-
PDF
- Split View
-
Views
-
Cite
Cite
Xiaoran Zhang, Alexandra Giantini Larsen, Natasha Kharas, Mark H Bilsky, William Christopher Newman, Separation surgery for metastatic spine tumors: How less became more, Neuro-Oncology Advances, Volume 6, Issue Supplement_3, October 2024, Pages iii94–iii100, https://doi.org/10.1093/noajnl/vdae017
Close - Share Icon Share
Abstract
Metastatic epidural spinal cord compression (MESCC) is an increasingly common clinical entity in cancer patients and is associated with significant morbidity and neurologic sequalae. Management of MESCC has undergone many significant paradigms shifts over the past 50 years and was at times managed exclusively with either surgery or radiation. Historically, aggressive surgical techniques to achieve en bloc or intralesional gross tumor resections were pursued but were associated with significant morbidity and poor tumor control rates when combined with conventional external beam radiation. However, improvements in radiation treatment delivery in the form of stereotactic body radiation therapy have allowed for the safe delivery of high-dose conformal photon beam radiation providing histology-independent ablative responses. This shifted the goals of surgery away from maximal tumor resection toward simple spinal cord decompression with reconstitution of the thecal to create a tumor target volume capable of being irradiated within the constraints of spinal cord tolerance. This new approach of creating space between the thecal sac and the tumor was termed separation surgery and when combined with postoperative SBRT, it is referred to as hybrid therapy. Herein, we will describe the evolution of the management of MESCC, the technique of separation surgery and its outcomes, and finish with an illustrative case example.
Metastatic disease causing epidural spinal cord compression is increasingly common.
Current treatment utilizing a combination of separation surgery and adjuvant radiation has proven to be highly effective at achieving durable local control while minimizing postoperative morbidity.
Spinal metastases are a major source of morbidity in patients with cancer with studies showing spinal metastases to be present in as much as 20% of patients with solid tumor malignancies.1,2 A subset of patients with spinal metastases may present with metastatic epidural spinal cord compression (MESCC), which is estimated to affect up to 5%–10% of patients with solid malignancies.2 These numbers likely will continue to increase given the continued improvements in systemic therapy options, surveillance imaging, and early cancer detection techniques and their impacts on overall survival. As the systemic management of cancer has evolved, so too has the treatment paradigm for metastatic spine disease undergone dramatic shifts.
The past several decades have seen advancements in the fields of spine surgery and radiation oncology that have combined to reduce the morbidity and mortality of those affected by metastatic spine disease.3 One of the biggest advancements in the management of metastatic spine disease comes at the intersection of surgery and radiation treatment in the form of separation surgery, which is the surgical technique of separating—or creating space between—the spinal cord and the metastatic disease to be treated.4,5 Herein, we will describe the evolution of separation surgery, the techniques used in surgery, and the ways in which we have attempted to optimize the recovery of these patients.
The Evolution of Separation Surgery
The first laminectomy for resection of an epidural tumor was performed by Sir Victor Horsley in the late 1800s.6 From then until the early 1980s, the surgical management of MESCC did not evolve significantly and relied heavily upon laminectomy without instrumentation. Laminectomy alone only offered direct posterior decompression of the spinal cord without the ability to address lesions anterior or lateral to the spinal cord. The surgical outcomes were poor with studies demonstrating that only 30% of patients experienced postoperative improvements in their clinical symptoms.7,8 Moreover, the procedure was associated with significant morbidity including iatrogenic instability, subsequent structural deformity, postoperative pain, and neurologic decline.8
Due to the relative ineffectiveness of laminectomy alone, when Gilbert et al. published their 1978 study comparing 235 patients with solid tumor MESCC treated with either laminectomy alone or conventional external beam radiation therapy (cEBRT), it should not come as a surprise that they found that clinical outcomes between the 2 groups were similar. Consequently, they concluded that cEBRT was as efficacious as laminectomy alone.9 This and other studies led to a shift away from surgical decompression and to cEBRT as the primary treatment for MESCC. Although cEBRT was associated with less morbidity than a laminectomy, cEBRT still suffered from similarly poor clinical outcomes and poor tumor control rates due to the relative radiation resistance from most solid tumor malignancies.9,10
In 2005, Patchell et al. released a landmark study comparing cEBRT alone to aggressive surgical decompression with instrumented fusion followed by cEBRT and demonstrated statistically significant improvements in preservation of neurological function, recovery of neurological function, and survival.11 With this data, the pendulum swung back in the direction of surgical interventions and ushered in an era of aggressive intralesional gross total and even en bloc tumor resections via anterior transcavitary approaches, transthoracic approaches, and vertebral column resections; however, the morbidity of some of these approaches proved to be prohibitively high.12–14 Moreover, with the aforementioned limitations of cEBRT, the ability to improve long-term local control proved elusive.
