Patients were included in the study if they had ≥1 claim with a diagnosis for RSV, had ≥6 months of continuous Medicare coverage (Part A, B, and D) prior to the index date, and were ≥60 years of age as of the index date.
Study Outcomes
Study outcomes included the proportion of medically attended RSV patients experiencing each of the RSV-related complications, predictors of developing ≥1 RSV-related complication, and pre- and post-index total all-cause and respiratory/infection-related healthcare costs.
RSV-related complications were evaluated during the observation period and were defined based on the occurrence of a new medical claim with a diagnosis of pneumonia, acute respiratory failure, congestive heart failure, hypoxia or dyspnea, non-RSV lower and upper respiratory tract infection, or chronic respiratory disease (ie, asthma or COPD) (Supplementary Table 1). To be considered for each specific complication, patients were required to have no claims with a diagnosis for the corresponding condition during the 6-month baseline period; that is, RSV-related complications represented diagnoses not previously observed during the baseline period. Patients with all 6 conditions observed during the baseline period were excluded from the at-risk population because by definition they would not be able to contribute an RSV-related complication. Of note, for complications such as chronic respiratory disease or congestive heart failure, although patients were required to not have diagnoses for these conditions during the baseline period to be included in the complication-specific analyses, the observation of a diagnosis post–RSV infection may likely reflect an exacerbation of the condition rather than new onset.
All-cause and respiratory/infection-related healthcare costs included medical (ie, inpatient, outpatient, ED, other [eg, hospice care, home health, skilled nursing facilities]) and pharmacy components. Respiratory/infection-related costs included all claims with a diagnosis for a respiratory condition (ICD-9-CM: 460–519; ICD-10-CM: Jxx), other respiratory-related comorbidities and infections not included in the range of diagnosis codes for respiratory conditions (ie, abnormal/decreased pulmonary function, adenovirus, congested or runny nose, cough, fever, severe acute respiratory syndrome–associated coronavirus, metapneumovirus, hypoxemia, Streptococcus/pneumococcus, wheezing, and pertussis), or procedures for respiratory support, intravenous fluids, or chest radiographs. Pre-index costs were measured during the 6-month period before the first RSV diagnosis, excluding the 30 most recent days before the index date. For patients without continuous coverage for an additional month before the 6-month baseline period, total costs were extrapolated based on the most recent 5 months of coverage. Post-index costs were measured during a maximum of 6 months of observation starting on the index date (this period also did not include the 1-month period pre-index). All costs were reported in 2019 US dollars with adjustment to inflation using the US Consumer Price Index [16].
Statistical Analyses
Analyses were conducted using SAS Enterprise Guide version 7.1 (SAS Institute, Cary, North Carolina). Mean, standard deviation, and median were reported for continuous variables, while frequencies and proportions were reported for categorical variables.
Predictors (ie, demographic and baseline clinical characteristics) of having ≥1 RSV-related complication were identified using multivariable Poisson regression. A stepwise multivariable Poisson regression model was used to compare the rate of having ≥1 RSV-related complication between patients with and without each predictor. At each iteration, variables with P < .30 were entered into the model and variables with P < .20 were retained. Age group, sex, race, US geographic region, index year, asthma, COPD, coronary artery disease, congestive heart failure, diabetes, chemotherapy, and prior diagnoses of RSV-related complications/comorbidities were forced into each iteration. Results were reported as incidence rate ratios (IRRs) with 95% confidence intervals (CIs) and P values. As part of a sensitivity analysis to assess the effect of having any potentially relevant baseline RSV-related complications/comorbidities versus not, having ≥1 RSV-related complication was assessed among patients without any of these conditions observed during the 6-month baseline period.
Differences between pre- and post-index total all-cause and respiratory/infection-related healthcare costs were assessed using ordinary least squares regression with generalized estimating equations. Results were reported as cost differences with 95% CIs and P values, estimated with a nonparametric bootstrap procedure with 500 replications.
RESULTS
Patient Characteristics
After applying the selection criteria, 175 392 patients with RSV were included in the study (Figure 1). A total of 279 patients had a diagnosis for all 6 RSV-related complications/comorbidities during the baseline period and were excluded from the analysis of RSV-related complications, leaving 175 113 patients eligible to have an RSV-related complication during the observation period. The mean age was 79.0 years and 64.8% were female (Table 1). Most patients (78.4%) were White, and the highest proportion of patients lived in the South (32.7%). Additionally, 12.1% of patients resided in a long-term facility. Regarding the type of Medicare healthcare plan, 20.8% had disability status, while 40.1% had dual Medicare/Medicaid coverage. During the baseline period, 76.9% of patients had prior diagnosis of an RSV-related complication/comorbidity, with the most common being hypoxia or dyspnea (52.8%), chronic respiratory disease (44.1%), and pneumonia (41.3%).
