-
PDF
- Split View
-
Views
-
Cite
Cite
Erik Senneby, Birger Eriksson, Erik Fagerholm, Magnus Rasmussen, Bacteremia with Aerococcus sanguinicola: Case Series with Characterization of Virulence Properties, Open Forum Infectious Diseases, Volume 1, Issue 1, Spring 2014, ofu025, https://doi.org/10.1093/ofid/ofu025
Close - Share Icon Share
Abstract
Background. Since Aerococcus sanguinicola was designated as a species in 2001, only a few cases of bacteremia have been reported. The aim with this study was to describe the clinical presentation of A sanguinicola bacteremia and to determine the antibiotic susceptibility and the capacity of the bacteria to form biofilm and to induce platelet aggregation.
Methods. Isolates of A sanguinicola from blood cultures were retrospectively identified from 2 clinical microbiology laboratories for 2006 to 2012. Species identity was confirmed through sequencing of the 16S rRNA gene. The medical charts of patients were reviewed. The minimum inhibitory concentration (MIC) for relevant antibiotics was determined. Biofilm formation was measured as the amount of crystal violet absorbed. Platelet aggregation was determined by aggregometry.
Results. Eleven cases of A sanguinicola bacteremia were identified. All patients were male and the median age was 82 years (range 67–93). Nine patients fulfilled criteria for severe sepsis, and 2 patients died at hospital. Two patients were diagnosed with infective endocarditis. Most patients had underlying urinary tract diseases or an indwelling urinary tract catheter. Five patients suffered from dementia. None of the patients was treated with immunosuppressive medications. The MIC values of the isolates were in line with previous reports, with low MICs for penicillin, cefotaxime, and vancomycin. All 11 isolates produced biofilms but not all could induce platelet aggregation.
Conclusions. A sanguinicola can cause severe infections in elderly men with urinary tract abnormalities and the bacteria possess potential virulence mechanisms.
Aerococcus sanguinicola is a cause of urinary tract infections, blood stream infections, and infective endocarditis (IE) [1–3]. Since it was designated as a species in 2001 [4], only a few cases of A sanguinicola bacteremia have been reported [4, 5] and only 1 case series, describing 6 patients, addresses the clinical presentation of such infection and has been published [2]. More cases of invasive infections with Aerococcus urinae have been previously described [6, 7]. From urinary samples, A sanguinicola and A urinae are isolated at similar frequencies [1, 2, 8]. Aerococci share features with other Gram-positive bacteria; they appear in clusters or tetrads as staphylococci, but they have similar colony morphology as α-hemolytic streptococci and are catalase negative. Aerococcal species are not easily distinguishable from each other when using conventional methods based on biochemistry [9]. Importantly, A sanguinicola is consistently being identified as Aerococcus viridans by several commercially available systems such as Vitek 2, API strep, and ID 32 [1, 8]. The misidentification of A sanguinicola in clinical microbiology laboratories has likely led to an underestimation of the incidence and the clinical importance of this species. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) has recently been showed to be a fast and reliable method for identification and correct species determination of aerococci including A sanguinicola [8, 10]. Aerococcus sanguinicola is sensitive to most antibiotics likely to be used for empirical treatment of septicaemia such as β-lactams and vancomycin [5]. However, A sanguinicola has often reduced susceptibility to fluoroquinolones [1, 3, 5]. High-level fluoroquinolone resistance has also been described [11]. Aerococcus urinae has been shown to form biofilm on plastic surfaces in vitro and to activate human platelets [12]. It is not known whether A sanguinicola also possess these potential virulence traits. The aim with this study was to investigate the incidence of A sanguinicola bacteremia in the south of Sweden and to describe the clinical presentation of such infections. Moreover, the antibiotic susceptibility and some virulence properties of the isolates were determined.
