-
PDF
- Split View
-
Views
-
Cite
Cite
Justin T Jordan, Julie J Miller, Tucker Cushing, Marlon Seijo, Tracy T Batchelor, Isabel C Arrillaga-Romany, Helen A Shih, Lisa B Nachtigall, Jay S Loeffler, Jorg Dietrich, Temozolomide therapy for aggressive functioning pituitary adenomas refractory to surgery and radiation: a case series, Neuro-Oncology Practice, Volume 5, Issue 1, March 2018, Pages 64–68, https://doi.org/10.1093/nop/npx013
Close - Share Icon Share
Abstract
Treatment of aggressive pituitary adenomas typically involves a multimodality approach based on histopathological features and may include pharmacotherapy, surgery, and occasionally radiation therapy. In cases of treatment-refractory tumor progression, chemotherapy may be considered; however, no standard chemotherapeutic regimen has been established. Literature review suggests that temozolomide may have a beneficial role in a subset of cases. To understand the efficacy of temozolomide in progressive pituitary tumors, we reviewed the outcomes of cases at our center.
We performed a retrospective chart review to report the outcome and unique features of 7 patients with aggressive functioning pituitary adenomas or carcinomas treated with temozolomide. Tumor pathology included somatotroph (n = 1), corticotroph (n = 3), and lactotroph (n = 3) tumors.
Four of the 7 patients had at least 2 prior resections, and all had prior radiation and surgery before treatment with temozolomide. Notably, all patients showed response to therapy, defined as either stable disease (43%) or partial response (57%). Median progression-free survival was 1.66 years, and median overall survival was 4 years.
Our data suggest that temozolomide has an important role in the management of aggressive functioning pituitary tumors that are resistant to standard therapies, and that optimization of therapy with temozolomide may involve individualized regimens. Future prospective clinical trials should be considered.
Pituitary tumors are the third-most-common class of primary intracranial tumors, the vast majority of which are nonmalignant adenomas.1 These tumors are thought to arise from adenohypophyseal cells, and as such may be hormone-secreting. Generally slow growing, pituitary adenomas may be incidentally identified by neuroimaging or in postmortem examination, with an estimated prevalence of 15% to 20% in the general population.2 Patients with symptomatic tumors present in either of two common scenarios: microadenomas (< 1 cm) typically present with symptoms of excessive hormone secretion, whereas macroadenomas (> 1 cm) do not usually secrete hormone and characteristically present with symptoms secondary to local mass effect in the area of the sella turcica. Such symptoms include headaches, vision impairment, pituitary stalk compression, or invasion into neighboring regions, such as the cavernous sinus.3,4 Finally, pituitary carcinoma is a rare and aggressive diagnosis, defined not by histologic change but by evidence of distant metastases from a pituitary adenoma.5
For symptomatic or hormone-producing tumors, which account for the majority of pituitary adenomas, initial treatment considerations include surgery and/or medical therapy aimed at suppressing hormonal secretion. While practices do vary, safely accessible tumors are commonly resected at presentation. Medical therapy to treat residual hormone dysfunction may be considered following a subtotal resection. Some medical therapies, such as dopamine agonists and somatostatin analogs, not only reduce hormone excess, but also may suppress tumor growth.6–8
Radiation therapy is typically used for pituitary tumors that are inoperable, or which persist or progress after surgery. Its use requires consideration of the risks of treatment effect to surrounding normal tissues, including normal pituitary gland and optic pathway structures. Radiation, either in fractionated course or delivered in a single treatment as stereotactic radiosurgery, has excellent efficacy toward tumor size control, but moderate and delayed efficacy in controlling hormonal markers. Combined case series suggest 80% to 100% local control rates regardless of tumor type or radiation technique used.9 Median duration of control is less frequently reported in these series, and less consistent, ranging from 22 months to 10 years. Hormone control is variable across studies, with an average reported rate of approximately 50% hormone normalization.9