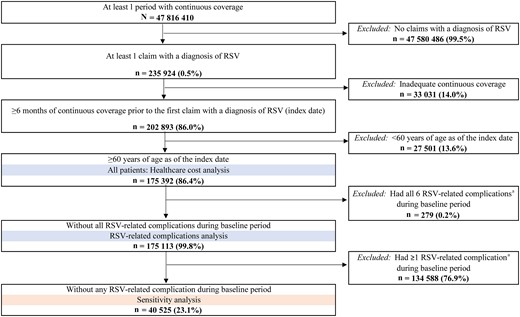
Patient selection. aRespiratory syncytial virus (RSV)–related complications included pneumonia, acute respiratory failure, congestive heart failure, hypoxia or dyspnea, non-RSV lower and upper respiratory tract infections, or chronic respiratory disease (ie, asthma or chronic obstructive pulmonary disease).
Baseline Demographic and Clinical Characteristics of Medicare Beneficiaries ≥60 Years Old With Respiratory Syncytial Virus (RSV) and Without All 6 RSV-Related Complications/Comorbidities During Baseline
| Characteristic . | Patients Without All 6 RSV-Related Complications/Comorbidities During Baseline . |
|---|---|
| (N = 175 113) . | |
| Age at index date, y, mean ± SD [median] | 79.0 ± 9.6 [79.0] |
| Age at index date, y, | |
| 60–64 | 9721 (5.6) |
| 65–69 | 24 441 (14.0) |
| 70–79 | 56 457 (32.2) |
| 80–89 | 56 881 (32.5) |
| ≥90 | 27 613 (15.8) |
| Female sex | 113 412 (64.8) |
| Race/ethnicity | |
| White | 137 227 (78.4) |
| Black/African American | 14 783 (8.4) |
| Hispanic | 13 548 (7.7) |
| Other/unknown | 9555 (5.5) |
| Year of index date | |
| 2007–2014 | 112 937 (64.5) |
| 2015–2019 | 62 176 (35.5) |
| US region | |
| South | 57 347 (32.7) |
| Northeast | 50 233 (28.7) |
| Midwest | 41 300 (23.6) |
| West | 25 386 (14.5) |
| Other/unknown | 847 (0.5) |
| Residence in long-term facilitya | 21 214 (12.1) |
| Type of healthcare plan | |
| Disability status | 36 446 (20.8) |
| Dual Medicare/Medicaid coverage | 70 303 (40.1) |
| Index RSV diagnosisb | |
| Pneumonia due to RSV | 72 383 (41.3) |
| Acute bronchitis or acute bronchiolitis due to RSV | 68 424 (39.1) |
| RSV or RSV as the cause of disease classified elsewhere | 34 306 (19.6) |
| Quan CCI, mean ± SD [median] | 1.8 ± 2.1 [1.0] |
| Previous diagnosis of the complications/comorbidities of interest | |
| Acute respiratory failure | 36 669 (20.9) |
| Chronic respiratory disease | 77 267 (44.1) |
| Asthma | 25 705 (14.7) |
| Chronic obstructive pulmonary disease | 67 724 (38.7) |
| Congestive heart failure | 62 119 (35.5) |
| Hypoxia or dyspnea | 92 519 (52.8) |
| Non-RSV lower and upper respiratory tract infectionsc | 5548 (3.2) |
| Pneumonia | 72 333 (41.3) |
| No. of previous complications/comorbidities of interest, mean ± SD [median] | 2.1 ± 1.7 [2.0] |
| Coronary artery disease | 64 151 (36.6) |
| Diabetes | 67 657 (38.6) |
| Immune system disorders | 18 454 (10.5) |
| Other respiratory-related conditions | |
| Acute upper respiratory illness (eg, nasopharyngitis, sinusitis) | 48 149 (27.5) |
| Bronchitis or bronchiolitis | 29 410 (16.8) |
| Cardiovascular-related conditions | |
| Hypertension | 127 884 (73.0) |
| Pulmonary hypertension | 10 528 (6.0) |
| Chemotherapy | 8880 (5.1) |
| Mental health–related conditions | 48 252 (27.6) |
| RSV-related symptoms | |
| Fever | 24 830 (14.2) |
| Abnormal/decreased pulmonary function | 792 (0.5) |
| Procedures | |
| Chest radiograph | 134 837 (77.0) |
| Respiratory support | 23 158 (13.2) |
| Intravenous fluids | 10 238 (5.8) |
| Testing | |
| Influenza | 10 180 (5.8) |
| RSV | 3840 (2.2) |
| Organ transplant | 699 (0.4) |
| Stem cell transplant | 122 (0.1) |
| Medication use | |
| Antibiotics | 171 105 (97.7) |
| Anti-asthmatics | 129 826 (74.1) |
| Corticosteroids | 124 289 (71.0) |
| Bronchodilators | |
| Short-acting β-agonists or muscarinic antagonists | 118 020 (67.4) |
| Aminophylline or theophylline | 5633 (3.2) |
| Antihistamines | 54 028 (30.9) |
| Antivirals | |
| Influenza agents | 30 244 (17.3) |
| Characteristic . | Patients Without All 6 RSV-Related Complications/Comorbidities During Baseline . |