MATERIAL AND METHODS
Bacterial Isolates
Isolates were identified by searching the databases of the 2 clinical microbiology laboratories belonging to University and Regional Laboratories of Skåne, Sweden. These laboratories are located in Malmö and Lund and serve all hospitals in a region with ∼1.2 million inhabitants. Searches were performed among all isolates from blood cultures drawn between March 2006 and November 2012. Both laboratories used the BacT/Alert blood culture system (bioMérieux, Marcy l’Etoile, France), and Gram stains were used to provide preliminary identification. Growth of catalase-negative bacteria, with colony appearance resembling α-hemolytic streptococci, in more than 1 bottle resulted routinely in species identification by sequencing of the 16S rRNA gene in Lund (2006–2011) or by Vitek2 (bioMérieux) in Malmö (2006–2011). Matrix-assisted laser desorption ionization time-of-flight mass spectrometry was introduced in both laboratories in 2011 and was the primary method of species identification during 2011–2012. Bacterial isolates were stored at −80oC. All isolates of A sanguinicola were subjected to sequencing of the 16S rRNA gene [13] to confirm species identity. Reclassification of older isolates from the same time period (2006–2012) was carried out with MALDI-TOF MS, as described in Senneby et al [8]. Reclassification was performed if growth was identified in more than 1 bottle and the isolates had been identified as A viridans by Vitek 2, or isolates that had been identified as α-hemolytic streptococci or Gram-positive coccus, although preliminary Gram stain had been interpreted as Gram-positive cocci in clusters. The local research ethical committee approved this study (registration number 2010/681).
Patient Information
The medical chart of each patient was reviewed to extract clinical presentation, underlying conditions, treatment, and outcome. Systemic inflammatory response syndrome (SIRS) was determined as described previously [14], and organ dysfunction caused by the infection was classified according to the Swedish Society of Infectious Diseases' guidelines as described by Senneby et al [7].
Antimicrobial Testing
The minimum inhibitory concentration (MIC) for penicillin, cefotaxime, vancomycin, clindamycin, gentamicin, and ciprofloxacin was determined by the use of Etests (bioMérieux, Marcy l'Etoile, France) according to the manufacturers’ instructions. Muller Hinton agar, supplemented with 5% horse blood, was used and MICs were determined after incubation for 24 hours at 35°C in 5% CO2.
Quantification of Biofilm Formation
Isolates were cultivated overnight in tryptic soy broth (Difco) with 0.5% glucose at 37°C with 5% CO2, and biofilm was determined using crystal violet as described by Holmberg [15] with modifications as described previously [16]. The experiment was repeated 3 times. Medium alone was used as negative control. The negative control absorbance values were subtracted from the isolates' absorbance values in each experiment. A strain of Enterococcus faecalis, known to be a potent biofilm producer, was used for positive control. Heparinized plasma was obtained by centrifugation of blood from a healthy donor at 1500 g for 10 minutes.
Platelet Aggregation
Platelet-rich plasma (PRP) and platelet-poor plasma were prepared from 3 healthy donors as described by Rasmussen et al [17]. Bacterial concentration was set as described [12], and platelet aggregation was determined by aggregometry as described by Rasmussen et al [17]. Soluble collagen was used as positive control.
RESULTS
A sanguinicola Blood Isolates
From the laboratory in Lund, 7 isolates of A sanguinicola were identified. Three isolates had already been identified through sequencing of the 16S rRNA gene, 2 isolates were originally identified as α-hemolytic streptococci, and 2 isolates had been identified with MALDI-TOF MS. Four isolates were retrieved in the Malmö laboratory. One isolate had been misidentified as A urinae by Vitek2, and 3 isolates were correctly identified by MALDI-TOF MS. Altogether, 11 A sanguinicola isolates were identified and confirmed through sequencing of the 16S rRNA gene. In 10 cases, A sanguinicola was found in more than 1 culture (only 1 blood culture was taken in 1 case). In 5 patients, A sanguinicola was the only organism isolated whereas 6 patients had additional pathogens isolated from their blood (Table 1).