Use of adjuvant chemotherapy has traditionally been reserved for patients with tumors refractory to prior multimodality therapy with surgery, radiation, and conventional hormone-suppressing medical therapy, however no specific chemotherapy regimen has been established. Temozolomide is a methylating chemotherapeutic agent that acts by delivering methyl groups to guanine bases along the DNA backbone, impairing cell replication and leading to cell death. Temozolomide has the ability to cross the blood-brain barrier, and is commonly used to treat intracranial tumors such as gliomas. Several authors have reported encouraging results with the use of temozolomide for individual patients with progressive pituitary adenomas,10–14 as well as with pituitary carcinomas.10,15–17 Larger case series and systematic reviews have further supported these data, suggesting a positive overall benefit of temozolomide treatment in this population.18–22 Published response rates are 48% to 65%,19,22 and progression-free survival (PFS) has been reported as 34 months, while overall survival (OS) could not be calculated due to insufficient number of events.22
O6-methylguanine-DNA methyltransferase (MGMT) is a DNA repair enzyme expressed throughout normal tissues that removes alkyl group adducts. Given the mechanism of action of temozolomide, normal MGMT activity may abrogate the antitumor efficacy of temozolomide.23 Some authors have evaluated the expression levels of MGMT within pituitary tumors and have identified a trend of increased MGMT promoter methylation with decreased MGMT expression, correlating with enhanced temozolomide antitumor activity.10,13,14,22,24,25 This issue, however, remains controversial.20,26
Here we review 7 cases of aggressive pituitary adenomas and carcinomas in which treatment with temozolomide was used in the setting of tumor progression after conventional treatments. These cases not only support previous research, but also add novel information to the available literature regarding the utility of dose and regimen alterations and neoadjuvant treatment in order to reduce radiation fields.
Materials and Methods
With institutional review board (IRB) approval, we queried our institutional clinical brain tumor database to identify all patients treated with temozolomide therapy for pituitary adenomas and carcinomas. Clinical information was recorded following review of electronic medical records. All patients had pathologically proven pituitary tumors with histopathologic determination of cell type. MGMT testing, where performed, was done with methylation-specific PCR testing. Further, all patients underwent pituitary imaging with magnetic resonance imaging with and without contrast at each interval scan, with images reviewed by neuroradiologists. Radiographic response was determined by an independent neuroradiologist using 2D measurements, and defined by either stabilization or reduction in the size of previously growing tumors.
Results
We identified 7 individuals who received or are actively receiving treatment with temozolomide for pituitary adenoma or pituitary carcinoma. The clinical characteristics of each patient are listed in Table 1. The median duration from pituitary tumor diagnosis to initiation of temozolomide therapy was 73 months, ranging from 14 to 276 months. Patients received treatment with temozolomide for an average of 21.1 months; 4 patients were treated intermittently based on overall response, tolerance, or alternative modalities of therapy, and no patients remain on active therapy. Each patient had at least 1 prior surgical procedure and at least 1 prior radiation treatment, and 6 of the 7 patients received at least 1 prior endocrine therapy before receiving temozolomide.
