-
PDF
- Split View
-
Views
-
Cite
Cite
William B McKean, Jingye Yang, Kenneth Boucher, Dennis C Shrieve, Gita Suneja, Karen Salzman, Randy Jensen, Howard Colman, Adam L Cohen, D-TERMINED, a phase 1 trial in newly diagnosed high-grade glioma with temozolomide, radiation, and minocycline followed by adjuvant minocycline/temozolomide, Neuro-Oncology Advances, Volume 6, Issue 1, January-December 2024, vdae063, https://doi.org/10.1093/noajnl/vdae063
Close - Share Icon Share
Abstract
Standard treatment for newly diagnosed high-grade gliomas remains suboptimal. Preclinical data indicate that mesenchymal transition and radiation resistance in glioblastoma are driven by NF-κB and microglia activation, which can be inhibited by minocycline. We assessed the safety and efficacy of minocycline combined with standard radiation and temozolomide in newly diagnosed high-grade gliomas.
Adults with newly diagnosed high-grade glioma were eligible. Minocycline was given with concurrent and adjuvant temozolomide. Minocycline doses were escalated using a 3 + 3 design and expanded to identify the maximum tolerated dose (MTD) and adverse event profile. Individual progression-free survival (PFS) was compared to predicted PFS based on RTOG RPA class using a binomial test. The relationships between mesenchymal and microglial biomarkers were analyzed with immunohistochemistry.
The MTD of minocycline was 150 mg twice per day (N = 20); 1 patient (5%) experienced CTCAE grade 3 + nausea and dizziness, and 2 patients (10%) demonstrated thrombocytopenia requiring temozolomide interruptions. Twelve patients exceeded their predicted PFS (60%), which did not meet the predefined efficacy endpoint of 70%. Symptoms increased during post-radiation treatment but remained mild. No significant correlation was seen between biomarkers and PFS. Expression levels of P-p65, a marker of NF-κB activation, were correlated with the microglia marker IBA-1.
Minocycline at 150 mg twice per day is well tolerated with standard chemoradiation in patients with newly diagnosed high-grade gliomas. PFS was not significantly increased with the addition of minocycline when compared to historical controls. NF-κB activation correlates with microglia levels in high-grade glioma.
Minocycline with chemoradiation is well tolerated in patients with high-grade glioma.
This intervention does not significantly change progression-free survival compared to historical medians.
There is significant correlation between P-p65 and IBA-1 in patient tissue samples.
High-grade gliomas are aggressive and incurable malignancies, and the overall prognosis remains poor despite advances in standard therapy. There is an unmet need to identify additional therapeutic targets to improve outcomes in this patient population. To this end, this phase 1 study assessed the safety, tolerability, and preliminary progression-free survival (PFS) effect of minocycline, an inhibitor of NF-κB mediated microglia activation, when combined with radiation and temozolomide. The treatment regimen was safe and demonstrated tolerable side effects; unfortunately, there was no significant change in the PFS when compared to historical matched controls. Molecular analysis of patient glioma samples did show a significant relationship between biomarkers of NF-κB signaling and microglial activation. This confirms preclinical data that has identified a role for NF-κB signaling in promoting microglial immunosuppression and subsequent glioma resistance and growth.
Despite extensive preclinical and clinical research, therapeutic advances for newly diagnosed high-grade astrocytomas have been limited. Standard of care remains maximal safe surgical resection followed by a combination of radiation and chemotherapy with temozolomide (TMZ).1,2 In glioblastoma (GBM), the addition of tumor-treatment fields also provides modest improvements in survival.3 Treatment resistance in GBM is multifactorial and depends on both tumor cell intrinsic and microenvironment factors.
Studies have identified several inter- and intratumoral subtypes of glioblastoma, which correlate with different genomic profiles and clinical behavior.4–6 Prior studies have identified that the mesenchymal gene expression subtype is associated with radiation resistance, and is associated with poor survival outcomes.4,7–10 Preclinical research shows that mesenchymal differentiation or transformation is driven by signaling from tumor necrosis factor alpha (TNFα) and transcriptional regulation via nuclear factor kappa B (NF-κB).8,11 TNFα also promotes invasion by tumor-associated microglia, which modify the tumor microenvironment, facilitate local immunosuppression, and predict poorer outcomes in patient populations.8,12,13
The FDA-approved antibiotic minocycline inhibits microglial activation and crosses the blood–brain barrier.14 It inhibits microglial expression of matrix metalloproteinase, reducing glioma migration and invasion.15 In C6 glioma cells, minocycline also blocks tumor cell growth, induces autophagy and cell death, and activates caspase-3.16 In rat xenograft models of intracranially implanted gliosarcoma, local administration of minocycline improved survival by 43% after resection.17 Subsequent studies using the same model also showed local minocycline increased median survival by 139% when delivered with radiation or by 38% with temozolomide.18
The addition of minocycline to repeat radiation with bevacizumab in patients with recurrent glioma was previously tested in the phase 1 trial RAMBO.19 This regimen was well tolerated and without significant effect on symptom burden or neuro-cognitive impairment. We investigated the addition of minocycline to upfront standard of care for newly diagnosed high-grade gliomas. This phase 1 trial assessed maximal tolerated dose, systemic toxicities, symptom burden and interference, and efficacy in unselected patients. Correlative studies evaluated the association between outcomes, molecular subtype (proneural vs mesenchymal), NF-κB activation, and microglial activation.