It was not until the advent of spine stereotactic radiosurgery (sSRS) and its ability to deliver highly conformal, ablative doses of radiation that the relative radioresistance of solid tumor malignancies could be overcome.5,15,16 sSRS brought with it histology agnostic local control rates that exceeded 86% across studied tumor histologies.16–18 As a result, the goals of surgery shifted from aggressive surgical resections to the need to create space between the spine metastatic disease and the adjacent critical neural structures (ie, the spinal cord and the cauda equina nerve roots) for postoperative radiation treatment. The need for this space is the basis upon which separation surgery is predicated, and when combined with postoperative radiation, it is termed hybrid surgery. Practically speaking, in patients with radioresistant histologies with Bilsky grade 0 to 1c MESCC, sSRS can be pursued as definitive therapy without the need for separation surgery. However, in those with Bilsky grade 2 and 3 MESCC (ie, high-grade MESCC), separation surgery is a critical technique for creating an appropriate radiation target within the dose constraints of the spinal cord (Figure 1).4,5,20
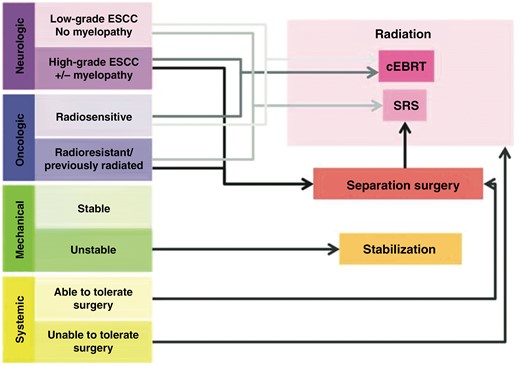
Schematic depiction of the Neurologic, Oncologic, Metastatic, and Systemic (NOMS) decision framework that links the various patient-level factors with the most appropriate treatment suggestion. cEBRT = conventional external beam radiation therapy; SRS = stereotactic radiosurgery. Taken from Laufer et al.19; used with permission.
Separation Surgery: Technique, Outcomes, and Surgical Advances
Technique
In practice, separation surgery entails the reconstitution of the cerebral spinal fluid space surrounding the spinal cord through circumferential decompression of the thecal sac. Most patients who undergo separation surgery will need spinal instrumentation as spinal stability can be compromised by both tumor invasion and the surgical removal of the pedicles and joints in order to safely access the ventral epidural space and vertebral body.4
Separation surgery utilizes a standard posterior midline exposure. In our practice, after the exposure, we proceed with spinal instrumentation of typically 1 to 2 levels above and below the index level depending on adjacent segment anatomy and tumor involvement. This is followed by a posterior osseous decompression using a high-speed drill. Lateral exposure is then obtained by performing a facetectomy and using the high-speed drill to remove the pedicle down to the level of the vertebral body. Drilling the bilateral pedicles allows for access to the ventral epidural space and the vertebral body. Tumor is then resected away from the dura using tenotomy scissors, Woodson dissector, and other surgical instruments. Ventral decompression is also achieved by depressing epidural tumor ventrally into the vertebral body using a Woodson dissector (Figure 2A). Once the decompression is completed, confirmation of the adequacy of the decompression is done using an intraoperative ultrasound (Figure 2B and C).
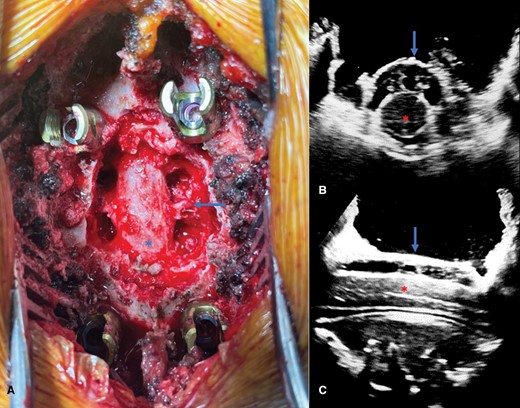
(A) Intraoperative photograph demonstrating separation surgery with circumferential decompression of the thecal sac (asterisk). Dorsal epidural tumor has been resected and the index-level pedicles have been removed bilaterally, thereby leaving the exiting nerve roots exposed (arrow) with access to the ventral epidural space. Axial (B) and sagittal (C) intraoperative ultrasound images taken after the decompression confirm circumferential decompression of the spinal cord (red asterisk) with reconstitution of the thecal sac as evidenced by the presence of the hypoechoic space between the thecal sac (blue arrow) and spinal cord (red asterisk).