| Patient # . | Age at TMZ . | Diagnosis . | Secretion Status . | MGMT Status . | Total Months on TMZ . | Prior Surgeries . | Prior XRT . | Prior Endocrine Treatments . |
|---|---|---|---|---|---|---|---|---|
| 1 | 32 | Pituitary carcinoma | ACTH | Unknown | 13 | 3 | 3 | Octreotide, Cabergoline, Ketoconazole, Metyrapone, Aminoglutethimide |
| 2 | 28 | Pituitary carcinoma | Prolactin | Not Methylated | 32 | 2 | 2 | Bromocriptine, Cabergoline |
| 3 | 29 | Pituitary adenoma | GH | Not Methylated | 25 | 4 | 1 | Octreotide |
| 4 | 61 | Pituitary adenoma | Prolactin | Unknown | 9 | 1 | 1 | Cabergoline |
| 5 | 60 | Pituitary adenoma | Prolactin | Not Methylated | 29 | 2 | 1 | Bromocriptine, Cabergoline, Octreotide |
| 6 | 58 | Pituitary adenoma | ACTH | Unknown | 19 | 1 | 1 | None |
| 7 | 62 | Pituitary carcinoma | ACTH | Unknown | 21 | 1 | 2 | Ketoconazole, Cabergoline, Metyrapone, Bilateral Adrenalectomy |
| Patient # . | Age at TMZ . | Diagnosis . | Secretion Status . | MGMT Status . | Total Months on TMZ . | Prior Surgeries . | Prior XRT . | Prior Endocrine Treatments . |
|---|---|---|---|---|---|---|---|---|
| 1 | 32 | Pituitary carcinoma | ACTH | Unknown | 13 | 3 | 3 | Octreotide, Cabergoline, Ketoconazole, Metyrapone, Aminoglutethimide |
| 2 | 28 | Pituitary carcinoma | Prolactin | Not Methylated | 32 | 2 | 2 | Bromocriptine, Cabergoline |
| 3 | 29 | Pituitary adenoma | GH | Not Methylated | 25 | 4 | 1 | Octreotide |
| 4 | 61 | Pituitary adenoma | Prolactin | Unknown | 9 | 1 | 1 | Cabergoline |
| 5 | 60 | Pituitary adenoma | Prolactin | Not Methylated | 29 | 2 | 1 | Bromocriptine, Cabergoline, Octreotide |
| 6 | 58 | Pituitary adenoma | ACTH | Unknown | 19 | 1 | 1 | None |
| 7 | 62 | Pituitary carcinoma | ACTH | Unknown | 21 | 1 | 2 | Ketoconazole, Cabergoline, Metyrapone, Bilateral Adrenalectomy |
Abbreviations: ACTH, adrenocorticotropic hormone; GH, growth hormone; MGMT, O-6-methylguanine-DNA methyltransferase; TMZ, temozolomide; XRT, radiation treatment.
| Patient # . | Age at TMZ . | Diagnosis . | Secretion Status . | MGMT Status . | Total Months on TMZ . | Prior Surgeries . | Prior XRT . | Prior Endocrine Treatments . |
|---|---|---|---|---|---|---|---|---|
| 1 | 32 | Pituitary carcinoma | ACTH | Unknown | 13 | 3 | 3 | Octreotide, Cabergoline, Ketoconazole, Metyrapone, Aminoglutethimide |
| 2 | 28 | Pituitary carcinoma | Prolactin | Not Methylated | 32 | 2 | 2 | Bromocriptine, Cabergoline |
| 3 | 29 | Pituitary adenoma | GH | Not Methylated | 25 | 4 | 1 | Octreotide |
| 4 | 61 | Pituitary adenoma | Prolactin | Unknown | 9 | 1 | 1 | Cabergoline |
| 5 | 60 | Pituitary adenoma | Prolactin | Not Methylated | 29 | 2 | 1 | Bromocriptine, Cabergoline, Octreotide |
| 6 | 58 | Pituitary adenoma | ACTH | Unknown | 19 | 1 | 1 | None |
| 7 | 62 | Pituitary carcinoma | ACTH | Unknown | 21 | 1 | 2 | Ketoconazole, Cabergoline, Metyrapone, Bilateral Adrenalectomy |
| Patient # . | Age at TMZ . | Diagnosis . | Secretion Status . | MGMT Status . | Total Months on TMZ . | Prior Surgeries . | Prior XRT . | Prior Endocrine Treatments . |
|---|---|---|---|---|---|---|---|---|
| 1 | 32 | Pituitary carcinoma | ACTH | Unknown | 13 | 3 | 3 | Octreotide, Cabergoline, Ketoconazole, Metyrapone, Aminoglutethimide |
| 2 | 28 | Pituitary carcinoma | Prolactin | Not Methylated | 32 | 2 | 2 | Bromocriptine, Cabergoline |
| 3 | 29 | Pituitary adenoma | GH | Not Methylated | 25 | 4 | 1 | Octreotide |
| 4 | 61 | Pituitary adenoma | Prolactin | Unknown | 9 | 1 | 1 | Cabergoline |
| 5 | 60 | Pituitary adenoma | Prolactin | Not Methylated | 29 | 2 | 1 | Bromocriptine, Cabergoline, Octreotide |
| 6 | 58 | Pituitary adenoma | ACTH | Unknown | 19 | 1 | 1 | None |
| 7 | 62 | Pituitary carcinoma | ACTH | Unknown | 21 | 1 | 2 | Ketoconazole, Cabergoline, Metyrapone, Bilateral Adrenalectomy |
Abbreviations: ACTH, adrenocorticotropic hormone; GH, growth hormone; MGMT, O-6-methylguanine-DNA methyltransferase; TMZ, temozolomide; XRT, radiation treatment.