Materials and Methods
Study Design
D-TERMINED (phase 1 trial in newly diagnosed high-grade glioma with temozolomide, radiation, and minocycline followed by adjuvant minocycline/temozolomide, NCT02272270) was a single-arm, open-label, dose-escalation, and dose-expansion phase 1 clinical trial. All patients were enrolled from a single institution, and the trial included adults (18 years or older) with newly diagnosed histopathologically proven World Health Organization (WHO) grade 3 or 4 gliomas. Subjects were required to have adequate bone marrow, coagulation, renal, and hepatic function, and a Karnofsky Performance Status (KPS) of 60 or greater. Patients were excluded from the study if there was a history of glioma treatment (radiation or chemotherapy), other invasive cancer within 2 years, serious arrhythmia, unstable angina/myocardial infarction, decompensated congestive heart failure, cerebrovascular accident, bleeding diatheses or coagulopathy, HIV/AIDS, liver disease, active connective tissue disorder, or uncontrolled infection. After the first two patients, the trial was amended to require KPS of 70 or greater.
Interventions
Following craniotomy and resection, all patients were started on continuous minocycline 7 days before the start of concurrent therapy. Oral doses were given twice per day (BID). The initial dose level was 200 mg BID. Dose escalation or de-escalation followed a standard 3 + 3 design. Chemoradiation was administered within 6 weeks of surgery, and subjects received 6000 centigray (cGy) over 30 fractions with daily TMZ at 75 mg/m2. Following concurrent therapy, minocycline was continued as monotherapy through the addition of adjuvant TMZ (15–-200 mg/m2), given for a maximum of 6 cycles. Minocycline was also maintained after the completion of adjuvant TMZ for as long as tolerated to a maximum of 12 cycles.
Safety Assessments
The primary endpoints were to identify the maximum tolerated dose (MTD) of minocycline and its adverse event rate over the course of treatment with radiation and TMZ. Safety was evaluated throughout concurrent therapy, monotherapy with minocycline, and up to 12 cycles of adjuvant therapy. Dose-limiting toxicities (DLTs) were defined as any grade 3/4 or intolerable grade 2 toxicities attributable to minocycline during the period of chemoradiation and up to 30 days afterward. With 29 patients at MTD, we had an 80% power to detect any adverse event affecting at least 10% of patients.
Efficacy
The secondary endpoint was progression-free survival (PFS). Specifically, for each Radiation Therapy Oncology Group recursive partitioning analysis (RTOG RPA) class the median PFS was determined based on published estimates.20 We then assigned to each patient a predicted PFS equal to the median PFS for their RTOG class. For RTOG class III, the predicted PFS was 10.2 months, for class IV 7.3 months, and for class V 6.3 months. This prescribed efficacy endpoint would be met if 70% or more of patients exceeded their predicted PFS, which gave 80% power with an alpha of 0.2 using an exact binomial distribution. In the trial population, PFS was measured from the date of surgery until tumor progression or death. Progression was defined by Response Assessment in Neuro-Oncology (RANO) criteria.21 PFS was only calculated in those who initiated concurrent chemoradiation on the MTD for minocycline. A Kaplan–Meier estimate for PFS and overall survival (OS) was also calculated from the MTD efficacy population, along with 95% confidence intervals (CI).
Symptom Burden
The brain tumor module of the MD Anderson Symptom Inventory (MDASI-BT) was used to evaluate patient symptoms burden.22 Participating patients completed the module before initiating therapy (baseline) and on subsequent treatment cycles. Three scales within the MDASI-BT module were analyzed—total symptom severity, brain tumor specific symptom severity, and symptom interference with daily life. The arithmetic mean and standard deviation of items within each scale were calculated and reported for each time point. An increase of 1 point in the mean scale score was considered the minimum clinically meaningful change, and the number of patients at each time point with such change was determined. Symptom scores were defined as mild (<4), moderate (4–6), or severe (>6) for each respective scale.
Immunohistochemistry
Patient glioma tissue samples were obtained from initial tumor resection, fixed in formalin, and embedded in paraffin. Slides from these blocks were deparaffinized with sequential washes of toluene and graded ethanol, then heated in unmasking buffer. Each slide was blocked with Background Sniper (Biocare Medical) or 2.5% horse serum (20 min). Samples were then incubated overnight at 4°C with primary antibodies against biomarkers for the proneural subtype (OLIG2 α-rabbit @1:1000; Millipore Sigma #AB9610), mesenchymal subtype (CD44, α-mouse @1:100; BD Biosciences #550392), NF-κB signaling (P-p65, α-rabbit @1:300; Abcam #ab194726), and activated microglia (IBA-1, α-rabbit @1:1000; Wako Chemicals #019-19741). The following day, slides were washed and sequentially incubated with ImmPRESS reagent (rabbit or mouse, 30 min) and ImmPACT DAB working solution (5–10 min). After further washing, slides were counterstained with hematoxylin and 0.1% toluidine blue. Samples were then transferred through graded ethanol and xylene, air dried, and sealed with Permount. Percent and number of high-positive or positive cells were counted by computer using ImageJ. These data were then averaged from between 5 and 21 images for each sample. The percent positive tumor cells were reported for OLIG2, CD44, and P-p65, while number of positive tumor cells/high-powered field (HPF) was measured for IBA-1. The mean and standard deviation of biomarker positivity were then calculated for patients who exceeded or did not exceed expected PFS, and these were compared using a 2-sided t-test. Additionally, pairwise comparison with Spearman correlation coefficients was performed on these 4 biomarkers. No additional glioma tissue samples were obtained for analysis upon progression of disease.