|---|---|
| (N = 175 113) . | |
| Age at index date, y, mean ± SD [median] | 79.0 ± 9.6 [79.0] |
| Age at index date, y, | |
| 60–64 | 9721 (5.6) |
| 65–69 | 24 441 (14.0) |
| 70–79 | 56 457 (32.2) |
| 80–89 | 56 881 (32.5) |
| ≥90 | 27 613 (15.8) |
| Female sex | 113 412 (64.8) |
| Race/ethnicity | |
| White | 137 227 (78.4) |
| Black/African American | 14 783 (8.4) |
| Hispanic | 13 548 (7.7) |
| Other/unknown | 9555 (5.5) |
| Year of index date | |
| 2007–2014 | 112 937 (64.5) |
| 2015–2019 | 62 176 (35.5) |
| US region | |
| South | 57 347 (32.7) |
| Northeast | 50 233 (28.7) |
| Midwest | 41 300 (23.6) |
| West | 25 386 (14.5) |
| Other/unknown | 847 (0.5) |
| Residence in long-term facilitya | 21 214 (12.1) |
| Type of healthcare plan | |
| Disability status | 36 446 (20.8) |
| Dual Medicare/Medicaid coverage | 70 303 (40.1) |
| Index RSV diagnosisb | |
| Pneumonia due to RSV | 72 383 (41.3) |
| Acute bronchitis or acute bronchiolitis due to RSV | 68 424 (39.1) |
| RSV or RSV as the cause of disease classified elsewhere | 34 306 (19.6) |
| Quan CCI, mean ± SD [median] | 1.8 ± 2.1 [1.0] |
| Previous diagnosis of the complications/comorbidities of interest | |
| Acute respiratory failure | 36 669 (20.9) |
| Chronic respiratory disease | 77 267 (44.1) |
| Asthma | 25 705 (14.7) |
| Chronic obstructive pulmonary disease | 67 724 (38.7) |
| Congestive heart failure | 62 119 (35.5) |
| Hypoxia or dyspnea | 92 519 (52.8) |
| Non-RSV lower and upper respiratory tract infectionsc | 5548 (3.2) |
| Pneumonia | 72 333 (41.3) |
| No. of previous complications/comorbidities of interest, mean ± SD [median] | 2.1 ± 1.7 [2.0] |
| Coronary artery disease | 64 151 (36.6) |
| Diabetes | 67 657 (38.6) |
| Immune system disorders | 18 454 (10.5) |
| Other respiratory-related conditions | |
| Acute upper respiratory illness (eg, nasopharyngitis, sinusitis) | 48 149 (27.5) |
| Bronchitis or bronchiolitis | 29 410 (16.8) |
| Cardiovascular-related conditions | |
| Hypertension | 127 884 (73.0) |
| Pulmonary hypertension | 10 528 (6.0) |
| Chemotherapy | 8880 (5.1) |
| Mental health–related conditions | 48 252 (27.6) |
| RSV-related symptoms | |
| Fever | 24 830 (14.2) |
| Abnormal/decreased pulmonary function | 792 (0.5) |
| Procedures | |
| Chest radiograph | 134 837 (77.0) |
| Respiratory support | 23 158 (13.2) |
| Intravenous fluids | 10 238 (5.8) |
| Testing | |
| Influenza | 10 180 (5.8) |
| RSV | 3840 (2.2) |
| Organ transplant | 699 (0.4) |
| Stem cell transplant | 122 (0.1) |
| Medication use | |
| Antibiotics | 171 105 (97.7) |
| Anti-asthmatics | 129 826 (74.1) |
| Corticosteroids | 124 289 (71.0) |
| Bronchodilators | |
| Short-acting β-agonists or muscarinic antagonists | 118 020 (67.4) |
| Aminophylline or theophylline | 5633 (3.2) |
| Antihistamines | 54 028 (30.9) |
| Antivirals | |
| Influenza agents | 30 244 (17.3) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CCI, Charlson Comorbidity Index; RSV, respiratory syncytial virus; SD, standard deviation; US, United States.
Residence in a long-term facility was assessed using place of service code 13 in the medical claims during the baseline period.
The type of RSV diagnosis identified on the index date was assessed using the following hierarchy: (1) RSV with pneumonia, (2) RSV with acute bronchiolitis or acute bronchitis, and (3) RSV alone or RSV as the cause of diseases classified elsewhere.
Includes the following non-RSV respiratory infections: influenza, parainfluenza, adenovirus, rhinovirus, severe acute respiratory syndrome–associated coronavirus, and human metapneumovirus.
