-
PDF
- Split View
-
Views
-
Cite
Cite
Erin S Murphy, Kailin Yang, John H Suh, Jennifer S Yu, Glen Stevens, Lilyana Angelov, Michael A Vogelbaum, Gene H Barnett, Manmeet S Ahluwalia, Gennady Neyman, Alireza M Mohammadi, Samuel T Chao, Phase I trial of dose escalation for preoperative stereotactic radiosurgery for patients with large brain metastases, Neuro-Oncology, Volume 26, Issue 9, September 2024, Pages 1651–1659, https://doi.org/10.1093/neuonc/noae076
Close - Share Icon Share
Abstract
Single-session stereotactic radiosurgery (SRS) or surgical resection alone for brain metastases larger than 2 cm results in unsatisfactory local control. We conducted a phase I trial for brain metastases(>2 cm) to determine the safety of preoperative SRS at escalating doses.
Radiosurgery dose was escalated at 3 Gy increments for 3 cohorts based on maximum tumor dimension starting at: 18 Gy for >2–3 cm, 15 Gy for >3–4 cm, and 12 Gy for >4–6 cm. Dose-limiting toxicity was defined as grade III or greater acute toxicity.
A total of 35 patients/36 lesions were enrolled. For tumor size >2–3 cm, patients were enrolled up to the second dose level (21 Gy); for >3–4 cm and >4–6 cm cohorts the third dose level (21 and 18 Gy, respectively) was reached. There were 2 DLTs in the >3–4 cm arm at 21 Gy. The maximum tolerated dose of SRS for >2–3 cm was not reached; and was 18 Gy for both >3–4 cm arm and >4–6 cm arm. With a median follow-up of 64.0 months, the 6- and 12-month local control rates were 85.9% and 76.6%, respectively. One patient developed grade 3 radiation necrosis at 5 months. The 2-year rate of leptomeningeal disease (LMD) was 0%.
Preoperative SRS with dose escalation followed by surgical resection for brain metastases greater than 2 cm in size demonstrates acceptable acute toxicity. The phase II portion of the trial will be conducted at the maximum tolerated SRS doses.
Dose-escalated preoperative SRS is safe for brain metastases ≥2 cm treated on a phase I trial.
Dose-escalated preoperative SRS followed by surgery results in good local control with no leptomeningeal failure seen at 2 years.
Postoperative radiotherapy either by whole brain or stereotactic radiosurgery (SRS) improves local control after surgical resection of brain metastases. There are several challenges associated with postoperative radiosurgery for brain metastases, including difficulty with targeting, the need to treat a margin of normal tissue, radiation necrosis, and leptomeningeal failure. Preoperative radiosurgery followed by surgical resection has several advantages including the fact that tumors are easier to target, no margin is needed, and potentially lower risk of leptomeningeal failure and radiation necrosis. A previously published series of preoperative SRS utilized a dose de-escalation approach and larger tumors were associated with local failure. Our dose-escalation preoperative SRS approach results in low rates of acute toxicity and good local control. The phase II portion of the trial will evaluate efficacy at the maximum tolerated doses for large brain metastases.
