-
PDF
- Split View
-
Views
-
Cite
Cite
Shinji Kawabata, Minoru Suzuki, Katsumi Hirose, Hiroki Tanaka, Takahiro Kato, Hiromi Goto, Yoshitaka Narita, Shin-Ichi Miyatake, Accelerator-based BNCT for patients with recurrent glioblastoma: a multicenter phase II study, Neuro-Oncology Advances, Volume 3, Issue 1, January-December 2021, vdab067, https://doi.org/10.1093/noajnl/vdab067
Close - Share Icon Share
Abstract
Boron neutron capture therapy (BNCT) utilizes tumor-selective particle radiation. This study aimed to assess the safety and efficacy of accelerator-based BNCT (AB-BNCT) using a cyclotron-based neutron generator (BNCT 30) and 10B-boronophenylalanine (SPM-011) in patients with recurrent malignant glioma (MG) (primarily glioblastoma [GB]).
This multi-institutional, open-label, phase II clinical trial involved 27 recurrent MG cases, including 24 GB cases, who were enrolled from February 2016 to June 2018. The study was conducted using the abovementioned AB-BNCT system, with 500 mg/kg SPM-011 (study code: JG002). The patients were bevacizumab-naïve and had recurrent MG after standard treatment. The primary endpoint was the 1-year survival rate, and the secondary endpoints were overall survival (OS) and progression-free survival (PFS). Results were compared to those of a previous Japanese domestic bevacizumab trial for recurrent GB (JO22506).
The 1-year survival rate and median OS of the recurrent GB cases in this trial were 79.2% (95% CI: 57.0–90.8) and 18.9 months (95% CI: 12.9–not estimable), respectively, whereas those of JO22506 were 34.5% (90% CI: 20.0–49.0) and 10.5 months (95% CI: 8.2–12.4), respectively. The median PFS was 0.9 months (95% CI: 0.8–1.0) by the RANO criteria. The most prominent adverse event was brain edema. Twenty-one of 27 cases were treated with bevacizumab following progressive disease.
AB-BNCT demonstrated acceptable safety and prolonged survival for recurrent MG. AB-BNCT may increase the risk of brain edema due to re-irradiation for recurrent MG; however, this appears to be controlled well with bevacizumab.
We conducted a clinical trial for a new medical drug and device approval using accelerator-based neutron source.
The results showed markedly excellent clinical results (median overall survival, 18.9 months) after treating recurrent glioblastoma.
Glioblastomas (GBs), particularly recurrent GBs, are difficult to manage and have no standard treatment. Boron neutron capture therapy (BNCT) is a biological cell-targeting radiotherapy and an ideal particle radiation modality that can exert cytocidal effects only on tumor cells and not on adjacent normal cells. Moreover, BNCT is ideal against highly malignant tumors, particularly infiltrative ones such as GB. We previously reported the effectiveness of BNCT for malignant gliomas using reactor-based neutron sources. A clinical trial was conducted that was aimed at a new medical drug and device approval using accelerator-based neutron source. Excellent clinical results (median overall survival, 18.9 months) were obtained after treating recurrent GBs.
Surgery and postoperative chemoradiotherapy are the standard of care for malignant glioma (MG). Temozolomide (TMZ) alone is the standard chemotherapeutic agent used for treating MG; however, in some cases, it is used in combination with bevacizumab (BEV). Although fractionated external beam X-ray radiation is generally used as an initial treatment, there are limited or no treatment options for recurrent MG. Thus, the development of a novel radiotherapy with sufficiently strong antitumor effects and acceptable toxicity, even for use in patients with recurrence, is strongly desired.
Boron neutron capture therapy (BNCT) selectively destroys tumor cells by irradiating with low-energy thermal neutrons and utilizing alpha (4He) and recoiling lithium-7 (7Li) particles generated by the nuclear reactions between thermalized neutron and boron-10 (10B) atoms. BNCT involves the generation of particles that have a higher relative biological effectiveness and higher linear energy transfer than photon irradiations and are expected to have strong antitumor effects at the cellular level. Its additional advantages are as follows: a high radiation dose can be provided in a single irradiation fraction and tumor cells can be biologically targeted by 10B-containing compound.
Surgery, radiotherapy, and standard chemotherapy are often ineffective in patients with recurrent MG, particularly glioblastoma (GB). Because radiotherapy is generally administered as a first-line treatment, radiotherapy at recurrence is usually discouraged. In such instances, there is a high expectation for chemotherapy as a treatment for relapse, and several new drugs have been tested, including BEV and the dose-dense TMZ. Moreover, a new medical device, the Novo-TTF-100A system, was recently developed; however, none of these treatments have yet been able to prolong the overall survival (OS) of patients with recurrent GB. The 1-year survival rate of patients with recurrent GB is 34.5% for those who receive BEV, 20% for those who receive Novo-TTF, and less than 30% for those who receive stereotactic radiotherapy.1–3 Therefore, we designed this study to investigate whether BNCT prolongs the OS of patients with recurrent MG, particularly GB.
Although the neutron source for BNCT has been reactor-based until now, this neutron source will probably never be approved for medical use. Therefore, the most important practical aim of this clinical trial is to obtain official approval of this new BNCT system as a novel medical drug and device from the Japanese government.
Methods
This is a multicenter, open-label study (study code: JG002) that aimed to confirm the clinical benefit of BNCT in patients with recurrent MG. This study was conducted in accordance with the guidelines of Good Clinical Practice as well as the Declaration of Helsinki. The study protocol was approved by the institutional review boards of all study sites (No. 15-1-07-0374, Osaka Medical College [others are listed in Acknowledgements]). Moreover, the study has been registered with the Japan Pharmaceutical Information Center—Clinical Trials Information (JapicCTI; trial number: JapicCTI-194742 [database.japic.or.jp]).
Eligibility
Eligible patients were aged 20–75 years with histologically confirmed MG who were previously treated with TMZ and radiation therapy. The inclusion criteria were as follows: the passing of at least 180 days from the irradiation start date of the previous radiation therapy at target lesion sites to the study screening date, passing of at least 30 days from the last surgery, and progression of the lesions after preceding treatments. Other key inclusion criteria were as follows: a Karnofsky Performance Status (KPS) of ≥60%; at least one measurable target contrast-enhanced lesion ≥1 cm in diameter, both in longitudinal and perpendicular directions; and presence of target lesions in the supratentorial hemisphere with adequate hematologic, renal, and hepatic function. Patients who had undergone BEV therapy were excluded.