Baseline Demographic and Clinical Characteristics of Medicare Beneficiaries ≥60 Years Old With Respiratory Syncytial Virus (RSV) and Without All 6 RSV-Related Complications/Comorbidities During Baseline
| Characteristic . | Patients Without All 6 RSV-Related Complications/Comorbidities During Baseline . |
|---|---|
| (N = 175 113) . | |
| Age at index date, y, mean ± SD [median] | 79.0 ± 9.6 [79.0] |
| Age at index date, y, | |
| 60–64 | 9721 (5.6) |
| 65–69 | 24 441 (14.0) |
| 70–79 | 56 457 (32.2) |
| 80–89 | 56 881 (32.5) |
| ≥90 | 27 613 (15.8) |
| Female sex | 113 412 (64.8) |
| Race/ethnicity | |
| White | 137 227 (78.4) |
| Black/African American | 14 783 (8.4) |
| Hispanic | 13 548 (7.7) |
| Other/unknown | 9555 (5.5) |
| Year of index date | |
| 2007–2014 | 112 937 (64.5) |
| 2015–2019 | 62 176 (35.5) |
| US region | |
| South | 57 347 (32.7) |
| Northeast | 50 233 (28.7) |
| Midwest | 41 300 (23.6) |
| West | 25 386 (14.5) |
| Other/unknown | 847 (0.5) |
| Residence in long-term facilitya | 21 214 (12.1) |
| Type of healthcare plan | |
| Disability status | 36 446 (20.8) |
| Dual Medicare/Medicaid coverage | 70 303 (40.1) |
| Index RSV diagnosisb | |
| Pneumonia due to RSV | 72 383 (41.3) |
| Acute bronchitis or acute bronchiolitis due to RSV | 68 424 (39.1) |
| RSV or RSV as the cause of disease classified elsewhere | 34 306 (19.6) |
| Quan CCI, mean ± SD [median] | 1.8 ± 2.1 [1.0] |
| Previous diagnosis of the complications/comorbidities of interest | |
| Acute respiratory failure | 36 669 (20.9) |
| Chronic respiratory disease | 77 267 (44.1) |
| Asthma | 25 705 (14.7) |
| Chronic obstructive pulmonary disease | 67 724 (38.7) |
| Congestive heart failure | 62 119 (35.5) |
| Hypoxia or dyspnea | 92 519 (52.8) |
| Non-RSV lower and upper respiratory tract infectionsc | 5548 (3.2) |
| Pneumonia | 72 333 (41.3) |
| No. of previous complications/comorbidities of interest, mean ± SD [median] | 2.1 ± 1.7 [2.0] |
| Coronary artery disease | 64 151 (36.6) |
| Diabetes | 67 657 (38.6) |
| Immune system disorders | 18 454 (10.5) |
| Other respiratory-related conditions | |
| Acute upper respiratory illness (eg, nasopharyngitis, sinusitis) | 48 149 (27.5) |
| Bronchitis or bronchiolitis | 29 410 (16.8) |
| Cardiovascular-related conditions | |
| Hypertension | 127 884 (73.0) |
| Pulmonary hypertension | 10 528 (6.0) |
| Chemotherapy | 8880 (5.1) |
| Mental health–related conditions | 48 252 (27.6) |
| RSV-related symptoms | |
| Fever | 24 830 (14.2) |
| Abnormal/decreased pulmonary function | 792 (0.5) |
| Procedures | |
| Chest radiograph | 134 837 (77.0) |
| Respiratory support | 23 158 (13.2) |
| Intravenous fluids | 10 238 (5.8) |
| Testing | |
| Influenza | 10 180 (5.8) |
| RSV | 3840 (2.2) |
| Organ transplant | 699 (0.4) |
| Stem cell transplant | 122 (0.1) |
| Medication use | |
| Antibiotics | 171 105 (97.7) |
| Anti-asthmatics | 129 826 (74.1) |
| Corticosteroids | 124 289 (71.0) |
| Bronchodilators | |
| Short-acting β-agonists or muscarinic antagonists | 118 020 (67.4) |
| Aminophylline or theophylline | 5633 (3.2) |
| Antihistamines | 54 028 (30.9) |
| Antivirals | |
| Influenza agents | 30 244 (17.3) |
| Characteristic . | Patients Without All 6 RSV-Related Complications/Comorbidities During Baseline . |
|---|---|
| (N = 175 113) . | |
| Age at index date, y, mean ± SD [median] | 79.0 ± 9.6 [79.0] |
| Age at index date, y, | |
| 60–64 | 9721 (5.6) |
| 65–69 | 24 441 (14.0) |
| 70–79 | 56 457 (32.2) |
| 80–89 | 56 881 (32.5) |
| ≥90 | 27 613 (15.8) |
| Female sex | 113 412 (64.8) |
| Race/ethnicity | |
| White | 137 227 (78.4) |
| Black/African American | 14 783 (8.4) |
| Hispanic | 13 548 (7.7) |
| Other/unknown | 9555 (5.5) |
| Year of index date | |
| 2007–2014 | 112 937 (64.5) |
| 2015–2019 | 62 176 (35.5) |
| US region | |
| South | 57 347 (32.7) |
| Northeast | 50 233 (28.7) |
| Midwest | 41 300 (23.6) |
| West | 25 386 (14.5) |