Ethical Standards
D-TERMINED was approved by the University of Utah Institutional Review Board (IRB) and registered on ClinicalTrials.gov as NCT02272270 before patient enrollment. Each patient provided written informed consent before study participation.
Results
Demographics and Clinical Characteristics
Between November 2014 and January 2017, 25 patients were screened, deemed eligible, and enrolled on trial. A CONSORT diagram for the trial is shown in Figure 1. The majority (19) of patients were diagnosed with glioblastoma (WHO grade 4, IDH-wildtype), 4 with IDH-mutant astrocytoma (WHO grade 3–4), and 2 with gliosarcoma (WHO grade 4, IDH-wildtype). IDH status was unknown in 2 patients. The majority of patients were male, with the median age of 62 and the median KPS 90. Complete patient characteristics are shown in Table 1. Four patients enrolled at 200 mg BID dosing; 1 withdrew consent before initiating chemoradiation and was censored. Seven patients were enrolled at 150 mg BID dosing after de-escalation, and an additional 14 patients at the same dose for expansion. One patient in this cohort experienced progression before initiating chemoradiation and was excluded from the MTD efficacy analysis. None of the patients used tumor treating fields.
| Characteristic . | All Patients (n = 25) . | MTD Efficacy Population (n = 20) . |
|---|---|---|
| Sex, n (%) | ||
| Male | 18 (72) | 15 (75) |
| Female | 7 (28) | 5 (25) |
| Age, y | ||
| Median (range) | 62 (28–77) | 62.5 (28–77) |
| Race | ||
| White | 25 (100) | 20 (100) |
| Ethnicity | ||
| Non-Hispanic | 25 (100) | 20 (100) |
| KPS at baseline, n | ||
| Median (range) | 90 (70-100) | 85 (70–100) |
| Histologic Grade, n (%) | ||
| 4 | 24 (96) | 20 (100) |
| 3 | 1 (4) | 0 (0) |
| RTOG RPA class, n (%) | ||
| V | 13 (52) | 10 (50) |
| IV | 10 (40) | 9 (45) |
| III | 1 (4) | 1 (5) |
| I | 1 (4) | 0 (0) |
| IDH status, n (%) | ||
| Wildtype | 19 (76) | 17 (85) |
| Mutant | 4 (16) | 2 (10) |
| Unknown | 2 (8) | 1 (5) |
| MGMT status, n (%) | ||
| Unmethylated | 16 (64) | 14 (70) |
| Methylated | 4 (16) | 2 (10) |
| Unknown | 5 (20) | 4 (20) |
| Characteristic . | All Patients (n = 25) . | MTD Efficacy Population (n = 20) . |
|---|---|---|
| Sex, n (%) | ||
| Male | 18 (72) | 15 (75) |
| Female | 7 (28) | 5 (25) |
| Age, y | ||
| Median (range) | 62 (28–77) | 62.5 (28–77) |
| Race | ||
| White | 25 (100) | 20 (100) |
| Ethnicity | ||
| Non-Hispanic | 25 (100) | 20 (100) |
| KPS at baseline, n | ||
| Median (range) | 90 (70-100) | 85 (70–100) |
| Histologic Grade, n (%) | ||
| 4 | 24 (96) | 20 (100) |
| 3 | 1 (4) | 0 (0) |
| RTOG RPA class, n (%) | ||
| V | 13 (52) | 10 (50) |
| IV | 10 (40) | 9 (45) |
| III | 1 (4) | 1 (5) |
| I | 1 (4) | 0 (0) |
| IDH status, n (%) | ||
| Wildtype | 19 (76) | 17 (85) |
| Mutant | 4 (16) | 2 (10) |
| Unknown | 2 (8) | 1 (5) |
| MGMT status, n (%) | ||
| Unmethylated | 16 (64) | 14 (70) |
| Methylated | 4 (16) | 2 (10) |
| Unknown | 5 (20) | 4 (20) |
MTD, maximum tolerated dose; KPS, Karnofsky performance status; RTOG RPA, Radiation Therapy Oncology Group recursive partitioning analysis; IDH, isocitrate dehydrogenase; MGMT, O6-methylguanine-DNA methyltransferase.