Treatment Protocol
This study used a cyclotron-based epithermal neutron source called BNCT 30 (Sumitomo Heavy Industries, Ltd)4,5 and10B-enriched 4-[10B] borono-l-phenylalanine (borofalan [10B]) containing ≥99% of 10B named SPM-011 (Stella Pharma Corporation) as the boron-carrying drug.
SPM-011 was administered as an intravenous infusion. After administering SPM-011 at 200 mg/kg/h for 2 h, the infusion rate was reduced to 100 mg/kg/h. During administration at the reduced infusion rate, neutron irradiation was performed using BNCT 30. In this phase II clinical trial, 8.5 Gy-Eq was decided as the maximum scalp dose for which tolerability and safety were demonstrated in our preceding phase I clinical trial, although the results of the phase I trial have not yet been published. The tumor/normal tissue ratio of borofalan (10B) was defined constantly as 3.5. For simulation on the dose planning system SERA (Idaho National Engineering and Environmental Laboratory), the10B concentration of normal tissue and blood was virtually fixed at 25 ppm. As a consequence, the value was replaced by the measurement from the laboratory data of the patients during the actual treatment. Based on the whole blood10B concentration at 2 h after the infusion of 10B, the irradiation time was determined by calculating the maximum scalp dose as 8.5 Gy-Eq. Tumor dose was then estimated from these10B concentrations in blood and the hypothesized tumor/normal tissue ratio of 3.5. Here, Gy-Eq denotes a biologically equivalent X-ray dose that is equivalent to the effects in total BNCT radiations. Based on the prescribed dose at the scalp surface, dose calculations were also performed for the tumor as well as the risk organs that were included in the irradiation field for each patient.
Treatment Planning
Patient treatment plan was formulated by SERA using magnetic resonance imaging (MRI) performed during the screening test. The setting of neutron irradiation conditions for irradiation simulation was performed considering the tumor size, positional relationship with the surrounding risk organs, and thermal neutron distribution. Subsequently, the tumor dose and normal tissue doses for the brain parenchyma, brain stem, pituitary gland, optic nerve, lens, and other organs, which are the risk organs, were evaluated. Surface remodeling images were generated by SERA, which was used as a reference for setting the patient’s posture for neutron irradiation. The compound biological effectiveness factors for boron derived from SPM-011 were 4.0 for the tumor, 2.5 for the skin, and 1.34 for other normal tissues.
Follow-up
Efficacy and safety were evaluated during the 1 year following BNCT, and if possible, the survival survey was conducted and continued up to 2 years after BNCT. During the efficacy evaluation, the use of (1) antineoplastic agents/medical devices, (2) other radiation therapies, (3) surgery of target lesions, and (4) other investigational drugs/devices were prohibited. However, if progressive disease (PD) was determined using the RANO criteria6 by the investigator in the 4 weeks following BNCT, the use of antineoplastic agents/medical devices was permitted, with the exception of surgical procedures and other radiation therapies.
Assessments
The primary endpoint of JG002 was the 1-year survival rate following BNCT in patients with recurrent GB. The data were independently evaluated by a central reviewer and the investigator. MRI was performed during screening as well as 4, 8, 12, 20, 28, 36, 44, and 52 weeks after BNCT (or at discontinuation). The RANO criteria were selected for tumor response evaluation by the investigators and the central reviewers separately.
To evaluate BNCT safety, adverse events (AEs) were recorded and laboratory testing was conducted at 1 week and every 4 weeks after BNCT. The data were evaluated using NCI-CTCAE (version 4.03).
Statistical Analysis
In patients with recurrent GB, the 1-year survival rate following BNCT and its 95% confidence interval (CI) was estimated using the Kaplan–Meier method. The estimated 1-year survival rate was compared with that of a Japanese domestic phase II BEV study (JO22506),2 in which similar inclusion criteria were used (one-sided significance level of 2.5%). Secondary endpoints were as follows: (1) OS was defined as the time from the date of BNCT to death. Subjects without documentation who were confirmed dead and subjects who remained alive at the completion of the survival survey period were censored at the last day of follow-up; (2) progression-free survival (PFS) was defined as the time from the date of enrollment to the date of the first observed disease progression judged by the RANO criteria or to death because of any cause; (3) the response rate was defined as the percentage of evaluable subjects with the best overall partial response (PR) or complete response (CR) using the RANO criteria (the response duration was defined as the time from the date of first confirmed CR or PR until the first date that recurrent disease or exacerbation is objectively observed); and (4) the disease control rate was defined as the percentage of evaluable subjects with the best overall CR, PR, or stable disease (SD) response. SAS Release 9.4 (SAS Institute Inc.) was used for statistical analyses.
Results
Patient Characteristics
Between February 2016 and June 2018, 27 patients with MG, including 3 with non-GB, received BNCT. The CONSORT diagram of this study and the protocol schema are shown in Supplementary Figures 1 and 2, respectively. The main analysis of efficacy was performed for 24 recurrent GB patients, whereas the safety analysis was performed for all 27 patients.
The baseline characteristics of the included patients are listed in Table 1. The median age of the patients with recurrent GB was 49.5 years (range: 25–68). The median tumor volume of the patients with recurrent GB was 7.3 mL (range: 1.3–30.0). The KPS of the patients with recurrent GB was 90–100 in 16 cases (66.7%), and the recursive partitioning analysis (RPA) classification for recurrent GB was the largest in Class 6 with 11 cases (45.8%).7 All the patients with recurrent GB had prior radiotherapy, with a median dose of 60.0 Gy (range: 55.8–60.0). The prescribed doses of BNCT adjusted by the planned boron concentration for tumor and risk organs are summarized in Supplementary Table 1.