Characteristics of Patients With Aerococcus sanguinicola Bacteremia in Skåne, Sweden From 2006–2012*
| Case . | Age (year)/ Gender . | Underlying Conditions . | Initial Symptoms . | SIRS Criteria . | Organ Dysfunction . | Antibiotic Treatment, iv . | Antibiotic Treatment, po . | Other Blood Culture Findings . | Diagnosis . | In-hospital Fatality and Other Remarks . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 75/M | UC, CVI, IHD, DM2, | Fe, back pain | 4/4 | Ren | Cf, Pt, PcG | 0 | 0 | Sepsis | Recovered |
| 2 | 93/M | BPH, UC, CHF, DM2, | Hematuria, dyspnoea | 2/3 | Resp, HT, Ren | Ct | 0 | 0 | Sepsis | Died after 24 h |
| 3 | 85/M | PC, dementia | Fe, vomiting, dyspnoea | 3/4 | Con, HT, HP, Ren, Resp | Ct, Ct + Va, PcG | 0 | 0 | IE | Recovered ICU care |
| 4 | 81/M | PC, DM2, IHD | Fe, vomiting, Ru, hematuria | 2/4 | Ren, HP | Ct, PcG + Gm | PcV | 0 | Sepsis | Recovered |
| 5 | 67/M | BC, UC, lung cancer, DM2, alcohol abuse, dementia | Fe, fatigue | 4/4 | HP, Ren | Ct | Am | 0 | Sepsis | Died after 15 days. Only 1 blood culture taken |
| 6 | 68/M | Trisomy 21 | Melena, fatigue | 0/4 | 0 | Ct + Me, PcG + Gm | Cm, Pc | CoNS | IE | Recovered |
| 7 | 89/M | UC, Alzheimer’s disease, CVI, IHD | Fe, Melena, hematemesis, hematuria | 4/4 | Ren, HP | Ct + Me | Ni + Am | Escherichia coli | UTI, melena | Recovered |
| 8 | 79/M | PC, UC, dementia, CHF, CVI | Fe, stop in UC | 4/4 | HT, Con, HP, Resp, Ren, Coa, Hep | Ct | 0 | Proteus mirabilis | Sepsis, pneumonia | Recovered |
| 9 | 82/M | UC, UC clot, pressure ulcers | Fe, hematuria | 2/3 | Ren, HP | Ct, Pt | Ci, Met | Staphylococcus aureus, Pseudomonas aeruginosa | Sepsis, UTI | Recovered |
| 10 | 84/M | UC, Alzheimer’s disease | Fe | 3/4 | 0 | PcG + Gm, Amp, Pt | Ci + Am | P mirabilis, Klebsiella pneumoniae, Enterococcus faecalis, Streptococcus sp | Sepsis | Recovered |
| 11 | 90/M | UC, BPH, CVI, kidney failure, | Fe, Hematuria, right side abdominal pain | 3/4 | HT | Ct + Gm, Pt | Ci + Met | Actinobaculum schaalii, Gram-positive coccus, Corynebacterium sp | Cholecystitis | Recovered |
| Case . | Age (year)/ Gender . | Underlying Conditions . | Initial Symptoms . | SIRS Criteria . | Organ Dysfunction . | Antibiotic Treatment, iv . | Antibiotic Treatment, po . | Other Blood Culture Findings . | Diagnosis . | In-hospital Fatality and Other Remarks . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 75/M | UC, CVI, IHD, DM2, | Fe, back pain | 4/4 | Ren | Cf, Pt, PcG | 0 | 0 | Sepsis | Recovered |
| 2 | 93/M | BPH, UC, CHF, DM2, | Hematuria, dyspnoea | 2/3 | Resp, HT, Ren | Ct | 0 | 0 | Sepsis | Died after 24 h |
| 3 | 85/M | PC, dementia | Fe, vomiting, dyspnoea | 3/4 | Con, HT, HP, Ren, Resp | Ct, Ct + Va, PcG | 0 | 0 | IE | Recovered ICU care |
| 4 | 81/M | PC, DM2, IHD | Fe, vomiting, Ru, hematuria | 2/4 | Ren, HP | Ct, PcG + Gm | PcV | 0 | Sepsis | Recovered |
| 5 | 67/M | BC, UC, lung cancer, DM2, alcohol abuse, dementia | Fe, fatigue | 4/4 | HP, Ren | Ct | Am | 0 | Sepsis | Died after 15 days. Only 1 blood culture taken |
| 6 | 68/M | Trisomy 21 | Melena, fatigue | 0/4 | 0 | Ct + Me, PcG + Gm | Cm, Pc | CoNS | IE | Recovered |
| 7 | 89/M | UC, Alzheimer’s disease, CVI, IHD | Fe, Melena, hematemesis, hematuria | 4/4 | Ren, HP | Ct + Me | Ni + Am | Escherichia coli | UTI, melena | Recovered |