| Characteristic . | All Patients (n = 25) . | MTD Efficacy Population (n = 20) . |
|---|---|---|
| Sex, n (%) | ||
| Male | 18 (72) | 15 (75) |
| Female | 7 (28) | 5 (25) |
| Age, y | ||
| Median (range) | 62 (28–77) | 62.5 (28–77) |
| Race | ||
| White | 25 (100) | 20 (100) |
| Ethnicity | ||
| Non-Hispanic | 25 (100) | 20 (100) |
| KPS at baseline, n | ||
| Median (range) | 90 (70-100) | 85 (70–100) |
| Histologic Grade, n (%) | ||
| 4 | 24 (96) | 20 (100) |
| 3 | 1 (4) | 0 (0) |
| RTOG RPA class, n (%) | ||
| V | 13 (52) | 10 (50) |
| IV | 10 (40) | 9 (45) |
| III | 1 (4) | 1 (5) |
| I | 1 (4) | 0 (0) |
| IDH status, n (%) | ||
| Wildtype | 19 (76) | 17 (85) |
| Mutant | 4 (16) | 2 (10) |
| Unknown | 2 (8) | 1 (5) |
| MGMT status, n (%) | ||
| Unmethylated | 16 (64) | 14 (70) |
| Methylated | 4 (16) | 2 (10) |
| Unknown | 5 (20) | 4 (20) |
| Characteristic . | All Patients (n = 25) . | MTD Efficacy Population (n = 20) . |
|---|---|---|
| Sex, n (%) | ||
| Male | 18 (72) | 15 (75) |
| Female | 7 (28) | 5 (25) |
| Age, y | ||
| Median (range) | 62 (28–77) | 62.5 (28–77) |
| Race | ||
| White | 25 (100) | 20 (100) |
| Ethnicity | ||
| Non-Hispanic | 25 (100) | 20 (100) |
| KPS at baseline, n | ||
| Median (range) | 90 (70-100) | 85 (70–100) |
| Histologic Grade, n (%) | ||
| 4 | 24 (96) | 20 (100) |
| 3 | 1 (4) | 0 (0) |
| RTOG RPA class, n (%) | ||
| V | 13 (52) | 10 (50) |
| IV | 10 (40) | 9 (45) |
| III | 1 (4) | 1 (5) |
| I | 1 (4) | 0 (0) |
| IDH status, n (%) | ||
| Wildtype | 19 (76) | 17 (85) |
| Mutant | 4 (16) | 2 (10) |
| Unknown | 2 (8) | 1 (5) |
| MGMT status, n (%) | ||
| Unmethylated | 16 (64) | 14 (70) |
| Methylated | 4 (16) | 2 (10) |
| Unknown | 5 (20) | 4 (20) |
MTD, maximum tolerated dose; KPS, Karnofsky performance status; RTOG RPA, Radiation Therapy Oncology Group recursive partitioning analysis; IDH, isocitrate dehydrogenase; MGMT, O6-methylguanine-DNA methyltransferase.
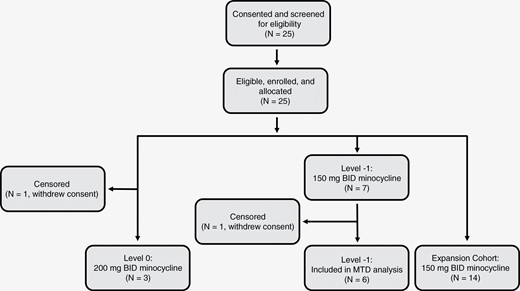
Study design and overview. Eligible patients (11) were assigned to dose escalation, which followed a 3 + 3 design. Additional patients (14) were assigned to dose expansion after determining the MTD. Patients were started on their respective minocycline doses before concurrent chemoradiation, and continued as tolerated through and after adjuvant temozolomide. MTD, maximum tolerated dose.
Safety
Dose changes for minocycline followed a 3 + 3 design, with eleven patients evaluated in the dose escalation cohort. Three patients of the MTD efficacy population received minocycline at 200 mg PO BID (level 0), with a fourth who withdrew from the study to enroll in hospice. Grade 3+adverse events occurred in the 3 (100%) evaluable patients, and related serious adverse events (SAE) were seen in 2 (67%). One person had dehydration with diarrhea, elevated liver enzymes, hyperkalemia, and hyperbilirubinemia; another had generalized weakness and GBM progression during concomitant treatment with radiation, temozolomide, and minocycline. The final patient at dose level 0 had grade 3 neutropenia. Due to the initial frequency of these adverse events and after discussion with the Data Safety and Monitoring Committee, dosing was reduced to 150 mg PO BID (level -1) for the remainder of patients in the escalation (N = 7) and expansion cohorts (N = 14). Related grade 3+ adverse events at this level were less frequent (45%), and no related SAEs were identified. The most common related grade 3+ adverse events included treatment-related thrombocytopenia prompting TMZ interruption (10%), nausea (5%), and dizziness (5%). Fatigue (5%) and muscle weakness (5%) were also seen. All grade 3+ symptoms or SAE resolved with supportive care or treatment interruption. For each minocycline dose, related low-grade (1/2) adverse events, high-grade (3/4) adverse events, and SAE are listed in Table 2.