| Characteristics . | GB (n = 24) . | Non-GB (n = 3) . | All Patients (n = 27) . |
|---|---|---|---|
| Median age, years (range) | 49.5 (25–68) | 44 (27–45) | 47 (25–68) |
| Sex, n (%) | |||
| Male | 14 (58.3) | 2 (66.7) | 16 (59.3) |
| Female | 10 (41.7) | 1 (33.3) | 11 (40.7) |
| KPS, n (%) | |||
| 100 | 3 (12.5) | 1 (33.3) | 4 (14.8) |
| 90 | 13 (54.2) | 1 (33.3) | 14 (51.9) |
| 80 | 6 (25.0) | 1 (33.3) | 7 (25.9) |
| 70 | 1 (4.2) | 0 (0.0) | 1 (3.7) |
| 60 | 1 (4.2) | 0 (0.0) | 1 (3.7) |
| Initial WHO classification | |||
| Grade II | 3 (12.5) | 1 (33.3) | 4 (14.8) |
| Grade III | 2 (8.3) | 2 (66.7) | 4 (14.8) |
| Grade IV | 19 (79.2) | 0 (0.0) | 19 (70.4) |
| Diagnostic method for recurrent MGa, n (%) | |||
| Histopathological diagnosis | 9 (37.5) | 1 (33.3) | 10 (37.0) |
| CT | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| MRI | 19 (79.2) | 2 (66.7) | 21 (77.8) |
| Others | 0 (0.0) | 1 (33.3) | 1 (3.7) |
| Treatment history of primary disease, n (%) | |||
| Surgery | 24 (100.0) | 3 (100.0) | 27 (100.0) |
| Radiation therapyb | 24 (100.0) | 3 (100.0) | 27 (100.0) |
| Median dose, Gy (range) | 60.0 (55.8–60.0) | 54.0 (50.0–62.8) | 60.0 (50.0–62.8) |
| Chemotherapyb | 24 (100.0) | 3 (100.0) | 27 (100.0) |
| Immunotherapy | 4 (16.7) | 0 (0.0) | 4 (14.8) |
| Others | 2 (8.3) | 0 (0.0) | 2 (7.4) |
| Relapse status, n (%) | |||
| First | 17 (70.8) | 1 (33.3) | 18 (66.7) |
| Second or more | 7 (29.2) | 2 (66.7) | 9 (33.3) |
| Median GTV, cm3 (range) | 7.3 (1.3–30.0) | 12.4 (5.2–56.8) | 7.8 (1.3–56.8) |
| RPA, n (%) | |||
| Class 1 | 3 (12.5) | 1 (33.3) | 4 (14.8) |
| Class 2 | 2 (8.3) | 2 (66.7) | 4 (14.8) |
| Class 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Class 4 | 6 (25.0) | 0 (0.0) | 6 (22.2) |
| Class 5 | 2 (8.3) | 0 (0.0) | 2 (7.4) |
| Class 6 | 11 (45.8) | 0 (0.0) | 11 (40.7) |
| Class 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Characteristics . | GB (n = 24) . | Non-GB (n = 3) . | All Patients (n = 27) . |
|---|---|---|---|
| Median age, years (range) | 49.5 (25–68) | 44 (27–45) | 47 (25–68) |
| Sex, n (%) | |||
| Male | 14 (58.3) | 2 (66.7) | 16 (59.3) |
| Female | 10 (41.7) | 1 (33.3) | 11 (40.7) |
| KPS, n (%) | |||
| 100 | 3 (12.5) | 1 (33.3) | 4 (14.8) |
| 90 | 13 (54.2) | 1 (33.3) | 14 (51.9) |
| 80 | 6 (25.0) | 1 (33.3) | 7 (25.9) |
| 70 | 1 (4.2) | 0 (0.0) | 1 (3.7) |
| 60 | 1 (4.2) | 0 (0.0) | 1 (3.7) |
| Initial WHO classification | |||
| Grade II | 3 (12.5) | 1 (33.3) | 4 (14.8) |
| Grade III | 2 (8.3) | 2 (66.7) | 4 (14.8) |
| Grade IV | 19 (79.2) | 0 (0.0) | 19 (70.4) |
| Diagnostic method for recurrent MGa, n (%) | |||
| Histopathological diagnosis | 9 (37.5) | 1 (33.3) | 10 (37.0) |
| CT | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| MRI | 19 (79.2) | 2 (66.7) | 21 (77.8) |
| Others | 0 (0.0) | 1 (33.3) | 1 (3.7) |
| Treatment history of primary disease, n (%) | |||
| Surgery | 24 (100.0) | 3 (100.0) | 27 (100.0) |
| Radiation therapyb | 24 (100.0) | 3 (100.0) | 27 (100.0) |
| Median dose, Gy (range) | 60.0 (55.8–60.0) | 54.0 (50.0–62.8) | 60.0 (50.0–62.8) |
| Chemotherapyb | 24 (100.0) | 3 (100.0) | 27 (100.0) |
| Immunotherapy | 4 (16.7) | 0 (0.0) | 4 (14.8) |
| Others | 2 (8.3) | 0 (0.0) | 2 (7.4) |
| Relapse status, n (%) | |||
| First | 17 (70.8) | 1 (33.3) | 18 (66.7) |
| Second or more | 7 (29.2) | 2 (66.7) | 9 (33.3) |
| Median GTV, cm3 (range) | 7.3 (1.3–30.0) | 12.4 (5.2–56.8) | 7.8 (1.3–56.8) |
| RPA, n (%) | |||
| Class 1 | 3 (12.5) | 1 (33.3) | 4 (14.8) |
| Class 2 | 2 (8.3) | 2 (66.7) | 4 (14.8) |
| Class 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Class 4 | 6 (25.0) | 0 (0.0) | 6 (22.2) |
| Class 5 | 2 (8.3) | 0 (0.0) | 2 (7.4) |
| Class 6 | 11 (45.8) | 0 (0.0) | 11 (40.7) |
| Class 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
GB, glioblastoma; non-GB, malignant glioma (WHO grade III); KPS, Karnofsky performance status; GTV, gross tumor volume; RPA, recursive partitioning analysis.
aOverlap aggregation.
bTreatment history of primary disease includes “radiotherapy + chemotherapy.”