| 8 | 79/M | PC, UC, dementia, CHF, CVI | Fe, stop in UC | 4/4 | HT, Con, HP, Resp, Ren, Coa, Hep | Ct | 0 | Proteus mirabilis | Sepsis, pneumonia | Recovered |
| 9 | 82/M | UC, UC clot, pressure ulcers | Fe, hematuria | 2/3 | Ren, HP | Ct, Pt | Ci, Met | Staphylococcus aureus, Pseudomonas aeruginosa | Sepsis, UTI | Recovered |
| 10 | 84/M | UC, Alzheimer’s disease | Fe | 3/4 | 0 | PcG + Gm, Amp, Pt | Ci + Am | P mirabilis, Klebsiella pneumoniae, Enterococcus faecalis, Streptococcus sp | Sepsis | Recovered |
| 11 | 90/M | UC, BPH, CVI, kidney failure, | Fe, Hematuria, right side abdominal pain | 3/4 | HT | Ct + Gm, Pt | Ci + Met | Actinobaculum schaalii, Gram-positive coccus, Corynebacterium sp | Cholecystitis | Recovered |
Abbreviations: Am, amoxicillin; Amp, ampicillin; BC, bladder cancer; BPH, benign prostate hyperplasia; Cf, cefuroxime; Ct, cefotaxime; CHF, congestive heart failure; Ci, ciprofloxacin; Cm, clindamycin; Coa, coagulation dysfunction; Con, confusion; CoNS, coagulase negative staphylococcus; Ct, cefotaxime; CVI, cerebrovascular insult with sequelae; DM2, diabetes mellitus type 2; Fe, fever; Gm, gentamicin; Hep, hepatic dysfunction; HP, hypoperfusion; HT, hypotension; ICU, intensive care unit; IE, infectious endocarditis; IHD, ischaemic heart disease; M, male; Me, meropenem; Met, metronidazole; Ni, nitrofurantoine; PC, prostate cancer; PcG, penicillin G; PcV, penicillin V; Pt, piperacillin-tazobactam; Ren, renal dysfunction; Resp, respiratory dysfunction; Ru, residual urine; UC, urinary catheter; UTI, urinary tract infection; Va, vancomycin.
* The SIRS criteria are expressed as number of criteria met through the total number of criteria given in the medical records. The commas indicate changes of antibiotic treatment, and a plus indicates a combined treatment.
Characteristics of Patients With Aerococcus sanguinicola Bacteremia in Skåne, Sweden From 2006–2012*
| Case . | Age (year)/ Gender . | Underlying Conditions . | Initial Symptoms . | SIRS Criteria . | Organ Dysfunction . | Antibiotic Treatment, iv . | Antibiotic Treatment, po . | Other Blood Culture Findings . | Diagnosis . | In-hospital Fatality and Other Remarks . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 75/M | UC, CVI, IHD, DM2, | Fe, back pain | 4/4 | Ren | Cf, Pt, PcG | 0 | 0 | Sepsis | Recovered |
| 2 | 93/M | BPH, UC, CHF, DM2, | Hematuria, dyspnoea | 2/3 | Resp, HT, Ren | Ct | 0 | 0 | Sepsis | Died after 24 h |
| 3 | 85/M | PC, dementia | Fe, vomiting, dyspnoea | 3/4 | Con, HT, HP, Ren, Resp | Ct, Ct + Va, PcG | 0 | 0 | IE | Recovered ICU care |
| 4 | 81/M | PC, DM2, IHD | Fe, vomiting, Ru, hematuria | 2/4 | Ren, HP | Ct, PcG + Gm | PcV | 0 | Sepsis | Recovered |
| 5 | 67/M | BC, UC, lung cancer, DM2, alcohol abuse, dementia | Fe, fatigue | 4/4 | HP, Ren | Ct | Am | 0 | Sepsis | Died after 15 days. Only 1 blood culture taken |
| 6 | 68/M | Trisomy 21 | Melena, fatigue | 0/4 | 0 | Ct + Me, PcG + Gm | Cm, Pc | CoNS | IE | Recovered |
| 7 | 89/M | UC, Alzheimer’s disease, CVI, IHD | Fe, Melena, hematemesis, hematuria | 4/4 | Ren, HP | Ct + Me | Ni + Am | Escherichia coli | UTI, melena | Recovered |
| 8 | 79/M | PC, UC, dementia, CHF, CVI | Fe, stop in UC | 4/4 | HT, Con, HP, Resp, Ren, Coa, Hep | Ct | 0 | Proteus mirabilis | Sepsis, pneumonia | Recovered |