| Toxicity code . | Dose level 0: 200 mg BID . | Dose level -1: 150 mg BID . |
|---|---|---|
| Patients experiencing related SAE | 2/4 | 0/21 |
| Dehydration | 1 | 0 |
| Diarrhea | 1 | 0 |
| Hyperbilirubinemia | 1 | 0 |
| Hyperkalemia | 1 | 0 |
| Neoplasms (benign/malignant) | 1 | 0 |
| Transaminitis | 1 | 0 |
| Patients experiencing related G3 + AE | 3/4 | 9/21 |
| Dehydration | 1 | 0 |
| Diarrhea | 1 | 0 |
| Dizziness | 0 | 1 |
| Fatigue | 0 | 1 |
| Hyperbilirubinemia | 1 | 0 |
| Hyperkalemia | 1 | 0 |
| Lymphopenia | 0 | 1 |
| Muscle weakness | 1 | 1 |
| Nausea | 0 | 1 |
| Neoplasms (benign/malignant) | 1 | 0 |
| Neutropenia | 1 | 0 |
| Thrombocytopenia | 0 | 2 |
| Patients experiencing related G1-2 AE | 4/4 | 19/21 |
| Edema | 1 | 0 |
| Fatigue | 1 | 5 |
| GERD | 0 | 2 |
| GI disorders | 0 | 2 |
| Headache | 1 | 0 |
| Hot flashes | 1 | 0 |
| Hypertriglyceridemia | 0 | 1 |
| Hypoalbuminemia | 1 | 0 |
| Hyponatremia | 1 | 0 |
| Injury, poisoning, and procedural | 0 | 1 |
| Insomnia | 1 | 0 |
| Nausea | 3 | 12 |
| Neutropenia | 1 | 0 |
| Oral pain | 0 | 1 |
| Pruritis | 1 | 4 |
| Rash, maculopapular | 0 | 7 |
| Scleral disorder | 1 | 0 |
| Skin hyperpigmentation | 0 | 1 |
| Thrombocytopenia | 1 | 2 |
| Tinnitus | 3 | 2 |
| Tremor | 0 | 1 |
| Vertigo | 1 | 2 |
| Vomiting | 1 | 3 |
| Weight loss | 0 | 2 |
| Toxicity code . | Dose level 0: 200 mg BID . | Dose level -1: 150 mg BID . |
|---|---|---|
| Patients experiencing related SAE | 2/4 | 0/21 |
| Dehydration | 1 | 0 |
| Diarrhea | 1 | 0 |
| Hyperbilirubinemia | 1 | 0 |
| Hyperkalemia | 1 | 0 |
| Neoplasms (benign/malignant) | 1 | 0 |
| Transaminitis | 1 | 0 |
| Patients experiencing related G3 + AE | 3/4 | 9/21 |
| Dehydration | 1 | 0 |
| Diarrhea | 1 | 0 |
| Dizziness | 0 | 1 |
| Fatigue | 0 | 1 |
| Hyperbilirubinemia | 1 | 0 |
| Hyperkalemia | 1 | 0 |
| Lymphopenia | 0 | 1 |
| Muscle weakness | 1 | 1 |
| Nausea | 0 | 1 |
| Neoplasms (benign/malignant) | 1 | 0 |
| Neutropenia | 1 | 0 |
| Thrombocytopenia | 0 | 2 |
| Patients experiencing related G1-2 AE | 4/4 | 19/21 |
| Edema | 1 | 0 |
| Fatigue | 1 | 5 |
| GERD | 0 | 2 |
| GI disorders | 0 | 2 |
| Headache | 1 | 0 |
| Hot flashes | 1 | 0 |
| Hypertriglyceridemia | 0 | 1 |
| Hypoalbuminemia | 1 | 0 |
| Hyponatremia | 1 | 0 |
| Injury, poisoning, and procedural | 0 | 1 |
| Insomnia | 1 | 0 |
| Nausea | 3 | 12 |
| Neutropenia | 1 | 0 |
| Oral pain | 0 | 1 |
| Pruritis | 1 | 4 |
| Rash, maculopapular | 0 | 7 |
| Scleral disorder | 1 | 0 |
| Skin hyperpigmentation | 0 | 1 |
| Thrombocytopenia | 1 | 2 |
| Tinnitus | 3 | 2 |
| Tremor | 0 | 1 |
| Vertigo | 1 | 2 |
| Vomiting | 1 | 3 |
| Weight loss | 0 | 2 |
SAE, serious adverse event; GERD, gastroesophageal reflux disease; GI, gastrointestinal.