Brain metastases develop in approximately 30% of patients with solid tumors, and their incidence continues to rise with longer survival time, improved prognosis, and increased use of advanced imaging modalities.1,2 Brain metastases are associated with severe mortality and morbidity, accounting for up to 50% of cancer-related deaths. Whole brain radiation therapy (WBRT) has been the historical standard of care for patients with brain metastasis, although decline in neurocognitive function is a common side effect with serious impact on quality of life.2 With the improvement of radiation delivery techniques, stereotactic radiosurgery (SRS) is shown to effectively treat brain metastasis with an optimal toxicity profile.2 Results from early trials including Radiation Therapy Oncology Group (RTOG) 90-05 established the maximum tolerated dose (MTD)s of single fraction SRS for re-irradiation to brain metastases as 24 Gy for lesions up to 2 cm in dimension (though the MTD was not reached in the trial for this subgroup), 18 Gy for lesions >2–3 cm, and 15 Gy for lesions >3–4 cm.3 Such doses have become the standard for clinical practice and subsequent clinical trials; however, our institutional analysis showed that local control rates are inferior for larger brain metastases of >2–3 and >3–4 cm receiving 18 and 15 Gy, respectively, with local control rates of only 49% and 45%.4
Surgery is another important management option for patients with brain metastases, particularly those with large or symptomatic lesions.2,5 Surgery was shown to improve overall survival for patients with single intracranial metastasis and good performance status.6 However, local control with surgery alone remains suboptimal, necessitating the addition of radiation to prevent local progression.7,8 Several phase III trials comparing postoperative SRS versus WBRT demonstrated that SRS is an effective adjuvant treatment that reduces local failure and has less impact on neurocognitive function without compromising overall survival.9–11 However, target delineation of the surgical cavity is a primary challenge of postoperative SRS that might have led to inferior local control as compared to WBRT on the prospective phase III trial, N107C.9 In addition, a significant risk of developing a unique form of leptomeningeal disease (LMD), referred to as “nodular LMD,” particularly in the posterior fossa has been recognized with postoperative SRS.2,12
To address these challenges, preoperative SRS has been proposed to increase local control through more accurate target delineation and potential sterilization effect on metastasis to minimize risk for iatrogenic spread during surgical resection.12 Retrospective comparison of institutional series showed that preoperative SRS conferred similar rates of local control as compared to postoperative SRS, but was associated with much lower rates of LMD and symptomatic radiation necrosis, further supporting the rationale of preoperative SRS.13 Since the standard doses of SRS (24, 18, and 15 Gy for lesions ≤2, >2–3, and >3–4 cm, respectively) were established in the re-irradiation setting,3 it remains unknown whether higher doses of preoperative SRS or SRS alone can be safely administered, in order to improve the local control rates for larger lesions.
We present the initial results of our institutional phase I clinical trial of dose escalation for preoperative SRS for large brain metastases. Our primary goal is to evaluate the safety of escalating doses for preoperative SRS to brain metastases that are subsequently resected.
Materials and Methods
Study Design
This prospective phase I clinical trial (NCT01891318) was approved by the Institutional Review Board at Cleveland Clinic (the protocol is provided as Supplementary Material). Patients at least 18 years of age with a prior histological diagnosis of cancer other than small cell lung cancer, lymphoma, or germ cell tumor, and with MRI evidence of 1 to 10 brain metastases with at least one lesion >2 and ≤6 cm in maximal dimension which is determined to be appropriate for SRS followed by gross total resection, and a Karnofsky performance score of ≥60 were eligible to participate in this study. Both SRS and surgery were performed at Cleveland Clinic Main Campus. Patients were allowed to have had prior SRS to lesions other than the one planned for preoperative SRS and surgical resection. Exclusion criteria included being medically unfit to undergo surgical resection, prior WBRT, contraindication to undergo brain MRI, tumors located in the brainstem, and radiographic or cytologic evidence of leptomeningeal disease prior to SRS. The date of the preoperative SRS was coded as day 0. The radiosurgery target was defined as the contrast-enhancing lesion only without an added margin. Patients underwent surgical resection of the selected brain metastasis within 2 weeks of SRS. Surgical resection was performed using standard contemporary minimal access technique with surgical navigation and the method of resection (en bloc vs. piecemeal) was recorded. Patients were followed at 2 months after surgical resection, and then at least every 3 months with regular brain MRIs with and without contrast for 2 years. After 2 years, patients were followed with MRI every 6 months or sooner if clinically indicated.
Study Endpoints
The primary endpoint of the phase I trial was safety of preoperative SRS at escalating doses followed by surgery, as determined by acute toxicity within the first 3 months from the preoperative SRS. Secondary endpoints included rates of local and distant brain failure, rate of radiation necrosis, and rate of LMD. For this study, local failure was defined as any new contrast-enhancing lesion within or adjacent to the resection cavity determined to be progressed by perfusion MRI and a multidisciplinary tumor board. Distant failure was defined as all other new enhancing lesions outside of the resection cavity. Radiation necrosis was defined as new enhancement within the radiosurgery target area determined to be treatment-related changes by perfusion imaging and a multidisciplinary tumor board. LMD was defined radiographically, using contrast-enhanced MRI scans.