| 9 | 82/M | UC, UC clot, pressure ulcers | Fe, hematuria | 2/3 | Ren, HP | Ct, Pt | Ci, Met | Staphylococcus aureus, Pseudomonas aeruginosa | Sepsis, UTI | Recovered |
| 10 | 84/M | UC, Alzheimer’s disease | Fe | 3/4 | 0 | PcG + Gm, Amp, Pt | Ci + Am | P mirabilis, Klebsiella pneumoniae, Enterococcus faecalis, Streptococcus sp | Sepsis | Recovered |
| 11 | 90/M | UC, BPH, CVI, kidney failure, | Fe, Hematuria, right side abdominal pain | 3/4 | HT | Ct + Gm, Pt | Ci + Met | Actinobaculum schaalii, Gram-positive coccus, Corynebacterium sp | Cholecystitis | Recovered |
| Case . | Age (year)/ Gender . | Underlying Conditions . | Initial Symptoms . | SIRS Criteria . | Organ Dysfunction . | Antibiotic Treatment, iv . | Antibiotic Treatment, po . | Other Blood Culture Findings . | Diagnosis . | In-hospital Fatality and Other Remarks . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 75/M | UC, CVI, IHD, DM2, | Fe, back pain | 4/4 | Ren | Cf, Pt, PcG | 0 | 0 | Sepsis | Recovered |
| 2 | 93/M | BPH, UC, CHF, DM2, | Hematuria, dyspnoea | 2/3 | Resp, HT, Ren | Ct | 0 | 0 | Sepsis | Died after 24 h |
| 3 | 85/M | PC, dementia | Fe, vomiting, dyspnoea | 3/4 | Con, HT, HP, Ren, Resp | Ct, Ct + Va, PcG | 0 | 0 | IE | Recovered ICU care |
| 4 | 81/M | PC, DM2, IHD | Fe, vomiting, Ru, hematuria | 2/4 | Ren, HP | Ct, PcG + Gm | PcV | 0 | Sepsis | Recovered |
| 5 | 67/M | BC, UC, lung cancer, DM2, alcohol abuse, dementia | Fe, fatigue | 4/4 | HP, Ren | Ct | Am | 0 | Sepsis | Died after 15 days. Only 1 blood culture taken |
| 6 | 68/M | Trisomy 21 | Melena, fatigue | 0/4 | 0 | Ct + Me, PcG + Gm | Cm, Pc | CoNS | IE | Recovered |
| 7 | 89/M | UC, Alzheimer’s disease, CVI, IHD | Fe, Melena, hematemesis, hematuria | 4/4 | Ren, HP | Ct + Me | Ni + Am | Escherichia coli | UTI, melena | Recovered |
| 8 | 79/M | PC, UC, dementia, CHF, CVI | Fe, stop in UC | 4/4 | HT, Con, HP, Resp, Ren, Coa, Hep | Ct | 0 | Proteus mirabilis | Sepsis, pneumonia | Recovered |
| 9 | 82/M | UC, UC clot, pressure ulcers | Fe, hematuria | 2/3 | Ren, HP | Ct, Pt | Ci, Met | Staphylococcus aureus, Pseudomonas aeruginosa | Sepsis, UTI | Recovered |
| 10 | 84/M | UC, Alzheimer’s disease | Fe | 3/4 | 0 | PcG + Gm, Amp, Pt | Ci + Am | P mirabilis, Klebsiella pneumoniae, Enterococcus faecalis, Streptococcus sp | Sepsis | Recovered |
| 11 | 90/M | UC, BPH, CVI, kidney failure, | Fe, Hematuria, right side abdominal pain | 3/4 | HT | Ct + Gm, Pt | Ci + Met | Actinobaculum schaalii, Gram-positive coccus, Corynebacterium sp | Cholecystitis | Recovered |
Abbreviations: Am, amoxicillin; Amp, ampicillin; BC, bladder cancer; BPH, benign prostate hyperplasia; Cf, cefuroxime; Ct, cefotaxime; CHF, congestive heart failure; Ci, ciprofloxacin; Cm, clindamycin; Coa, coagulation dysfunction; Con, confusion; CoNS, coagulase negative staphylococcus; Ct, cefotaxime; CVI, cerebrovascular insult with sequelae; DM2, diabetes mellitus type 2; Fe, fever; Gm, gentamicin; Hep, hepatic dysfunction; HP, hypoperfusion; HT, hypotension; ICU, intensive care unit; IE, infectious endocarditis; IHD, ischaemic heart disease; M, male; Me, meropenem; Met, metronidazole; Ni, nitrofurantoine; PC, prostate cancer; PcG, penicillin G; PcV, penicillin V; Pt, piperacillin-tazobactam; Ren, renal dysfunction; Resp, respiratory dysfunction; Ru, residual urine; UC, urinary catheter; UTI, urinary tract infection; Va, vancomycin.