| Characteristics . | GB (n = 24) . | Non-GB (n = 3) . | All Patients (n = 27) . |
|---|---|---|---|
| Median age, years (range) | 49.5 (25–68) | 44 (27–45) | 47 (25–68) |
| Sex, n (%) | |||
| Male | 14 (58.3) | 2 (66.7) | 16 (59.3) |
| Female | 10 (41.7) | 1 (33.3) | 11 (40.7) |
| KPS, n (%) | |||
| 100 | 3 (12.5) | 1 (33.3) | 4 (14.8) |
| 90 | 13 (54.2) | 1 (33.3) | 14 (51.9) |
| 80 | 6 (25.0) | 1 (33.3) | 7 (25.9) |
| 70 | 1 (4.2) | 0 (0.0) | 1 (3.7) |
| 60 | 1 (4.2) | 0 (0.0) | 1 (3.7) |
| Initial WHO classification | |||
| Grade II | 3 (12.5) | 1 (33.3) | 4 (14.8) |
| Grade III | 2 (8.3) | 2 (66.7) | 4 (14.8) |
| Grade IV | 19 (79.2) | 0 (0.0) | 19 (70.4) |
| Diagnostic method for recurrent MGa, n (%) | |||
| Histopathological diagnosis | 9 (37.5) | 1 (33.3) | 10 (37.0) |
| CT | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| MRI | 19 (79.2) | 2 (66.7) | 21 (77.8) |
| Others | 0 (0.0) | 1 (33.3) | 1 (3.7) |
| Treatment history of primary disease, n (%) | |||
| Surgery | 24 (100.0) | 3 (100.0) | 27 (100.0) |
| Radiation therapyb | 24 (100.0) | 3 (100.0) | 27 (100.0) |
| Median dose, Gy (range) | 60.0 (55.8–60.0) | 54.0 (50.0–62.8) | 60.0 (50.0–62.8) |
| Chemotherapyb | 24 (100.0) | 3 (100.0) | 27 (100.0) |
| Immunotherapy | 4 (16.7) | 0 (0.0) | 4 (14.8) |
| Others | 2 (8.3) | 0 (0.0) | 2 (7.4) |
| Relapse status, n (%) | |||
| First | 17 (70.8) | 1 (33.3) | 18 (66.7) |
| Second or more | 7 (29.2) | 2 (66.7) | 9 (33.3) |
| Median GTV, cm3 (range) | 7.3 (1.3–30.0) | 12.4 (5.2–56.8) | 7.8 (1.3–56.8) |
| RPA, n (%) | |||
| Class 1 | 3 (12.5) | 1 (33.3) | 4 (14.8) |
| Class 2 | 2 (8.3) | 2 (66.7) | 4 (14.8) |
| Class 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Class 4 | 6 (25.0) | 0 (0.0) | 6 (22.2) |
| Class 5 | 2 (8.3) | 0 (0.0) | 2 (7.4) |
| Class 6 | 11 (45.8) | 0 (0.0) | 11 (40.7) |
| Class 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Characteristics . | GB (n = 24) . | Non-GB (n = 3) . | All Patients (n = 27) . |
|---|---|---|---|
| Median age, years (range) | 49.5 (25–68) | 44 (27–45) | 47 (25–68) |
| Sex, n (%) | |||
| Male | 14 (58.3) | 2 (66.7) | 16 (59.3) |
| Female | 10 (41.7) | 1 (33.3) | 11 (40.7) |
| KPS, n (%) | |||
| 100 | 3 (12.5) | 1 (33.3) | 4 (14.8) |
| 90 | 13 (54.2) | 1 (33.3) | 14 (51.9) |
| 80 | 6 (25.0) | 1 (33.3) | 7 (25.9) |
| 70 | 1 (4.2) | 0 (0.0) | 1 (3.7) |
| 60 | 1 (4.2) | 0 (0.0) | 1 (3.7) |
| Initial WHO classification | |||
| Grade II | 3 (12.5) | 1 (33.3) | 4 (14.8) |
| Grade III | 2 (8.3) | 2 (66.7) | 4 (14.8) |
| Grade IV | 19 (79.2) | 0 (0.0) | 19 (70.4) |
| Diagnostic method for recurrent MGa, n (%) | |||
| Histopathological diagnosis | 9 (37.5) | 1 (33.3) | 10 (37.0) |
| CT | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| MRI | 19 (79.2) | 2 (66.7) | 21 (77.8) |
| Others | 0 (0.0) | 1 (33.3) | 1 (3.7) |
| Treatment history of primary disease, n (%) | |||
| Surgery | 24 (100.0) | 3 (100.0) | 27 (100.0) |
| Radiation therapyb | 24 (100.0) | 3 (100.0) | 27 (100.0) |
| Median dose, Gy (range) | 60.0 (55.8–60.0) | 54.0 (50.0–62.8) | 60.0 (50.0–62.8) |
| Chemotherapyb | 24 (100.0) | 3 (100.0) | 27 (100.0) |
| Immunotherapy | 4 (16.7) | 0 (0.0) | 4 (14.8) |
| Others | 2 (8.3) | 0 (0.0) | 2 (7.4) |
| Relapse status, n (%) | |||
| First | 17 (70.8) | 1 (33.3) | 18 (66.7) |
| Second or more | 7 (29.2) | 2 (66.7) | 9 (33.3) |
| Median GTV, cm3 (range) | 7.3 (1.3–30.0) | 12.4 (5.2–56.8) | 7.8 (1.3–56.8) |
| RPA, n (%) | |||
| Class 1 | 3 (12.5) | 1 (33.3) | 4 (14.8) |
| Class 2 | 2 (8.3) | 2 (66.7) | 4 (14.8) |
| Class 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Class 4 | 6 (25.0) | 0 (0.0) | 6 (22.2) |
| Class 5 | 2 (8.3) | 0 (0.0) | 2 (7.4) |
| Class 6 | 11 (45.8) | 0 (0.0) | 11 (40.7) |
| Class 7 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
GB, glioblastoma; non-GB, malignant glioma (WHO grade III); KPS, Karnofsky performance status; GTV, gross tumor volume; RPA, recursive partitioning analysis.
aOverlap aggregation.
bTreatment history of primary disease includes “radiotherapy + chemotherapy.”
Efficacy
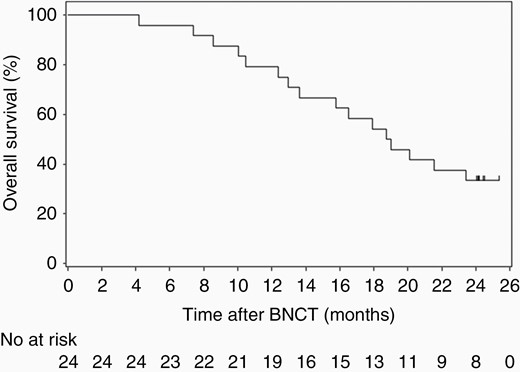
The Kaplan–Meier survival curves of OS and PFS of the 24 patients with recurrent GBs are shown in Figures 1 and 2. The 1-year survival rate was 79.2% (95% CI: 57.0–90.8) and the median OS (mOS) was 18.9 months (12.9–not estimable) with BNCT. In addition, the 18-month and 2-year survival rates were 54.2% (32.7%–71.4%) and 33.3% (15.9%–51.9%), respectively. The 6-month PFS rate and mPFS of patients with recurrent GB were 5.3% (95% CI: 0.4–21.4) and 0.9 months (95% CI: 0.8–1.0), respectively, using the RANO criteria.