Clinical responses to therapy are depicted in Fig. 1. Each patient achieved at least stable disease radiographically, with tumor regression seen in 4 of the 7 patients (57%) following temozolomide initiation. Temozolomide treatment was associated with a decrease in abnormal hormone production in 3 patients (2 with ACTH-secreting and 1 with prolactin-secreting tumor). Among the patients assessed for MGMT promoter methylation status (3/7), none demonstrated promoter methylation, but all patients had radiographic response to temozolomide. Median PFS after temozolomide initiation was 1.6 years, and median OS was 4 years, though 3 individuals remain alive but off therapy (range, 2.8–4.5 years). All 7 patients survived at least 3 years from temozolomide initiation.
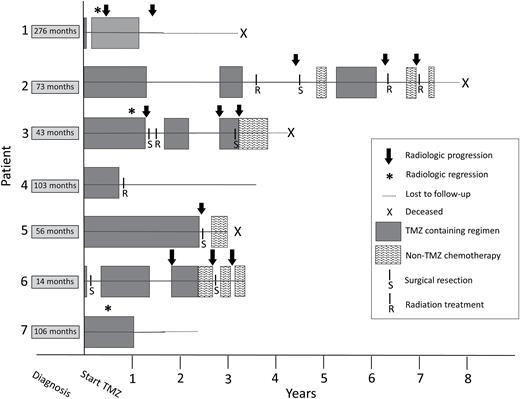
Graphical representation of clinical response experienced by patients treated with temozolomide (TMZ) for refractory pituitary tumors. Time is represented on x-axis, with time 0 denoting the point of first treatment with temozolomide. The time between diagnosis of the pituitary tumor and initiation of temozolomide is noted on the left. Four patients in the series are deceased (“X”), 3 are living. Radiologic progression is notated by a black arrow, while radiologic regression, as occurred in 3 patients during treatment, in notated by an asterisk.
Treatment dose and regimen were varied in these 7 patients. Most patients were treated at conventional dosing of 150 to 200 mg/m2 on 5 sequential days of a 28-day cycle. However, modifications were made based on individual responsiveness and 2 noteworthy patterns were identified among our patients. First, in patients 1 and 5, temozolomide dosing was increased at tumor progression, and demonstrated additional or renewed antitumor effect. In patient 1, temozolomide was increased slowly with progression from 100 mg/m2 daily (given on alternate weeks in a metronomic fashion), to 200 mg/m2 daily on alternate weeks. While radiographic progression and ACTH elevation during the slow dose increase were noted, by the time of the maximum dose, both MRI and ACTH levels stabilized. This response lasted for another 7 months, until the patient elected to discontinue therapy. For patient 5, the temozolomide dose was started at 75 mg/m2 daily (in a dose-dense fashion), was decreased after 9 months to reduce toxicity, and then returned to a 75 mg/m2 daily dose at tumor progression 6 months later. The MRI was stable by 4 months later.
Another unique pattern of temozolomide use was seen in patient 2, where clinicians saw repeated intracranial tumor stabilization upon serial challenges with temozolomide over the course of several years. Unfortunately, the patient’s systemic metastases progressed during treatment with temozolomide and the patient ultimately passed away as a result.
A final noteworthy point was observed in patient 4, for whom 9 cycles of temozolomide therapy resulted in such marked radiographic response that the patient’s tumor receded from the optic structures sufficiently to allow for safe delivery of radiotherapy, with reduced risk to visual pathway damage (Fig. 2).