| Other/unknown | 847 (0.5) |
| Residence in long-term facilitya | 21 214 (12.1) |
| Type of healthcare plan | |
| Disability status | 36 446 (20.8) |
| Dual Medicare/Medicaid coverage | 70 303 (40.1) |
| Index RSV diagnosisb | |
| Pneumonia due to RSV | 72 383 (41.3) |
| Acute bronchitis or acute bronchiolitis due to RSV | 68 424 (39.1) |
| RSV or RSV as the cause of disease classified elsewhere | 34 306 (19.6) |
| Quan CCI, mean ± SD [median] | 1.8 ± 2.1 [1.0] |
| Previous diagnosis of the complications/comorbidities of interest | |
| Acute respiratory failure | 36 669 (20.9) |
| Chronic respiratory disease | 77 267 (44.1) |
| Asthma | 25 705 (14.7) |
| Chronic obstructive pulmonary disease | 67 724 (38.7) |
| Congestive heart failure | 62 119 (35.5) |
| Hypoxia or dyspnea | 92 519 (52.8) |
| Non-RSV lower and upper respiratory tract infectionsc | 5548 (3.2) |
| Pneumonia | 72 333 (41.3) |
| No. of previous complications/comorbidities of interest, mean ± SD [median] | 2.1 ± 1.7 [2.0] |
| Coronary artery disease | 64 151 (36.6) |
| Diabetes | 67 657 (38.6) |
| Immune system disorders | 18 454 (10.5) |
| Other respiratory-related conditions | |
| Acute upper respiratory illness (eg, nasopharyngitis, sinusitis) | 48 149 (27.5) |
| Bronchitis or bronchiolitis | 29 410 (16.8) |
| Cardiovascular-related conditions | |
| Hypertension | 127 884 (73.0) |
| Pulmonary hypertension | 10 528 (6.0) |
| Chemotherapy | 8880 (5.1) |
| Mental health–related conditions | 48 252 (27.6) |
| RSV-related symptoms | |
| Fever | 24 830 (14.2) |
| Abnormal/decreased pulmonary function | 792 (0.5) |
| Procedures | |
| Chest radiograph | 134 837 (77.0) |
| Respiratory support | 23 158 (13.2) |
| Intravenous fluids | 10 238 (5.8) |
| Testing | |
| Influenza | 10 180 (5.8) |
| RSV | 3840 (2.2) |
| Organ transplant | 699 (0.4) |
| Stem cell transplant | 122 (0.1) |
| Medication use | |
| Antibiotics | 171 105 (97.7) |
| Anti-asthmatics | 129 826 (74.1) |
| Corticosteroids | 124 289 (71.0) |
| Bronchodilators | |
| Short-acting β-agonists or muscarinic antagonists | 118 020 (67.4) |
| Aminophylline or theophylline | 5633 (3.2) |
| Antihistamines | 54 028 (30.9) |
| Antivirals | |
| Influenza agents | 30 244 (17.3) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CCI, Charlson Comorbidity Index; RSV, respiratory syncytial virus; SD, standard deviation; US, United States.
Residence in a long-term facility was assessed using place of service code 13 in the medical claims during the baseline period.
The type of RSV diagnosis identified on the index date was assessed using the following hierarchy: (1) RSV with pneumonia, (2) RSV with acute bronchiolitis or acute bronchitis, and (3) RSV alone or RSV as the cause of diseases classified elsewhere.
Includes the following non-RSV respiratory infections: influenza, parainfluenza, adenovirus, rhinovirus, severe acute respiratory syndrome–associated coronavirus, and human metapneumovirus.
RSV-Related Complications
During the observation period, 47.9% of patients had ≥1 RSV-related complication, with a mean (median) time to first RSV-related complication of 1.0 (0.1) month. The most common RSV-related complications were pneumonia (24.0%), chronic respiratory disease (23.6%), and hypoxia or dyspnea (22.0%) (Figure 2). Among the 40 525 patients without any baseline RSV-related conditions, 42.7% of patients had ≥1 RSV-related complication, with a mean (median) time to first RSV-related complication of 1.1 (0.2) months (Supplementary Figure 1).
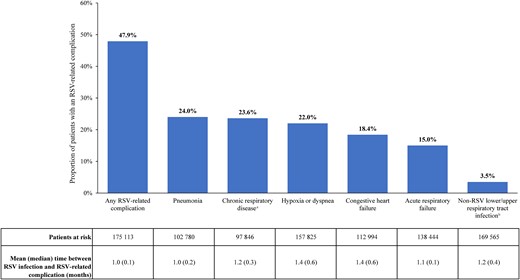
Respiratory syncytial virus (RSV)–related complications among Medicare beneficiaries aged ≥60 years. aIncludes asthma and chronic obstructive pulmonary disease. bIncludes the following non-RSV respiratory infections: influenza, parainfluenza, adenovirus, rhinovirus, severe acute respiratory syndrome–associated coronavirus, and human metapneumovirus.