* The SIRS criteria are expressed as number of criteria met through the total number of criteria given in the medical records. The commas indicate changes of antibiotic treatment, and a plus indicates a combined treatment.
A sanguinicola in Urinary Cultures
None of the 11 patients had recorded growth of A sanguinicola in a urinary culture. In 5 patients, no urinary culture was performed and 3 patients had sterile urine. In 2 patients, the urine grew more than 2 species that were not further characterized, and in 1 patient Pseudomonas aeruginosa was identified in the urine culture.
Patient Characteristics
The clinical presentation is summarized in Table 1. All patients were male and the median age was 82 years (range, 67–93). Six patients had underlying urinary tract diseases; prostatic malignancy (n = 3), bladder malignancy (n = 1), or benign prostatic hyperplasia (n = 2). Eight of the 11 patients had an indwelling urinary tract catheter. Five patients suffered from Alzheimer's disease or other types of dementia, and another 2 patients had memory impairment. One patient had Trisomy 21. None of the patients was treated with immunosuppressive medications. At presentation in the hospital, 9 patients were febrile. Hematuria was an initial symptom in 5 patients, 1 patient had complains of back pain, and 1 patient had obstruction of his urinary catheter. Ten patients fulfilled the criteria for SIRS and 9 of them had signs of organ dysfunction, most commonly renal failure (n = 8). Thus, 10 patients fulfilled criteria for sepsis and 9 fulfilled criteria for severe sepsis. One patient had septic shock. Nine patients recovered from their infections and 2 patients died at hospital (after 24 h and 15 days of care, respectively). The nonsurvivors were both terminally ill with cancer. The median duration of the hospital stay was 12 days (range, 6–61) for the surviving patients.
IE With A sanguinicola
Two of the 11 patients were diagnosed with IE according to Duke's criteria for IE [18]. In 1 case, a 68-year-old man with Trisomy 21 presented with fatigue and fever. The patient had no history of cardiac anomalies. A heart murmur was noted for the first time upon clinical examination, and a transesophageal echocardiography (TEE) visualized a suspected vegetation on the mitral valve. After initial empiric treatment with a broad-spectrum cephalosporin, the patient received penicillin for 1 month in combination with gentamicin the first week and recovered without complications. One year later, the patient was treated for suspected recurrent IE due to fever and elevated levels of C-reactive protein. However, in the second episode no bacteria were isolated from the blood. The other case was an 85-year-old man with prostatic cancer, but with no medical history of cardiac disease, who was admitted to the intensive care unit due to fever, respiratory failure, and septic shock. Twice, a TEE was performed without conclusive evidence of IE. However, the aortic valve was too sclerotic to safely disregard the possibility of IE. This patient received penicillin for 1 month in combination with gentamicin the first week and recovered from the infection.
Antibiotic Susceptibility and Antibiotic Treatment
The MICs for tested antibiotics are presented in Table 2. The results are in line with previous reports [1, 3, 5] with the bacteria displaying low MICs for penicillin, cefotaxime, and vancomycin. We observed high MICs for ciprofloxacin (range, 4–>32 mg/L). The cefotaxime MICs for the isolates from the 2 patients who died at hospital were 0.125 mg/L and 2 mg/L. All 11 patients received empirical treatment with broad-spectrum β-lactam antibiotic. In 5 cases, this treatment was shifted to intravenous penicillin. Four patients were given gentamycin in combination with penicillin or cefotaxime. As follow-up oral treatment, penicillin or ampicillin were most commonly used, followed by ciprofloxacin. The median treatment length with intravenous antibiotics was 9 days (range, 2–36 days) and the median total treatment length was 14 days (range, 2–36 days).