In the early days of separation surgery, surgical fixation using posterior segmental instrumentation relied on long, multisegment constructs to overcome the intrinsic limitations of bone quality as well as the issue of adjacent segment tumor involvement in patients with metastatic spine disease.21 However, longer constructs necessitated longer operative times, longer incisions, and higher blood loss. The development of fenestrated screws to allow for the safe delivery of poly-methylmethacrylate through the screw overcame the bone quality limitations by allowing for the cement extruded through the screw to interdigitate with the screw and the vertebral body, thereby improving its pullout strength as well as reinforcing the vertebral body architecture22–24 (Figure 3). Our institutional data reviewing the failure rates of these cement-augmented constructs demonstrates a revision rate of 2.8% for long-segment fixations and 2.2% for short-segment fixations, rates that are not significantly different.24
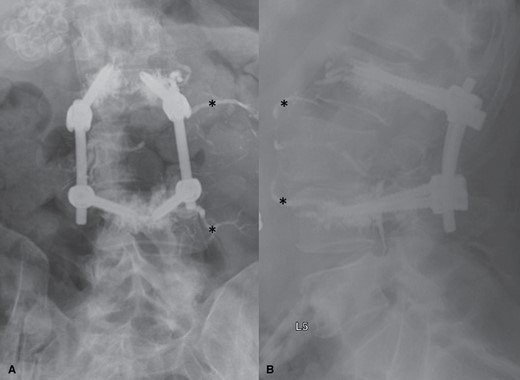
AP (A) and lateral (B) postoperative x-rays demonstrating the appearance of fenestrated screws with cement augmentation used in the spinal reconstruction portion of separation surgery. X-rays show cement extruded into the vertebral body at the L2 and L4 levels with the cement surrounding the distal screw circumferentially. Asterisks indicate endovascularly delivered embolisate placed preoperatively for embolization of this highly vascular tumor. AP = antero-posterior.
A major limitation of hybrid surgery is the risk of wound complications due to radiation treatment. From a preoperative radiation treatment perspective, Ghogawala et al. performed a retrospective review of 123 patients undergoing preoperative cEBRT within 6 weeks of open surgical treatment and demonstrated a 46% risk of wound complications or infection.25 A subsequent study by Keam et al. analyzing 165 patients undergoing preoperative radiation using either cEBRT or sSRS demonstrates a wound complication rate of 17% and 6% (P = .11), respectively, suggesting a trend toward reduced wound complication rates with more conformal radiation delivery.26 While not statistically significant, when combined with the ability of short-segment fixation to allow for a more minimal surgical footprint, the ability to spare the operative corridor with image-guided radiation therapy should allow for continued reductions in postoperative complication rates.
Durable Local-Tumor Control Using Hybrid Therapy
In addition to a reduction in surgical morbidity, hybrid therapy also provides better long-term durable tumor control, thereby reducing both local recurrences and the need for reoperation for tumor progression resulting in high-grade ESCC. Klekamp and Samii (1998) demonstrated that aggressive surgery followed by cEBRT resulted in only 30% 1-year tumor control with one of the most significant predictors of recurrence being radioresistant tumor histology.27 Laufer et al. presented the first large series evaluating the strategy of separation surgery followed by sSRS (ie, hybrid therapy) in 186 patients operated on for high-grade ESCC for predominantly radioresistant tumor histologies, with 50% of those patients having failed prior radiation therapy.5 The 3 dose strategies used were high-dose single fraction (24 Gy), high-dose hypofractionated (24 to 30 Gy in 3 fractions), and low-dose hypofractionated (18 to 36 Gy in 5 to 6 fractions). The 1-year cumulative recurrence rate was 16.4%, but patients undergoing high-dose single fraction or high-dose hypofractionated radiation had local recurrence rates of 9% and 4%, respectively.
Multiple studies from MSKCC subsequently examined histology-specific outcomes in patients undergoing hybrid therapy with a median postoperative adjuvant dose of 27 Gy in 3 fractions. Hussain et al. recently reported hybrid therapy for RCC in 90 patients using the same dose demonstrating 1-year and 2-year cumulative incidence of recurrence of 4.6% and 8.2%, respectively.18 Overall, 90% of patients remained ambulatory with an ECOG of 0 to 2 at 1-year follow-up. Similarly, Chakravarthy et al. demonstrated similarly good outcomes in 103 patients undergoing hybrid therapy for non–small cell lung carcinoma demonstrating 2-year local control rates of 94.6% (95% CI: 89.8–99.3).17 Overall survival was impacted by initiating EGFR-targeted therapy in patients who harbored the mutation and were EGFR-inhibitor naïve. Similarly, Chakravarthy et al. examined outcomes in colorectal carcinoma demonstrating LC in 86.7% at 2 years.28 Of note, adenomatous polyposis coli gene mutation conveyed a poor prognosis.