Predictors of Having ≥1 RSV-Related Complication
Having an RSV-related complication during the observation period was more likely for patients with a previous diagnosis of any of the RSV-related complications/comorbidities of interest (except asthma) during the baseline period (IRR range, 1.09 [95% CI, 1.05–1.13] for pneumonia to 1.47 [95% CI, 1.42–1.52] for hypoxia or dyspnea; all P < .001; Figure 3). Additionally, patients were more likely to have an RSV-related complication if they were ≥80 years old (IRR, 1.26 [95% CI, 1.22–1.31] for 80–89 years old and 1.43 [95% CI, 1.38–1.49] for ≥90 years old, compared to patients 60–64 years old; both P < .001) or had disability Medicare coverage (IRR, 1.11 [95% CI, 1.09–1.13]; P < .001), hypoxemia (IRR, 1.28 [95% CI, 1.24–1.32]; P < .001), chemotherapy treatment (IRR, 1.19 [95% CI, 1.15–1.22]; P < .001), a chest radiograph (IRR, 1.68 [95% CI, 1.64–1.71]; P < .001), stem cell transplant (IRR, 1.48 [95% CI, 1.15–1.90]; P = .002), anti-asthmatic medication use (IRR, 1.28 [95% CI, 1.24–1.32]; P < .001), or aminophylline/theophylline bronchodilator use (IRR, 1.20 [95% CI, 1.16–1.25]; P < .001) during the baseline period (Figure 3).
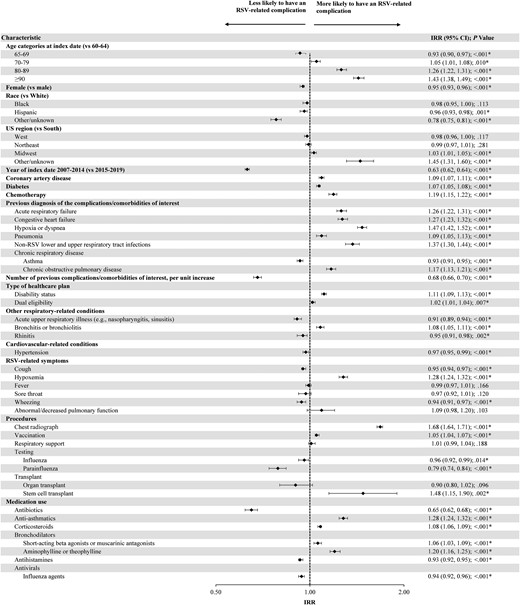
Predictors of developing ≥1 respiratory syncytial virus–related complication. *Significant at the 5% level. Abbreviations: CI, confidence interval; IRR, incidence rate ratio; RSV, respiratory syncytial virus; US, United States.
Patients were less likely to have an RSV-related complication during the observation period if they were diagnosed with RSV during 2007–2014 versus 2015–2019 (IRR, 0.63 [95% CI, 0.62–0.64]; P < .001), had an increasing number of baseline RSV-related complications/comorbidities (IRR, 0.68 [95% CI, 0.66–0.70]; P < .001), had a parainfluenza test (IRR, 0.79 [95% CI, 0.74–0.84]; P < .001), or used antibiotics (IRR, 0.65 [95% CI, 0.62–0.68]; P < .001) during the baseline period (Figure 3).
Pre- and Post-index Total All-Cause and Respiratory/Infection-Related Costs
For the cost analysis, all 175 392 patients who met the selection criteria were included (see Supplementary Table 2 for baseline characteristics). After a mean (median) post-index observation period of 5.1 (6.0) months, mean total all-cause healthcare costs were $7797 (95% CI, $7591–$8019) higher (P < .001) post-index ($29 968) relative to the pre-index period ($22 171), driven by $5736 (95% CI, $5565–$5925) higher inpatient costs (pre-index: $10 372; post-index: $16 108; P < .001; Figure 4A). Mean total respiratory/infection-related healthcare costs were $8863 (95% CI, $8706–$9046) higher (P < .001) post-index ($18 766) relative to the pre-index period ($9903), also driven by $6256 (95% CI, $6102–$6400) higher inpatient costs (pre-index: $6436; post-index: $12 692; P < .001; Figure 4B). Respiratory/infection-related costs accounted for a greater proportion of post-index costs (62.6%) than pre-index costs (44.7%). A similar trend was observed for inpatient costs, where respiratory/infection-related inpatient costs accounted for a greater proportion of post-index inpatient costs (78.8%) then pre-index inpatient costs (62.1%).