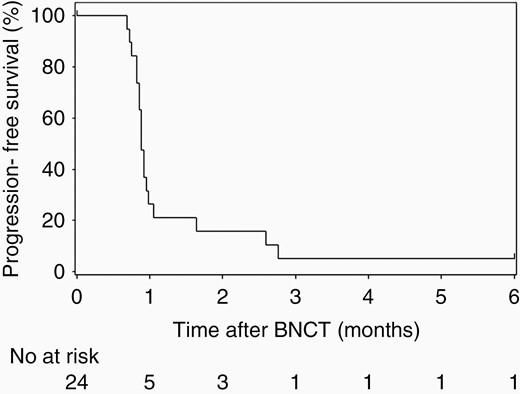
Progression-free survival was evaluated by the RANO criteria in patients with glioblastoma.
Figure 3 shows the illustrated time courses of all patients. This figure shows the timing of PD by the RANO assessment/initiation of posttreatment and the changes in KPS over time in each patient after BNCT. Of the 24 patients with recurrent GB, 11 patients completed the efficacy and safety evaluation in the year following BNCT and 13 patients discontinued the evaluation within 1 year. However, survival survey was conducted in all patients. Of the 27 patients with recurrent MG, 21 received posttreatment during the study after PD; BEV was adopted for these 21 cases at the physicians’ discretion. In 5 cases, TMZ was added with BEV after PD.
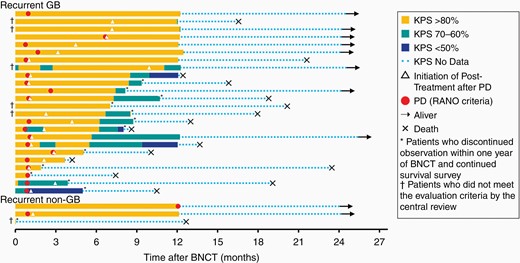
Illustrated time series of all patients, including 24 with recurrent glioblastoma and 3 with recurrent malignant glioma (WHO grade III). Illustration showing the timing of progressive disease (PD) by the radiographical assessment/initiation of posttreatment as well as the changes in Karnofsky Performance Status (KPS) over time in each patient after BNCT. The symbols are illustrated in the figure caption.
Two of the 3 non-GB patients completed the efficacy and safety evaluation in the year following BNCT and were observed for survival, whereas the other patient discontinued the evaluation within 1 year of BNCT because of the use of contraindicated therapy; and only survival survey was continued in this patient. Two patients survived for 2 years and 1 patient died of primary disease 384 days after BNCT; this patient had intratumoral hemorrhage early after BNCT and underwent surgical removal. The follow-up details of these patients are shown in Figure 3.
Based on the RANO assessment, because only 1 patient showed PR, the response rate (CR + PR) was 3.7% for 12–36 weeks. Moreover, the disease control rate (CR + PR + SD) was 18.5%, 14.8%, 7.4%, 3.7%, and 0% for 4, 8, 12–20, 28–44, and 52 weeks, respectively.
Safety
All 27 patients experienced treatment-related AEs (Table 2). Frequent treatment-related AEs included amylase increased (22 cases, 81.5%), alopecia (18 cases, 66.7%), brain edema (13 cases, 48.1%), and others. One patient died from thalamic hemorrhage, but this was unrelated to the treatment. Treatment-related severe AEs (SAEs) occurred in 9 patients (33.3%), the most common of which was brain edema in 4 patients (14.8%). Grade ≥3 treatment-related AEs occurred in 22 patients (81.5%); frequent events were amylase increased (18 cases, 66.7%), lymphocyte count decreased (4 cases, 14.8%), and brain edema (3 cases, 11.1%). Transient hyperamylasemia was commonly observed and was presumably the result of irradiation of the salivary gland, as indicated in previous reports.8
| . | n = 27 . | . | . |
|---|---|---|---|
| Patients, n (%) . | All Grades . | Grade 3 . | Grade 4 . |
| Amylase increased | 22 (81.5) | 5 (18.5) | 13 (48.1) |
| Alopecia | 18 (66.7) | 0 (0.0) | 0 (0.0) |
| Brain edema | 13 (48.1) | 3 (11.1) | 0 (0.0) |
| Nausea | 7 (25.9) | 0 (0.0) | 0 (0.0) |
| Decreased appetite | 7 (25.9) | 0 (0.0) | 0 (0.0) |
| Lymphocyte count decreased | 7 (25.9) | 4 (14.8) | 0 (0.0) |
| Crystal urine present | 6 (22.2) | 0 (0.0) | 0 (0.0) |
| Vomiting | 4 (14.8) | 0 (0.0) | 0 (0.0) |
| Dizziness | 4 (14.8) | 0 (0.0) | 0 (0.0) |
| Deafness unilateral | 3 (11.1) | 0 (0.0) | 0 (0.0) |
| Parotitis | 3 (11.1) | 0 (0.0) | 0 (0.0) |
| Headache | 3 (11.1) | 0 (0.0) | 0 (0.0) |
| Blood prolactin increased | 2 (7.4) | 0 (0.0) | 0 (0.0) |
| Back pain | 2 (7.4) | 0 (0.0) | 0 (0.0) |
| Tumor hemorrhage | 2 (7.4) | 2 (7.4) | 0 (0.0) |
| Cerebral infarction | 2 (7.4) | 1 (3.7) | 0 (0.0) |
| Epilepsy | 1 (3.7) | 1 (3.7) | 0 (0.0) |
| Seizure | 1 (3.7) | 1 (3.7) | 0 (0.0) |
| Optic nerve disorder | 1 (3.7) | 1 (3.7) | 0 (0.0) |
| . | n = 27 . | . | . |
|---|---|---|---|
| Patients, n (%) . | All Grades . | Grade 3 . | Grade 4 . |
| Amylase increased | 22 (81.5) | 5 (18.5) | 13 (48.1) |
| Alopecia | 18 (66.7) | 0 (0.0) | 0 (0.0) |
| Brain edema | 13 (48.1) | 3 (11.1) | 0 (0.0) |
| Nausea | 7 (25.9) | 0 (0.0) | 0 (0.0) |
| Decreased appetite | 7 (25.9) | 0 (0.0) | 0 (0.0) |
| Lymphocyte count decreased | 7 (25.9) | 4 (14.8) | 0 (0.0) |
| Crystal urine present | 6 (22.2) | 0 (0.0) | 0 (0.0) |
| Vomiting | 4 (14.8) | 0 (0.0) | 0 (0.0) |
| Dizziness | 4 (14.8) | 0 (0.0) | 0 (0.0) |
| Deafness unilateral | 3 (11.1) | 0 (0.0) | 0 (0.0) |