Aiding Postoperative Recovery: Enhanced Recovery After Surgery
To further improve outcomes, the Metastatic Spine Enhanced Recovery After Surgery (ERAS) protocol was launched in April of 2019 at Memorial Sloan Kettering Cancer Center.29 This protocol focused on a multidisciplinary approach to optimizing 3 phases of spine cancer patient care: preoperative, intraoperative, and postoperative. Before surgery, patients were given written information regarding the preoperative, intraoperative, and postoperative components of the ERAS pathway and the expectations for each step. On the day of surgery, patients were administered preoperative neuropathic pain medications (ie, gabapentin or celecoxib) as well as 1000 mg of acetaminophen. Intraoperatively, goal-directed fluid therapy was determined based on arterial line blood pressure measurements, and maintenance analgesia was followed by the use of local anesthetics infiltrated into the surgical site(s). The postoperative protocol involved having the patient sit up in a chair on postoperative (POD) 0, resumption of a normal diet by POD 0, early removal of the foley catheter (by 6 am on POD 1 at the latest), and early ambulation on POD 1.
From January of 2018 to May of 2020, 390 patients (177 consecutive patients enrolled in ERAS and 213 consecutive patients pre-ERAS) were compared. There was no significant difference in mean case duration. However, the ERAS cohort had significantly decreased estimated blood loss (P = .003), earlier ambulation (P = .0001), earlier discontinuation of urinary catheters (P < .001), decreased postoperative cumulative mean opioid use from POD 0 to POD 5 (P < .0001), and shorter lengths of stay (P < .0001). The results were significant for both open and minimally invasive spinal surgery. Given the improvement in clinical quality data, most of our patients undergoing metastatic spine tumor surgery are enrolled in the ERAS pathway unless patient considerations would prevent their full participation.
Case Example
A 59-year-old male with salivary gland cancer presents with severe back pain found to have ESCC grade 3 at T7 on T2 sagittal (Figure 4A) and axial (Figure 4C ) and T1 with contrast sagittal (Figure 4B) and axial (Figure 4D) demonstrating spinal cord compression without cerebrospinal fluid present around the spinal cord. Evaluating him using the Neurologic, Oncologic, Mechanical, and Systemic (NOMS) framework,19 he has high-grade spinal cord compression, a radioresistant tumor, no evidence of preop instability, and no systemic contraindication to surgery. He underwent separation surgery with posterolateral epidural decompression with partial thecal sac reconstitution and stabilization with T6-8 posterior segmented fusion with cement augmentation on CT myelogram sagittal (Figure 4E) and axial (Figure 4F) followed by postoperative sSRS. Postoperative MRI sagittal (Figure 4G) and axial (Figure 4H) shows complete thecal sac reconstitution with decompression of the spinal cord.
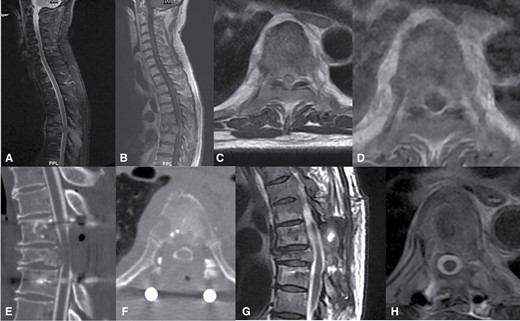
Fifty-nine-year-old male with salivary gland cancer presents with severe back pain found to have epidural spinal cord compression (ESCC) grade 3 at T7 on T2 sagittal (A) and axial (C) and T1 with contrast sagittal (B) and axial (D) demonstrating spinal cord compression without cerebrospinal fluid present around the spinal cord. He underwent separation surgery with posterolateral epidural decompression with partial thecal sac reconstitution and stabilization with T6-8 posterior segmented fusion with cement augmentation on CT myelogram sagittal (E) and axial (F) followed by postoperative spine stereotactic radiosurgery. Postoperative MRI sagittal (G) and axial (H) shows complete thecal sac reconstitution with decompression of the spinal cord.
Conclusion
The treatment paradigm for management of MESCC has changed numerous times over the past 5 decades from laminectomy to cEBRT to aggressive surgical resection and cEBRT and now to hybrid therapy in the form of separation surgery and sSRS. Each shift was propelled not only by advancements in surgery, radiation, and oncology but also by more nuanced approaches to treatment decision-making. Separation surgery in the form of hybrid therapy is the current standard treatment paradigm for MESCC, though as treatments continue to evolve, so too will the standards by which we practice.
Funding
None declared.
Conflict of interest statement
M.H.B.: Globus Medical Royalties; Deputy-Spine: Royalties; Icotec Spine: Talk.
Authorship statement
X.Z., A.G.L., N.K., M.H.B., and W.C.N. all contributed to the drafting of the article. M.H.B. and W.C.N. contributed to the generation of images and critically editing and revising the manuscript. All authors have reviewed and approved of the final submitted manuscript draft.