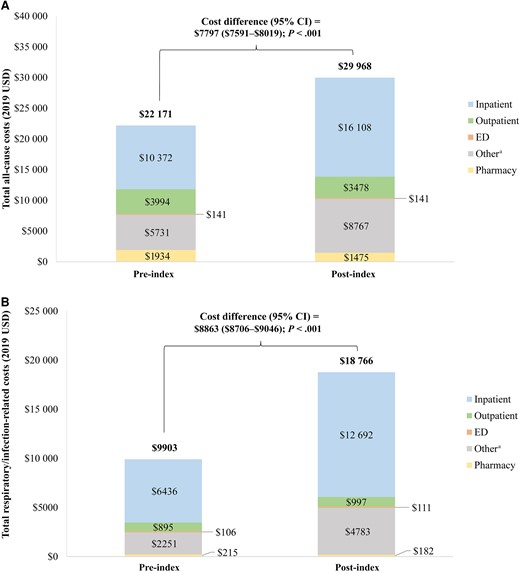
Pre- and post-index total all-cause (A) and respiratory/infection-related (B) costs. aOther services included hospice, home health, and skilled nursing facility care. Abbreviations: CI, confidence interval; ED, emergency department; USD, United States dollars.
DISCUSSION
In this large, real-world study of Medicare beneficiaries aged ≥60 years with medically attended RSV, almost half (47.9%) developed an RSV-related complication, most within an average of 1 month (median, 0.1 month) following RSV infection. Patients with a diagnosis for a complication/comorbidity of interest at baseline, who accounted for >75% of patients evaluated in this cohort, were at higher risk of developing a different complication during the observation period. Last, the post-index RSV period was associated with significantly increased all-cause and respiratory/infection-related healthcare costs, largely driven by hospitalization costs, as compared to the period before RSV diagnosis.
The rates of different RSV-related complications among older adults have previously been characterized, but in smaller populations or among very specific patient populations (ie, RSV patients who experienced a hospitalization). In 1 study of community-dwelling adults aged ≥60 years with RSV who were hospitalized between 2011 and 2015 in California, 48.7%–55.9% had pneumonia and 77.7%–81.2% had hypoxia during their hospital stay [5]. However, results from this analysis may not be generalizable to the entire population of patients with RSV in the US given that only hospitalized patients (who may have more severe disease) from 1 state were analyzed. In a separate retrospective analysis of adults with Medicare coverage between 2011 and 2015 (82% were aged ≥65 years) by Wyffels et al, the most common complications during the 180 days following RSV diagnosis were upper and lower respiratory tract infection (38%–88% depending on hospitalization and risk status), lower respiratory tract infection (15%–84%), dyspnea (35%–75%), and pneumonia (6%–77%) [6]. Given that the 5% Medicare sample was used, this study had a relatively smaller sample of Medicare beneficiaries (n = 1628) and did not include more recent years of data. The current study addresses the limitations of the studies mentioned above, as it is more generalizable to the entire population of Medicare beneficiaries ≥60 years old with RSV in the US, given that it included all medically attended patients, who may or may not have been hospitalized, and captured all Medicare beneficiaries with coverage between 2007 and 2019 from all US states, resulting in a larger sample size.
To our knowledge, the current study is among the first using the entire Medicare population with medically attended RSV to identify predictors of RSV-related complications, including prior cardiovascular disease (ie, congestive heart failure), respiratory conditions (ie, pneumonia, hypoxia or dyspnea, acute respiratory failure, lower and upper respiratory tract infections, COPD), and older age. While previous studies have not evaluated predictors of specific RSV-related complications, many of the factors from the present analysis have been identified in the literature as predictors of other outcomes such as having severe RSV or hospitalization in adults. Indeed, in a study of adults aged ≥60 years in Wisconsin, new/increased dyspnea and age ≥75 years were identified as predictors of severe RSV outcomes (ie, hospital admission, ED visit, or pneumonia diagnosis within 28 days of enrollment) based on a reduced penalized model [8]. In the study by Wyffels et al, comorbidities like COPD (odds ratio [OR], 2.12; P < .001) and congestive heart failure (OR, 2.06; P = .002) were identified as predictors of hospitalization among Medicare beneficiaries [6]. Last, cardiac disease (ie, coronary artery disease or congestive heart failure; OR, 7.5; P = .01), diabetes (OR, 5.0; P = .03), and COPD (OR, 4.6; P = .02) were found to be independent predictors of hospitalization among a cohort of adult patients with RSV in New York [7]. Taken together, even though different outcomes have been evaluated in the literature, common factors have been identified to predict negative RSV outcomes, whether it be complications or hospitalization. This study adds to the existing body of literature by specifically identifying predictors of RSV-related complications, regardless of whether they resulted in hospitalization, among all medically attended Medicare beneficiaries ≥60 years of age with RSV. These findings emphasize the substantial disease burden of specific groups of patients with medically attended RSV who may have characteristics that predispose them to further RSV complications.
In addition to the clinical burden, the present study also described the significantly higher healthcare costs incurred after RSV diagnosis. Wyffels et al conducted a similar analysis and found that Medicare beneficiaries (82% were ≥65 years of age) had $2072–$19 141 higher all-cause healthcare costs during the 180-day period following RSV diagnosis compared to before, which is within range of the $7797 increase observed in the current study [6]. Similarly, the primary cost driver among Medicare beneficiaries was hospitalization costs for both the Wyffels et al [6] and the current study. In a different claims-based study, Wyffels et al reported that the mean cost of an RSV-related hospitalization was $7683 among a sample of 954 older adults ≥65 years old [17]. Results from the current study, however, provide broader overview of all types of healthcare costs, beyond only hospitalization costs, in addition to identifying costs that are specifically respiratory-related. Additionally, a recent claims-based study of adults with medically attended RSV recently conducted by Mesa-Frias et al found that costs associated with RSV were generally highest among older patients (ie, aged ≥65 years) [18]. The current study adds to the previous literature on healthcare costs of RSV by identifying excess costs for all services associated with RSV infection between the 6-month period before and after RSV diagnosis, which may help identify the impact that RSV infection has on the economic burden of RSV among Medicare beneficiaries.