| Parotitis | 3 (11.1) | 0 (0.0) | 0 (0.0) |
| Headache | 3 (11.1) | 0 (0.0) | 0 (0.0) |
| Blood prolactin increased | 2 (7.4) | 0 (0.0) | 0 (0.0) |
| Back pain | 2 (7.4) | 0 (0.0) | 0 (0.0) |
| Tumor hemorrhage | 2 (7.4) | 2 (7.4) | 0 (0.0) |
| Cerebral infarction | 2 (7.4) | 1 (3.7) | 0 (0.0) |
| Epilepsy | 1 (3.7) | 1 (3.7) | 0 (0.0) |
| Seizure | 1 (3.7) | 1 (3.7) | 0 (0.0) |
| Optic nerve disorder | 1 (3.7) | 1 (3.7) | 0 (0.0) |
| . | n = 27 . | . | . |
|---|---|---|---|
| Patients, n (%) . | All Grades . | Grade 3 . | Grade 4 . |
| Amylase increased | 22 (81.5) | 5 (18.5) | 13 (48.1) |
| Alopecia | 18 (66.7) | 0 (0.0) | 0 (0.0) |
| Brain edema | 13 (48.1) | 3 (11.1) | 0 (0.0) |
| Nausea | 7 (25.9) | 0 (0.0) | 0 (0.0) |
| Decreased appetite | 7 (25.9) | 0 (0.0) | 0 (0.0) |
| Lymphocyte count decreased | 7 (25.9) | 4 (14.8) | 0 (0.0) |
| Crystal urine present | 6 (22.2) | 0 (0.0) | 0 (0.0) |
| Vomiting | 4 (14.8) | 0 (0.0) | 0 (0.0) |
| Dizziness | 4 (14.8) | 0 (0.0) | 0 (0.0) |
| Deafness unilateral | 3 (11.1) | 0 (0.0) | 0 (0.0) |
| Parotitis | 3 (11.1) | 0 (0.0) | 0 (0.0) |
| Headache | 3 (11.1) | 0 (0.0) | 0 (0.0) |
| Blood prolactin increased | 2 (7.4) | 0 (0.0) | 0 (0.0) |
| Back pain | 2 (7.4) | 0 (0.0) | 0 (0.0) |
| Tumor hemorrhage | 2 (7.4) | 2 (7.4) | 0 (0.0) |
| Cerebral infarction | 2 (7.4) | 1 (3.7) | 0 (0.0) |
| Epilepsy | 1 (3.7) | 1 (3.7) | 0 (0.0) |
| Seizure | 1 (3.7) | 1 (3.7) | 0 (0.0) |
| Optic nerve disorder | 1 (3.7) | 1 (3.7) | 0 (0.0) |
| . | n = 27 . | . | . |
|---|---|---|---|
| Patients, n (%) . | All Grades . | Grade 3 . | Grade 4 . |
| Amylase increased | 22 (81.5) | 5 (18.5) | 13 (48.1) |
| Alopecia | 18 (66.7) | 0 (0.0) | 0 (0.0) |
| Brain edema | 13 (48.1) | 3 (11.1) | 0 (0.0) |
| Nausea | 7 (25.9) | 0 (0.0) | 0 (0.0) |
| Decreased appetite | 7 (25.9) | 0 (0.0) | 0 (0.0) |
| Lymphocyte count decreased | 7 (25.9) | 4 (14.8) | 0 (0.0) |
| Crystal urine present | 6 (22.2) | 0 (0.0) | 0 (0.0) |
| Vomiting | 4 (14.8) | 0 (0.0) | 0 (0.0) |
| Dizziness | 4 (14.8) | 0 (0.0) | 0 (0.0) |
| Deafness unilateral | 3 (11.1) | 0 (0.0) | 0 (0.0) |
| Parotitis | 3 (11.1) | 0 (0.0) | 0 (0.0) |
| Headache | 3 (11.1) | 0 (0.0) | 0 (0.0) |
| Blood prolactin increased | 2 (7.4) | 0 (0.0) | 0 (0.0) |
| Back pain | 2 (7.4) | 0 (0.0) | 0 (0.0) |
| Tumor hemorrhage | 2 (7.4) | 2 (7.4) | 0 (0.0) |
| Cerebral infarction | 2 (7.4) | 1 (3.7) | 0 (0.0) |
| Epilepsy | 1 (3.7) | 1 (3.7) | 0 (0.0) |
| Seizure | 1 (3.7) | 1 (3.7) | 0 (0.0) |
| Optic nerve disorder | 1 (3.7) | 1 (3.7) | 0 (0.0) |
Discussion
MG, particularly GB, characteristically infiltrates and invades into the brain parenchyma. As described above, BNCT is considered a good treatment option for the tumors of infiltrative nature; thus, reactor-based BNCT has been applied aggressively for both newly diagnosed9,10 and recurrent MG.11–14 Among patients with MG, those with recurrent GB have a poor prognosis. Indeed, the latest Japanese registry of brain tumors15 reported the mPFS and mOS of patients with newly diagnosed GB as 11 months and 18 months, respectively. Simply put, the approximate OS after recurrence is only 7 months. In addition, no standard treatment has yet been established for this disease. In a previous study, we applied reactor-based BNCT to recurrent MG and reported excellent results, particularly for those with poor prognosis (RPA Class 3 + 7 in Carson’s report).7,13 Moreover, we reported that BNCT showed excellent antitumor effects even for large-sized tumors, as reported previously using reactors as a neutron source (the median and range of gross tumor volume [GTV] 42.0 and 4.1–84.6 mL, respectively),13 although this clinical trial (JG002) included only relatively small-sized GB, as given in Table 1. Unfortunately, reactor-based BNCT cannot possibly be approved for use as a new medical device because nuclear reactors cannot be set up in hospitals. Therefore, the main purpose of this clinical trial was to obtain the approval of a novel medical device and drug combination (BNCT 30 and SPM-011) for treating recurrent GB from the regulatory authority of the Japanese government. BNCT 30 has slightly different neutron energy spectrum characteristics compared with that generated by a nuclear reactor; therefore, we applied for phase I and II clinical trials for recurrent MG, primarily GB. This combination was approved as a new medical device and a new drug for refractory or advanced head and neck cancers in 2020 (see Acknowledgements).