| Toxicity code . | Dose level 0: 200 mg BID . | Dose level -1: 150 mg BID . |
|---|---|---|
| Patients experiencing related SAE | 2/4 | 0/21 |
| Dehydration | 1 | 0 |
| Diarrhea | 1 | 0 |
| Hyperbilirubinemia | 1 | 0 |
| Hyperkalemia | 1 | 0 |
| Neoplasms (benign/malignant) | 1 | 0 |
| Transaminitis | 1 | 0 |
| Patients experiencing related G3 + AE | 3/4 | 9/21 |
| Dehydration | 1 | 0 |
| Diarrhea | 1 | 0 |
| Dizziness | 0 | 1 |
| Fatigue | 0 | 1 |
| Hyperbilirubinemia | 1 | 0 |
| Hyperkalemia | 1 | 0 |
| Lymphopenia | 0 | 1 |
| Muscle weakness | 1 | 1 |
| Nausea | 0 | 1 |
| Neoplasms (benign/malignant) | 1 | 0 |
| Neutropenia | 1 | 0 |
| Thrombocytopenia | 0 | 2 |
| Patients experiencing related G1-2 AE | 4/4 | 19/21 |
| Edema | 1 | 0 |
| Fatigue | 1 | 5 |
| GERD | 0 | 2 |
| GI disorders | 0 | 2 |
| Headache | 1 | 0 |
| Hot flashes | 1 | 0 |
| Hypertriglyceridemia | 0 | 1 |
| Hypoalbuminemia | 1 | 0 |
| Hyponatremia | 1 | 0 |
| Injury, poisoning, and procedural | 0 | 1 |
| Insomnia | 1 | 0 |
| Nausea | 3 | 12 |
| Neutropenia | 1 | 0 |
| Oral pain | 0 | 1 |
| Pruritis | 1 | 4 |
| Rash, maculopapular | 0 | 7 |
| Scleral disorder | 1 | 0 |
| Skin hyperpigmentation | 0 | 1 |
| Thrombocytopenia | 1 | 2 |
| Tinnitus | 3 | 2 |
| Tremor | 0 | 1 |
| Vertigo | 1 | 2 |
| Vomiting | 1 | 3 |
| Weight loss | 0 | 2 |
| Toxicity code . | Dose level 0: 200 mg BID . | Dose level -1: 150 mg BID . |
|---|---|---|
| Patients experiencing related SAE | 2/4 | 0/21 |
| Dehydration | 1 | 0 |
| Diarrhea | 1 | 0 |
| Hyperbilirubinemia | 1 | 0 |
| Hyperkalemia | 1 | 0 |
| Neoplasms (benign/malignant) | 1 | 0 |
| Transaminitis | 1 | 0 |
| Patients experiencing related G3 + AE | 3/4 | 9/21 |
| Dehydration | 1 | 0 |
| Diarrhea | 1 | 0 |
| Dizziness | 0 | 1 |
| Fatigue | 0 | 1 |
| Hyperbilirubinemia | 1 | 0 |
| Hyperkalemia | 1 | 0 |
| Lymphopenia | 0 | 1 |
| Muscle weakness | 1 | 1 |
| Nausea | 0 | 1 |
| Neoplasms (benign/malignant) | 1 | 0 |
| Neutropenia | 1 | 0 |
| Thrombocytopenia | 0 | 2 |
| Patients experiencing related G1-2 AE | 4/4 | 19/21 |
| Edema | 1 | 0 |
| Fatigue | 1 | 5 |
| GERD | 0 | 2 |
| GI disorders | 0 | 2 |
| Headache | 1 | 0 |
| Hot flashes | 1 | 0 |
| Hypertriglyceridemia | 0 | 1 |
| Hypoalbuminemia | 1 | 0 |
| Hyponatremia | 1 | 0 |
| Injury, poisoning, and procedural | 0 | 1 |
| Insomnia | 1 | 0 |
| Nausea | 3 | 12 |
| Neutropenia | 1 | 0 |
| Oral pain | 0 | 1 |
| Pruritis | 1 | 4 |
| Rash, maculopapular | 0 | 7 |
| Scleral disorder | 1 | 0 |
| Skin hyperpigmentation | 0 | 1 |
| Thrombocytopenia | 1 | 2 |
| Tinnitus | 3 | 2 |
| Tremor | 0 | 1 |
| Vertigo | 1 | 2 |
| Vomiting | 1 | 3 |
| Weight loss | 0 | 2 |
SAE, serious adverse event; GERD, gastroesophageal reflux disease; GI, gastrointestinal.
Efficacy
The PFS and OS were calculated for all patients assigned to treatment at 150 mg BID dosing and who started treatment with concurrent chemoradiation (N = 20). The median PFS was 7.1 months (95% CI 5.4–14.7 months). There were 12 patients (60%) who exceeded the predicted PFS for their RTOG RPA class (1 class III, 5 class IV, and 6 class V). This rate did not meet the prescribed secondary efficacy endpoint of 70%. Of the 8 patients (40%) with PFS less than predicted, 4 were RPA class IV and 4 RPA class V. Within the MTD efficacy population, 16 (80%) were evaluated for best radiographic response to treatment by RANO. Of these, 2 (12%) showed partial response (PR), 10 (62%) showed stable disease (SD), and 4 (25%) showed progressive disease (PD). The median OS was 14.3 months (95% CI 9.5–23.8 months). Kaplan–Meier graphs as well as estimates for median PFS and OS are shown in Figure 2.
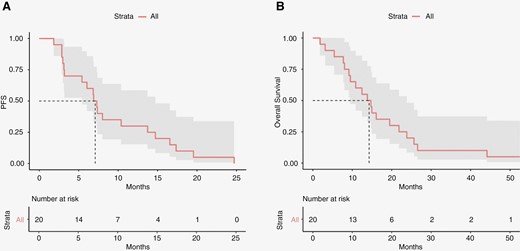
Kaplan–Meier survival analysis. Progression-free survival (A) and overall survival (B) estimates for the MTD efficacy population. MTD, maximum tolerated dose.