Dose Escalation of Stereotactic Radiosurgery
Dose was escalated at 3 Gy increments with the starting dose as 18 Gy for >2–3 cm, 15 Gy for >3–4 cm, and 12 Gy for >4–6 cm. This scheme was based on the MTDs established by RTOG 90-05 for intact brain metastases up to 4 cm in size and 12 Gy was chosen for lesions >4–6 cm as an extrapolation. A cohort of 2–6 patients was treated at each dose. For the initial dose, 2 patients were treated and followed for 3 months. If one patient developed dose-limiting toxicity (DLT), an additional patient could be enrolled. If a second DLT was observed, that branch of the trial would be suspended, and the prior dose level would be considered the MTD. If no DLT was observed, the dose would be escalated at a 3 Gy increment with a new cohort of patients. DLT was defined as any neurological or surgically related grade 3–5 toxicity assessed using common terminology criteria for adverse events version 4.0,14 which was possibly, probably, or definitely related to the SRS treatment or surgery within 3 months. The maximum planned dose escalation for each respective group was 6 Gy above the initial dose.
Statistical Analysis
Continuous variables were reported using median and range. Categorical variables were summarized using count and percentage. The Kaplan–Meier method was used to determine overall survival, local failure, and distant brain failure from the date of SRS treatment. Statistical analyses were performed using SPSS (version 24, SPSS Inc.).
Results
We enrolled 35 patients from September 2013 to June 2022, of whom 5 were in the >2–3 cm group, 18 were in the >3–4 cm group, and 13 were in the > 4–6 cm group (Table 1). One patient with 2 lesions (one of 3.8 cm and the other of 4.4 cm) was enrolled simultaneously into the 15 Gy arm of the > 3–4 cm group and the 12 Gy arm of the > 4–6 cm group, respectively. Both lesions were managed with preoperative SRS followed by surgical resection per protocol. Median follow-up time was 64.0 months, interquartile range (IQR) 43.0–85.0 months. The majority (85.7%) of patients had follow-up of greater than 6 months. The characteristics of enrolled patients and lesions are presented in Tables 2 and 3, respectively. Median age was 63 years, IQR 56–68 years, and non-small cell lung cancer was the most prevalent histology (20 patients, 57.1%). Only 12 out of 35 patients had actionable mutations and these 12 all received targeted therapy and/or immunotherapy. A total of 10 patients received targeted therapy either before or after study treatment for their brain metastasis. A total of 11 patients received immunotherapy either before or after the study treatment. The median tumor volume was 5.0, 16.6, and 24.4 cc for the >2–3, >3–4, and >4–6 cm groups, respectively. The median tumor diameter was 3.80 cm, range 2.21–5.60 cm, IQR 3.44–4.30 cm. All patients successfully underwent surgical resection after preoperative SRS, at a median time of 2 days (range 1–8 days). For 14 (38.9%) out of 36 lesions, surgery was performed in a piecemeal approach.
Summary of Study Design and Patient Enrollment for SRS Dose Escalation (n = 35)
| Study cohort* . | Preoperative SRS dose (Gy) . | No. of patients enrolled . |
|---|---|---|
| >2–3 cm | 18 | 2 |
| 21 | 3 | |
| >3–4 cm | 15 | 4# |
| 18 | 9 | |
| 21 | 5 | |
| >4–6 cm | 12 | 2# |
| 15 | 7 | |
| 18 | 4 | |
| Total | 35# |
| Study cohort* . | Preoperative SRS dose (Gy) . | No. of patients enrolled . |
|---|---|---|
| >2–3 cm | 18 | 2 |
| 21 | 3 | |
| >3–4 cm | 15 | 4# |
| 18 | 9 | |
| 21 | 5 | |
| >4–6 cm | 12 | 2# |
| 15 | 7 | |
| 18 | 4 | |
| Total | 35# |
*Grouped by maximal dimension of the index lesion.
#1 patient with 2 lesions was enrolled in 15 Gy of >3–4 cm cohort and 12 Gy of >4–6 cm cohort at the same time.