Recurrent GB has poor prognosis, and several randomized controlled trials (RCTs) have shown pessimistic results without any effectiveness.16–20 From these reports, we estimated that the natural course of recurrent GB has a 1-year survival rate of 21% and an mOS of 3–7 months. Moreover, a previous RCT found no clinical benefit of Novo-TTF for recurrent GB, with an mOS of approximately 6 months.3 Our data showed superiority in terms of OS in comparison with these previous data.
BNCT is a type of irradiation, and as such, radiation damage—chiefly brain radiation necrosis and symptomatic pseudoprogression—is relatively common.21,22 Furthermore, radiation damage is particularly likely in patients with recurrent MG because they generally have a history of full-dose X-ray treatment (XRT). Therefore, we predicted the enlargement of edema or contrast-enhanced lesions after BNCT, which may lead to a diagnosis of PD by the RANO criteria. From our experience of reactor-based BNCT, we know that these deteriorations on MR and ADL can be recovered by BEV administration.23,24 As anticipated, 21 of 27 patients with MG were treated with BEV following PD diagnosis. A representative case of this clinical trial is depicted in Figure 4. Although BNCT controlled the lesions for a while, the enlargement of edema and contrast-enhanced lesions soon followed in this patient. However, BEV treatment could control these aggravated magnetic resonance findings, and the patient survived, with stable neurological status and without further deterioration over the subsequent 3 years.
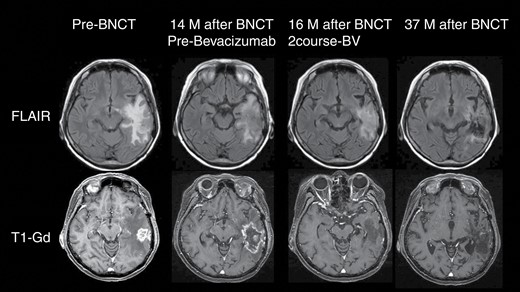
Representative case of this trial. A 63-year-old female patient underwent surgery for a left temporal tumor with GB histology. She was followed up with a standard of care comprising fractionated XRT and TMZ. Unfortunately, 14 months after craniotomy, recurrence was recognized on follow-up MRI. She then entered into this clinical trial and received BNCT. BNCT could control the mass 1 year after treatment, although brain swelling occurred subsequently because of radiation injury. After PD assessment, she was administered BEV periodically. Edema was well-controlled, and no re-aggravation was observed. The patient continues to do well, with stabilized neurological status in the 3 years following BNCT. Some MR images in this figure were obtained outside the scope of this clinical trial; informed consent was obtained for use in this report from the patient.
PD was diagnosed using the RANO assessment. Based on RANO, PD was defined as the MRI progression or deterioration of clinical performance status. However, we were unsure whether each PD assessment was dependent on the former or the latter. As a consequence, the performance status was preserved even on PD assessment as shown in Figure 3. Therefore, PD assessment has been presumed to be decided primarily based on MRI deterioration. The timing of BEV administration appeared to be immediately after PD (Figure 3). In addition, stabilized performance status and prolonged OS were observed even after PD on RANO assessment. In addition, the Mini-Mental State Examination (MMSE) was adopted to assess the mental state of the patients before and after BNCT. MMSE was stable during the patient’s survival (Supplementary Table 2). Stabilized KPS and MMSE may be considered to be the merits of BNCT.
Several recent studies have attempted re-irradiation for recurrent MG with the addition of BEV.25–28 However, even the combination of XRT and BEV did not dramatically improve the OS of recurrent GB cases. Kazmi et al.29 published a meta-analysis of re-irradiation for recurrent GB, in which the 1-year OS rate was 36% (95% CI: 32%–40%); the outcome of the present study surpassed this result.
Tipping et al.30 performed a meta-analysis regarding the use of BEV for recurrent GB, in which they summarized the effect of BEV on OS for recurrent GB, including BEV alone, in combination with BEV and TMZ as well as interferon and BEV. They reported the mOS to be 4.07–9.28 months with several BEV administration methods. Other RCTs regarding the use of BEV for recurrent GB showed an mOS of 6.9–12.6 months.31–35 Moreover, RCT interim analysis of RT + BEV versus BEV alone has recently been published online (C. Tsein, et al. Randomized Phase II Trial of Re-Irradiation and Concurrent Bevacizumab versus Bevacizumab Alone as Treatment for Recurrent Glioblastoma [NRG Oncology/RTOG 1205]: Initial Outcomes and RT Plan Quality Report.) This prospective phase II RCT demonstrated that the hypofractionated RT + BEV arm and the BEV alone arm showed an mOS of 10.1 and 9.7 months, respectively, for recurrent GB, with no significant difference in effectiveness between arms.
As mentioned above, BEV with and without XRT could not prolong the OS of patients with recurrent GB; therefore, why the BNCT and BEV combination can prolong the OS of recurrent GB but XRT with BEV cannot remains an important question. The only explanation that we could come up with was that BNCT can provide an effective dose for tumor tissue but XRT cannot. Indeed, in the present study, we could apply the median of minimum tumor dose as a single fraction (39.8 Gy-Eq [range: 23.1–63.2]), whereas that of maximum dose for normal brain tissue was only 10.9 Gy-Eq (9.6–11.6) (Supplementary Table 1). The former value might be a curative dose even for GB, and there is an extraordinary difference in the maximum dose for the tumor tissue and normal tissue. Thus, we conclude that XRT cannot provide such high radiation doses for tumor tissue without leading to serious AEs. To summarize, BNCT enabled high dose radiation in a single fraction, leading to apparent antitumor effects; however, it was also associated with inevitable deterioration on magnetic resonance image. This should be the reason for the dissociation of good OS and poor PFS.