Symptom Burden
All eligible patients completed the MDASI-BT at baseline. Completion rates at other cycles ranged from 19% (cycle 3) to 100% (cycles 6 and 10). The average baseline MDASI-BT score was mild for total symptom severity (1.73 ± 1.54), brain tumor specific symptom severity (1.58 ± 1.45), and symptom interference with daily life (2.27 ± 1.92). Following initiation of concurrent chemoradiation with minocycline, these scores remained mild but increased (2.83 ± 1.47 for total symptom severity, 2.45 ± 1.30 for brain tumor specific symptom severity, and 3.51 ± 2.07 for symptom interference). This increase was driven by clinically meaningful changes in 7 (43%), 11 (68%), and 8 (50%) patients for each score at cycle 1. After cycle 1, the average symptom burden remained relatively stable in the mild range. The arithmetic mean and standard deviations for each MDASI-BT subscale at separate time points are represented by box-and-whisker plots in Figure 3.
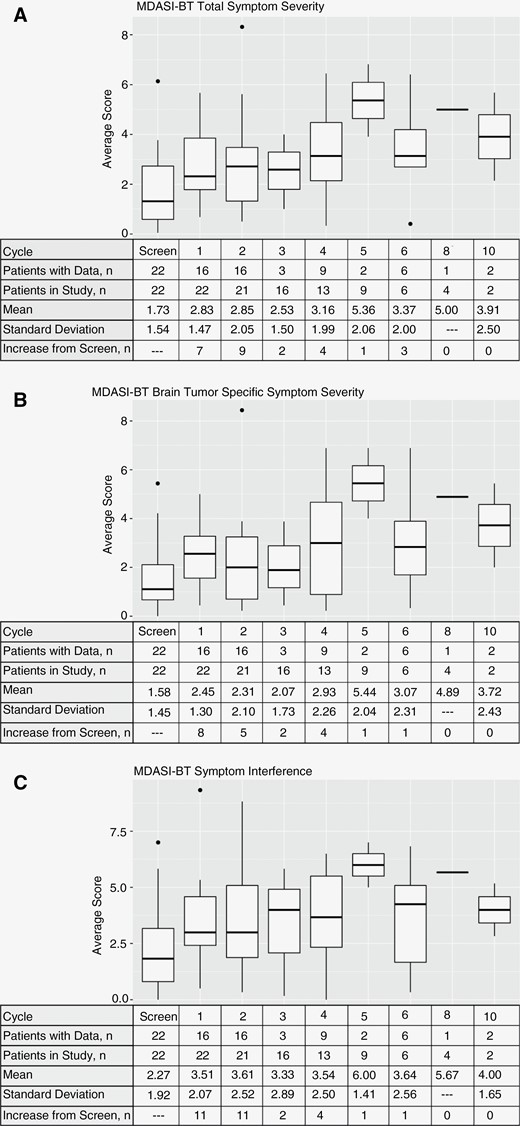
MDASI-BT survey results. Patient data and subscore for total symptom severity (A), brain tumor specific symptom severity (B), and symptom interference (C). Number of respondents, mean values, standard deviation, and number of patients with subscore increases from baseline are presented for each cycle. MDASI-BT, MD Anderson Symptom Inventory—Brain Tumor.
Efficacy Analysis Within Molecular Subtypes
Biomarkers correlating to proneural subtype (OLIG-2), mesenchymal subtype (CD44), microglial activation (IBA-1), and NF-κB signaling (P-p65) were evaluated for relationship with PFS. Example images demonstrating high and low immunohistochemical tissue expression are shown in Figure 4A. The percent (OLIG-2, CD44, P-p65) or number (IBA-1) of positive cells did not differ between people with high PFS and those with low PFS. Percents and P-values are shown in Figure 4B. Pairwise comparisons were also made between each combination of molecular subtypes. There was a significant positive correlation between P-p65 and IBA-1, with a Spearman rank correlation of 0.637 (P = .007, shown in Figure 4C).
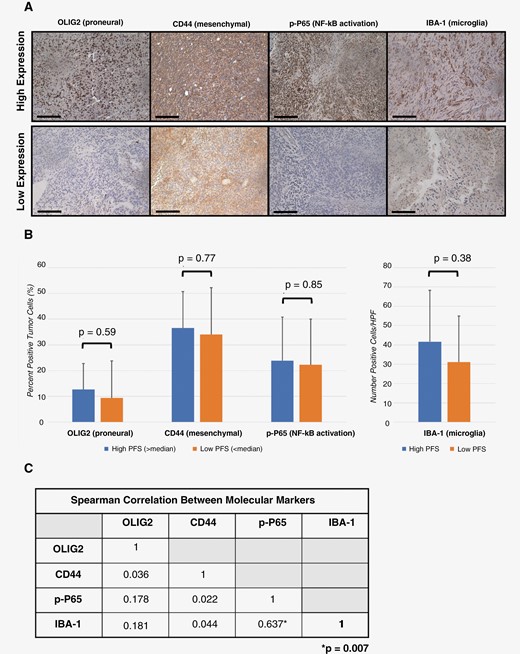
Molecular subtype efficacy analysis. Resected tumors were stained using antibodies against markers for glioblastoma subtypes, NF-κB transcription, and microglia activation. Representative samples with strong or weak expression are shown. (A) Percent or number of positive cells were averaged from tissue corresponding to patients with PFS greater or less than median historical data (scale bar = 100 μm). (B) Pairwise comparison was made between each immunohistochemical marker using Spearman rank correlation. (C) Statistical significance was considered if P ≤ .05. OLIG2, oligodendrocyte transcription factor 2; CD44, cluster of differentiation 44; P-p65, phospho-p65; NF-κB, nuclear factor kappa B; IBA-1, ionized calcium-binding adapter molecule 1; PFS, progression-free survival.