Minimum Inhibitory Concentration (MIC) in Milligrams per Liter Determined for 11 Isolates of Aerococcus sanguinicola
| . | MIC50 . | MIC90 . | Range . |
|---|---|---|---|
| Penicillin | 0.032 | 0.125 | 0.016–0.125 |
| Cefotaxime | 0.125 | 2 | 0.125–2 |
| Vancomycin | 0.5 | 0.5 | 0.5–1 |
| Clindamycin | 0.25 | 1 | 0.064–2 |
| Gentamicin | 8 | 16 | 8–16 |
| Ciprofloxacin | >32 | >32 | 4–>32 |
| . | MIC50 . | MIC90 . | Range . |
|---|---|---|---|
| Penicillin | 0.032 | 0.125 | 0.016–0.125 |
| Cefotaxime | 0.125 | 2 | 0.125–2 |
| Vancomycin | 0.5 | 0.5 | 0.5–1 |
| Clindamycin | 0.25 | 1 | 0.064–2 |
| Gentamicin | 8 | 16 | 8–16 |
| Ciprofloxacin | >32 | >32 | 4–>32 |
Minimum Inhibitory Concentration (MIC) in Milligrams per Liter Determined for 11 Isolates of Aerococcus sanguinicola
| . | MIC50 . | MIC90 . | Range . |
|---|---|---|---|
| Penicillin | 0.032 | 0.125 | 0.016–0.125 |
| Cefotaxime | 0.125 | 2 | 0.125–2 |
| Vancomycin | 0.5 | 0.5 | 0.5–1 |
| Clindamycin | 0.25 | 1 | 0.064–2 |
| Gentamicin | 8 | 16 | 8–16 |
| Ciprofloxacin | >32 | >32 | 4–>32 |
| . | MIC50 . | MIC90 . | Range . |
|---|---|---|---|
| Penicillin | 0.032 | 0.125 | 0.016–0.125 |
| Cefotaxime | 0.125 | 2 | 0.125–2 |
| Vancomycin | 0.5 | 0.5 | 0.5–1 |
| Clindamycin | 0.25 | 1 | 0.064–2 |
| Gentamicin | 8 | 16 | 8–16 |
| Ciprofloxacin | >32 | >32 | 4–>32 |
Biofilm Formation
All 11 clinical isolates produced biofilms that were firmly attached to the plastic surface and visible to the eye after 24, 48, and 72 hours. There was a variation in the amount of absorbed crystal violet between the isolates, indicating a difference in the capacity to form biofilm (Figure 1). For 4 isolates, there was a statistically significant increase in the amount of biofilm formed in the presence of plasma compared to the amount of biofilm formed in medium alone (Mann-Whitney U test; P < .05) at 48 hours. At 72 hours, the same comparison resulted in a significant difference for 7 of the 11 isolates (Figure 1).
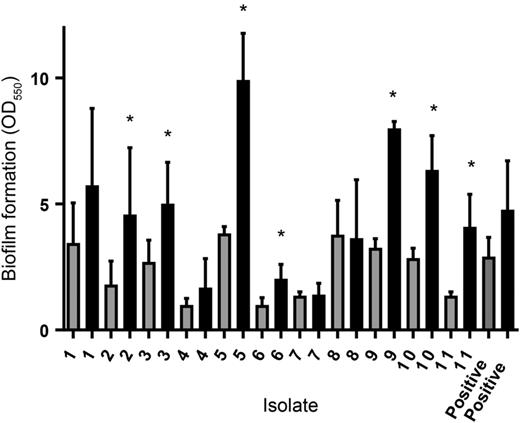
Biofilm formation by Aerococcus sanguinicola after 72 hours of incubation in the presence of medium (gray bars) or medium containing 10% human plasma (black bars). The error bars represent the standard deviation from 3 different experiments. The asterisks indicate a statistically significant increase in the amount of biofilm formed in the presence of plasma compared to the amount of biofilm formed in medium alone. The mean negative control absorbance value was 0.083 (range, 0.055–0.31).
Platelet Aggregation
The 11 isolates were tested for their capacity to aggregate human platelets from 3 donors. Three isolates failed to induce platelet aggregation in any donor, 2 isolates induced aggregation of platelets from 1 donor, 5 isolates induced aggregation in 2 donors, and 1 isolate induced aggregation of platelets from all 3 donors. The median time to aggregation for all isolates was 12 minutes (range, 8–24 min). The isolate that induced aggregation from all 3 donors was from one of the patients with IE. The other patients with IE had an isolate that induced aggregation after 8 minutes in 2 donors.