The present study fills a gap in the literature by providing one of the first characterizations of the complication and cost burden of older adult patients with medically attended RSV, who have a particularly high risk of poor outcomes and hospitalization [19]. Moreover, these patients have a large unmet medical need and may benefit from prophylactic options, including vaccines currently under review by the FDA [1, 9]. Additionally, the recent rise in cases of cocirculating RSV, influenza, and COVID-19 in the US has increasingly strained hospital systems, further impeding optimal management [20]. Improved recognition of the considerable burden of RSV and efficient identification of patients at risk for severe disease and RSV-related complications will be instrumental in improving the management of RSV.
Certain limitations of the study design should be noted. Claims databases only contain diagnostic and procedure codes that are recorded for reimbursement purposes; therefore, additional information needed to fully confirm RSV diagnosis, such as diagnostic polymerase chain reaction testing results, which may not be universally available in hospitals and may be restricted to certain at-risk populations, were not available. RSV may have also been misdiagnosed as influenza given the similar etiology, epidemiology, and presence of symptoms. Moreover, as with all claims-based studies, billing inaccuracies or omissions in coded procedures, diagnoses, and pharmacy claims may have occurred. The reliance on diagnosis codes to identify RSV may have led to the inclusion of patients with more severe RSV (ie, requiring medical care), as opposed to patients with milder RSV who did not seek care. As such, the rates of RSV-related complications reported here may not be applicable to patients with milder RSV. While patients were required to have no claims with a diagnosis for the complication/comorbidity of interest during the 6-month baseline period to identify new RSV-related complications, patients with the pre-existing disease who did not receive medical care for that condition during the baseline period may have been included in the analysis; therefore, the complication observed may not have been new, but rather a worsening or exacerbation of a prior condition. This limitation may mostly apply to chronic conditions such as chronic respiratory disease (ie, asthma and COPD) and congestive heart failure. Given that the analysis of pre-index costs did not include the month prior to RSV diagnosis, which may include healthcare resource utilization related to RSV symptoms and diagnostic testing, the full economic burden of RSV may have been underestimated. This analysis also did not include any comparator groups without RSV or with other similar infections, such as influenza. Last, given the use of 100% Medicare data, the study findings may be generalizable to Medicare beneficiaries aged ≥60 years with medically attended RSV but may not be representative of the overall population of patients with RSV.
CONCLUSIONS
This large, real-world study of Medicare beneficiaries aged ≥60 years with medically attended RSV demonstrated that almost half of all patients experienced an RSV-related complication within 1 month of RSV diagnosis. Over three-quarters of patients experienced a complication/comorbidity of interest prior to RSV diagnosis, which predicted a higher risk of developing a different complication following diagnosis. Furthermore, RSV was associated with a substantial economic burden among Medicare beneficiaries ≥60 years old, with excess costs post–RSV infection driven by respiratory/infection-related inpatient stays. These findings suggest that many older adults with medically attended RSV experience a significant clinical and economic burden that impacts both the patient and the healthcare system.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. All authors have made substantial contributions to the conception or design of the study or the acquisition, analysis, or interpretation of data. All authors made substantial contributions in drafting the manuscript and revising it critically for important intellectual content, and have provided final approval of this version to be published and agree to be accountable for all aspects of the work.
Acknowledgments. Medical writing assistance was provided by a professional medical writer, Christine Tam, an employee of Analysis Group, Inc, a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC, which funded the development and conduct of this study and manuscript.
Patient consent. Since the data contain actual beneficiary-specific and physician-specific information, an institutional review board (IRB) exemption was obtained from the Western Copernicus Group IRB. Data were de-identified and comply with the patient requirements of the Health Insurance Portability and Accountability Act. (HIPAA) of 1996.
Financial support. This study was funded by Janssen Scientific Affairs, LLC.
References
Author notes
Presented in part: IDWeek, Washington, DC, 19–23 October 2022; Academy of Managed Care Pharmacy Nexus, National Harbor, Maryland, 11–14 October 2022.
Potential conflicts of interest. J. K. D. and G. K. are employees of Janssen Scientific Affairs, LLC, and stockholders of Johnson & Johnson. M.-H. L., B. E., C. R., and P. L. are employees of Analysis Group, Inc, a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC, which funded the development and conduct of this study and manuscript. J. W. and S. L. were employees of Analysis Group, Inc, at the time the study was conducted.





Comments