The Japanese domestic BEV trial for recurrent GB (JO22506) showed an mOS of 10.5 months (95% CI: 8.2–12.4) for recurrent GB, which compared favorably to other BEV series and even to re-irradiation. The inclusion criteria of JG002 and JO22506 were BEV-naïve recurrent GB, and both studies included similar patient populations. Therefore, it appears adequate to compare our results with those of JO22506, although the tumor volume in JO22506 was uncertain from the published data. The current JG002 trial surpassed OS data compared with that of the JO22506 trial. In addition, re-irradiation with XRT with and without BEV did not prolong the OS of patients with recurrent GB, whereas BNCT showed an excellent prolongation. Moreover, as described above, the present JG002 trial showed excellent data in comparison with all other reports on OS after recurrent GB treatment, although JG002 is not an RCT.
There are certain limitations in the present study. In this study, the molecular information of the tumors is lacking, including the MGMT promoter methylation and IDH mutation status. In addition, this single-arm clinical trial might have a selection bias with regard to tumor size. It has been previously reported that re-irradiation therapy using stereotactic radiation techniques may be effective for small-sized tumors. Although this JG002 study did not set an upper limit on the GTV of the recurrent tumor, the contrast-enhanced lesions in patients treated with BNCT were rather small. Stereotactic radiosurgery (SRS) as a single fraction, which is commonly used in re-irradiation for recurrent MG, has been shown to be effective, although its indications are limited to small-sized tumors from a safety viewpoint. In other words, radiotherapy can be effective for recurrent GB if a sufficient dose can be delivered only to the tumor cells. In previous reports on using SRS with the mOS for recurrent GB more than 12 months, the target tumor size was approximately 2–10 mL, and 16–18 Gy was administered as a single fraction.26,36,37 Fractionated stereotactic radiotherapies can be administered for relatively large-sized tumors, such as those with volumes of 30–35 mL, without serious AEs, whereas the therapeutic effects may be limited in comparison with a single fraction of SRS.38–40 In the future, these novel accelerator-based BNCT techniques should be evaluated for relatively larger-sized recurrent GB, similar to that conducted using reactor-based BNCT.13
Conclusions
The results of this BNCT clinical trial using a novel boron-carrying drug (SPM-011) and a cyclotron-based epithermal neutron source (BNCT 30) showed a 1-year survival rate of 79.2% (95% CI: 57.0–90.8) and an mOS of 18.9 months for recurrent GB. Although direct comparison with other treatments is difficult, the results appeared to be favorable.
Acknowledgments
The authors thank Dr. Yoshihiro Takai for his technical support in applying BNCT for this clinical trial in Southern Tohoku BNCT Research Center. The authors also thank Mrs Kanako Sho, Osaka Medical College; Ai Sekido and Yoshimi Yamaguchi, National Cancer Center Hospital; and Mika Tanaka, Southern Tohoku Research Institute for Neuroscience for their support as clinical research coordinators.
Institutional Review Board Approval
Osaka Medical College (Takatsuki, Osaka, Japan) and Institute for Integrated Radiation and Nuclear Science, Kyoto University (Kumatori, Osaka, Japan) December 21, 2015 (15-1-07-0374). Southern Tohoku Research Institute for Neuroscience (Koriyama, Fukushima, Japan) and Southern Tohoku BNCT Research Center (Koriyama, Fukushima, Japan) December 24, 2015. National Cancer Center Hospital (Chuo-ku, Tokyo, Japan) 2016/1/27 (T4268).
Radiological Review Office: Micron, Inc., Central Shin-Osaka Building, 4-5-36, Miyahara, Yodogawa-ku, Osaka-shi, Osaka 532-0003.
To receive, manage, and store the MRI imaging data provided by the medical institutions; hold committee meetings; and improve/coordinate the evaluation environment: the details of these duties are specified in the written procedures for the Data and Safety Monitoring Committee.
Data and Safety Monitoring Committee: An independent committee was established to evaluate the efficacy data and safety data obtained from the clinical trial and to assess at appropriate intervals and provide recommendations to the sponsor as to whether to continue, modify, or discontinue the clinical trial. The Data and Safety Monitoring Committee comprises the following committee members: Dr. Yasumasa Nishimura, Professor, Department of Radiation Oncology, Kindai University Faculty of Medicine; Dr. Akio Asai, Professor, Department of Neurosurgery, Kansai Medical University Hospital; Dr. Kazuo Arakawa, Visiting Professor, Gunma University Heavy Ion Medical Center; and Dr. Yukio Miki, Professor, Department of Diagnostic and Interventional Radiology, Osaka City University Graduate School of Medicine.
Medical experts: Dr. Mitsuyuki Abe (until December 31, 2017), Professor Emeritus, Kyoto University. Dr. Koji Ono (from January 1, 2018), Professor Emeritus, Kyoto University; and Dr. Takanori Ohnishi, Sadamoto Hospital, Washokai Medical Corporation.
To provide medical advice on planning and implementation of the clinical trial, preparation of a clinical study report, and signature of the clinical study report.
Pathology review expert: Dr. Takanori Hirose, Institute Professor, Pathology for Regional Communication, Kobe University School of Medicine/Director, Department of Diagnostic Pathology, Hyogo Cancer Center.
To perform a central review for pathological tissues from subjects receiving BNCT and to report the results to the sponsor.
Sumitomo Heavy Industries, Ltd. obtained Medical Device Approval for the Manufacturing and Sales of Accelerator-Based BNCT System and the dose Calculation Program in Japan. World’s First BNCT System as Medical Device. https://www.shi.co.jp/english/info/2019/6kgpsq0000002ji0.html. Accessed May 19, 2020.
Stella Pharma received Marketing and Manufacturing Approval in Japan for Steboronine Intravenous Drip Bag 9000 mg/300 mL. World’s First BNCT Drug. https://stella-pharma.co.jp/cp-bin/wordpress5/wp-content/uploads/2020/03/Press-release_Steboronine-approvalENG.pdf. Accessed May 19, 2020.
This trial has previously been reported at the American Society of Clinical Oncology Annual Meeting (2020) and the Society for Neuro-Oncology Annual Meeting (2020).
The authors would like to thank Enago (www.enago.jp) for the English language review.
Funding
Stella Pharma Corporation (Osaka, Japan) and Sumitomo Heavy Industries, Ltd (Tokyo, Japan) are the legal entities that are responsible for this trial.
Conflict of interest statement. All authors report nonfinancial support from Stella Pharma and Sumitomo Heavy Industry during the conduct of the study.
Authorship statement. Design: S.K. and S.-I.M.; implementation: S.K., M.S., K.H., H.T., T.K., H.G., and Y.N.; analysis of the data: S.K. and S.-I.M.; interpretation of the data: S.K. and S.-I.M.