DISCUSSION
Only a few clinical cases of bacteremia with A sanguinicola have been previously presented [2], and in this study we report an additional 11 cases. The scarcity of reports could be explained by the fact that the bacterium was quite recently designated its own species and that it is easily misidentified as A viridans. Because MALDI-TOF MS is a reliable method of identifying A sanguinicola and A urinae [8, 10], the introduction of this method in clinical microbiology laboratories will lead to a more accurate identification of aerococcal species and also a better determination of the incidence of these infections. Our study was carried out retrospectively and may have underestimated the actual number of cases with blood stream infections caused by A sanguinicola. Based on our data, the estimated incidence is 1.4 cases per 1 000 000 inhabitants per year. In our material, all patients were elderly men and a majority of them had underlying urological conditions or an indwelling urinary catheter. Neurological disorders were common, 5 patients had some form of dementia, and 1 patient had Trisomy 21. Similar findings were presented by Ibler et al [2]. Four of their 6 patients were of male gender, and all of them had neurological conditions, including 1 patient with Trisomy 21. It is interesting to note that both of the patients with this genetic disorder were diagnosed with IE. Aerococcus urinae, which has been more extensively studied, also infects elderly men with urinary tract abnormalities, but patients with A urinae bacteremia seem to have less neurological disease [7]. Most patients in our study presented fever as a primary symptom of infection, and several had complaints or symptoms from the urinary tract. Aerococcus sanguinicola is most commonly encountered in urinary cultures, and most studies suggest that it has a pathogenic capacity in the urinary tract [1, 3]. Taken together, this result implies that the primary focus of blood stream infections with A sanguinicola is most likely the urinary tract. However, we could not find support for this speculation in our material because no aerococci were isolated from urinary cultures. This result could partly be explained by shortcomings in the clinical management of these patients upon arrival at hospital and possibly also failure to recognize the bacteria in urinary cultures. Ten patients fulfilled the criteria for SIRS and 9 of them had organ dysfunction, which points out the severity of bacteremia with A sanguinicola. Two patients did not survive the hospital stay; however, both of these patients were terminally ill with cancer. In both nonsurvivors, A sanguinicola was isolated in pure cultures from blood. In our hospitals, the fatality of A sanguinicola (2 of 11) is similar to that of A urinae (1 of 16) [7], although the small number of cases does not permit any definite conclusions. The pathogenic role of A sanguinicola in the 6 patients with polymicrobial bacteremia is less clear, and some of these patients had bacteremia with bacteria of relatively high pathogenic potential such as Escherichia coli and Staphylococcus aureus. Notably, none of the patients with polymicrobial bacteremia succumbed, and consequently the case fatality in bacteremia with A sanguinicola in pure culture was 2 of 5 in this material. Two of the 11 patients were diagnosed with IE, and in an additional 3 patients a TTE was performed, making the possibility of IE in those patients less likely. Thus, in 6 patients, the possibility of IE was not investigated by diagnostic echocardiography and potential IEs could have been missed. Ibler et al [2] reported that 2 of 6 patients had IE, and we have previously reported that 3 of 16 patients with A urinae bacteremia had IE [7]. We find it reasonable to asses all cases of aerococcal bacteremia with diagnostic echocardiography.
In our experiment, the 11 clinical isolates of A sanguinicola were all biofilm producers, which probably could facilitate the colonization of indwelling urinary catheters. For most isolates, the biofilm after 72 hours was more pronounced in the presence of human plasma, which indicates that the bacteria could also form biofilm in a situation where plasma exudates due to local inflammation. For S aureus [19, 20] and Propionibacterium acnes [15], serum or plasma inhibit biofilm formation, but in A urinae, biofilm formation is strongly stimulated by human plasma [12]. The mechanisms behind plasma effects on bacterial biofilm formation remains to be explored, but the fact that aerococci can form biofilm in the presence of plasma supports the possibility that biofilm formation could have a role in IE caused by aerococci. It is interesting to note that the isolates from the 2 patients with IE induced aggregation in PRP from 2 or 3 donors, respectively. These patients had predisposing factors for IE, but it could support the theory that induction of platelet aggregation is to be considered as a virulence property of aerococci [21]. In conclusion, our study shows that A sanguinicola can cause severe infections in elderly men with urinary tract abnormalities and neurological diseases and that the bacterium has virulence mechanisms of potential importance in IE.
Notes
Acknowledgments. We acknowledge the staff at the Clinical Microbiology Laboratories in Lund and Malmö who collected the isolates and performed sequencing of the 16S rRNA gene.
Financial support. This work was financed by the Swedish Government Funds for Clinical Research (ALF), the Royal Physiographic Society in Lund, the Scandinavian Society for Antimicrobial Chemotherapy, and the foundations of Österlund and Groschinsky.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.





Comments