Discussion
This trial demonstrates the safety and tolerability of continuous minocycline at 150 mg BID as the MTD when combined with standard chemoradiation in newly diagnosed high-grade gliomas. At the 150 mg BID dose, there were few related high-grade adverse events and no related serious adverse events. However, the addition of minocycline to standard upfront chemoradiation did not significantly improve PFS compared to historical benchmarks in unselected patients or in those classified by expression levels of OLIG2, CD44, P-p65, or IBA-1 by immunohistochemistry.
The use of minocycline for the treatment of high-grade gliomas was previously investigated in the RAMBO study, where it was combined with repeat irradiation and bevacizumab in heavily pretreated patients.19 In the RAMBO trial, the MTD with bevacizumab and reirradiation was determined to be 400 mg BID; at this dose PFS at 3 and 6 months was 64.6% and 27.7%, respectively. During the dose escalation phase (100–200 mg BID), only 1 patient showed a grade 3+ adverse event (hypokalemia). In the present study, hypokalemia was not seen, and more patients experienced grade 3+ or SAE at equivalent dosing (150–200 mg BID), likely due to differences in population and the concomitant use of temozolomide.
There may be confounding factors that limited the benefit of minocycline in this trial. Prior studies indicate that other factors and pathways beyond NF-κB may promote mesenchymal phenotype in GBM. For instance, one recent publication utilizing monocyte-deficient CRISPR/Cas9-edited mouse strains suggests that redundant signaling through neutrophil invasion may promote the proneural-to-mesenchymal transition in glioblastoma.23 Other recent publications highlight the potential importance of microRNAs and extracellular vesicles in the TNFα/NF-κB cascade, which could also play a role in glioblastoma subtype determination.24,25 Ongoing trials are examining drugs that inhibit upstream effectors of NF-κB, proteins that interact with NF-κB, or nuclear import of NF-κB (NCT03782415, NCT03535350, NCT04216329).
Strengths of our trial include the prospective nature of the primary and secondary analyses. Weaknesses include the high proportion of White men, which may limit generalizability. The small numbers mean signals of activity in subgroups could be missed.
The lack of correlation between CD44 and OLIG2 has been seen in other cohorts, suggesting either that they are imperfect markers of the proneural and mesenchymal phenotype or that they are demonstrating intratumoral phenotypic heterogeneity.26 An important outcome of this trial was confirmation of the relationship between NF-κB signaling and microglia activation in glioma samples. This relationship has been previously shown using in vitro samples and xenograft models.8,27 However, this study marks the first instance where NF-κB and microglia activity are shown to correlate in human patients. It reinforces the role of microglia in NF-κB activation in high-grade gliomas and the importance of further study into treatments targeting the immunosuppressive effects of microglia on high-grade gliomas.
D-TERMINED shows inclusion of minocycline during radiation with concurrent and adjuvant TMZ is safe in patients with newly diagnosed high-grade gliomas. However, this regimen does not significantly change PFS when compared to historical data, or within select molecular subtypes. Further research on alternative or combination approaches to inhibiting NF-κB signaling is needed.
Funding
This work was supported by the Musella Foundation (to A.L.C.) and National Institute of Health (P30CA042014).
Conflict of Interest statement
A.L.C. has institutional research funding from Chimerix, Nuvox, Novartis, Acrotech, and BPGbio. H.C. is an advisor/consultant for Best Doctors/Teladoc, Orbus Therapeutics, Bristol Myers Squibb, Regeneron, Novocure, PPD/Chimerix, AnHeart Therapeutics, and Alpha Biopharma, and has institutional research funding from Orbus, GCAR, Array BioPharma, Nuvation Bio, Bayer, Bristol Myers Squibb, Sumitomo Dainippon Pharma Oncology, Samus Therapeutics, Erasca, and AnHeart Therapeutics.
Authorship statement
Conception and Design: K.B., D.C.S., G.S., K.S., R.J., H.C., and A.L.C. Collection and Assembly of Data: J.Y., K.B., H.C., and A.L.C. Data analysis and interpretation: All authors. Figures: W.B.M., J.Y., and K.B. Manuscript writing and revising: All authors.
References
Author notes
Howard Colman and Adam L Cohen contributed equally to this work.