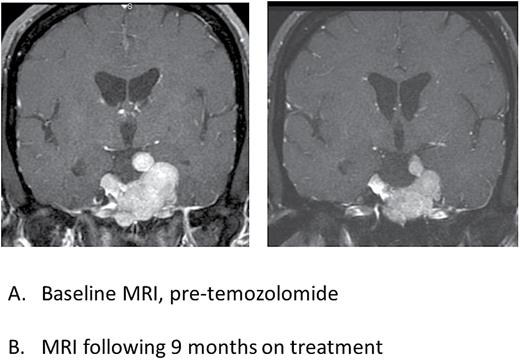
Postcontrast T1-weighted coronal MR images of patient 4 showing radiographic response to temozolomide treatment. Panel A (left) is the baseline MRI prior to start of temozolomide and Panel B (right) is an MRI performed following 9 months of treatment with temozolomide, showing regression of the tumor and reduced mass effect on the optic nerves and chiasm.
Hematologic data were reviewed for those individuals for whom such data were retrievable during the time of their therapy. No toxicities above grade 3 were seen. Thrombocytopenia was seen in 3 individuals (43%; two grade 1, one grade 2), leukopenia in 1 individual (14%; grade 2), neutropenia in 1 individual (14%; grade 1), and anemia in 2 individuals (29%; grade 1). There were no cases in which temozolomide treatment was terminated secondary to adverse side effects.
Discussion
Management of patients with aggressive pituitary tumors can be challenging and tumors may progress or recur despite pharmacological therapy, surgical resection, and adjuvant radiation. We report a series of patients treated at a single institution with adjuvant temozolomide at various stages in their disease. Our data suggest that temozolomide treatment of these refractory tumors was associated with an excellent rate of disease stabilization and even regression. Further, hormonal response was additionally seen in 3 of the 4 patients for whom this was followed.
We were unable to confirm prior reports that tumor response to therapy correlates with MGMT promoter methylation in the tumor. All tested patients in our cohort were negative for MGMT promoter methylation status, yet had radiographic tumor response to temozolomide. This confirms the inconsistency of the relationship between MGMT expression status and response to temozolomide in pituitary tumors, differing from what is known for malignant gliomas. However, a similar inconsistency has been recently reported in recurrent ependymoma patients treated with temozolomide.27
Given the paucity of prospective data in the use of temozolomide therapy for pituitary tumors, no standardized dosing regimen has been established. The most commonly prescribed regimen in our patients was adopted from glioblastoma treatment. However, in 2 patients temozolomide dosing was increased following tumor progression, with subsequent benefit as determined by radiographic stabilization. Thus, PFS estimates are shorter than duration of received therapies due to repeat exposures. Whereas stepwise dose increase is not a standard approach in other neuro-oncologic diseases such as glioblastoma, the data from these 2 patients may suggest that the extrapolated dose of temozolomide from glioblastoma literature may lead to borderline or insufficient concentrations for optimal antitumor effect.
Additional noteworthy observations in this series include the utility of repeat challenges of temozolomide for repeatedly progressive disease, and the neoadjuvant use of temozolomide to reduce tumor size and reduce the risk of radiation-associated injury to the optic structures. Both of these points challenge traditional use of temozolomide in neuro-oncology, and the latter may be worth evaluating in a clinical trial for large or invasive tumors.
While our survival data are encouraging for this group of patients with aggressive tumors, median PFS (1.66 years) was shorter than reported in the systematic review by Ji et al, indicating a median PFS of 2.8 years. Median OS could not be calculated for that study due to insufficient deaths. Whereas the present study summarizes a single institution case series, this can be challenging to compare directly to an aggregate of individual reports, which often highlight exceptional cases.
Our data are limited by the retrospective nature of the analysis. However, the use of temozolomide in this patient population deserves further attention given the overall high response rate to temozolomide, which generally has been well tolerated. These data not only align with several other authors’ findings, but also highlight potentially new avenues for the use of temozolomide in this population. Future prospective clinical trials, such as a prospective study comparing various dosing regimens for progressive tumors, should be considered to increase our understanding on how temozolomide could be incorporated into the management of patients with pituitary tumors.
Conflict of interest statement. The authors have no conflicts of interest or funding sources to report.
References
Author notes
These authors contributed equally to this work.