Summary of Study Design and Patient Enrollment for SRS Dose Escalation (n = 35)
| Study cohort* . | Preoperative SRS dose (Gy) . | No. of patients enrolled . |
|---|---|---|
| >2–3 cm | 18 | 2 |
| 21 | 3 | |
| >3–4 cm | 15 | 4# |
| 18 | 9 | |
| 21 | 5 | |
| >4–6 cm | 12 | 2# |
| 15 | 7 | |
| 18 | 4 | |
| Total | 35# |
| Study cohort* . | Preoperative SRS dose (Gy) . | No. of patients enrolled . |
|---|---|---|
| >2–3 cm | 18 | 2 |
| 21 | 3 | |
| >3–4 cm | 15 | 4# |
| 18 | 9 | |
| 21 | 5 | |
| >4–6 cm | 12 | 2# |
| 15 | 7 | |
| 18 | 4 | |
| Total | 35# |
*Grouped by maximal dimension of the index lesion.
#1 patient with 2 lesions was enrolled in 15 Gy of >3–4 cm cohort and 12 Gy of >4–6 cm cohort at the same time.
| Characteristic . | All patients (n = 35)* . | >2–3 cm (n = 5) . | >3–4 cm (n = 18)# . | >4–6 cm (n = 13)# . |
|---|---|---|---|---|
| Age, median (range), y | 63 (25-76) | 67 (56-72) | 63.5 (34-76) | 63 (25-72) |
| Sex | ||||
| Female | 23 (66%) | 4 | 12 | 7 |
| Male | 12 (34%) | 1 | 6 | 6 |
| Race | ||||
| White | 28 (80%) | 4 | 18 | 7 |
| Other | 7 (20%) | 1 | 0 | 6 |
| Primary cancer | ||||
| Non-small cell lung cancer | 20 (57%) | 4 | 9 | 8 |
| Breast cancer | 5 (14%) | 1 | 3 | 1 |
| Colon cancer | 3 (9%) | 0 | 1 | 2 |
| Esophageal cancer | 3 (9%) | 0 | 2 | 0 |
| Endometrial cancer | 2 (5%) | 0 | 1 | 2 |
| Melanoma | 1 (3%) | 0 | 1 | 0 |
| Prostate cancer | 1 (3%) | 0 | 1 | 0 |
| KPS | ||||
| 90 | 6 (17%) | 0 | 3 | 2 |
| 80 | 17 (49%) | 5 | 7 | 6 |
| 70 | 11 (31%) | 0 | 7 | 5 |
| 60 | 1 (3%) | 0 | 1 | 0 |
| Characteristic . | All patients (n = 35)* . | >2–3 cm (n = 5) . | >3–4 cm (n = 18)# . | >4–6 cm (n = 13)# . |
|---|---|---|---|---|
| Age, median (range), y | 63 (25-76) | 67 (56-72) | 63.5 (34-76) | 63 (25-72) |
| Sex | ||||
| Female | 23 (66%) | 4 | 12 | 7 |
| Male | 12 (34%) | 1 | 6 | 6 |
| Race | ||||
| White | 28 (80%) | 4 | 18 | 7 |
| Other | 7 (20%) | 1 | 0 | 6 |
| Primary cancer | ||||
| Non-small cell lung cancer | 20 (57%) | 4 | 9 | 8 |
| Breast cancer | 5 (14%) | 1 | 3 | 1 |
| Colon cancer | 3 (9%) | 0 | 1 | 2 |
| Esophageal cancer | 3 (9%) | 0 | 2 | 0 |
| Endometrial cancer | 2 (5%) | 0 | 1 | 2 |
| Melanoma | 1 (3%) | 0 | 1 | 0 |
| Prostate cancer | 1 (3%) | 0 | 1 | 0 |
| KPS | ||||
| 90 | 6 (17%) | 0 | 3 | 2 |
| 80 | 17 (49%) | 5 | 7 | 6 |
| 70 | 11 (31%) | 0 | 7 | 5 |
| 60 | 1 (3%) | 0 | 1 | 0 |
*Unless otherwise indicated, data were presented as number (percentage) of patients.
#1 patient with 2 lesions was enrolled in 15 Gy of >3–4 cm cohort and 12 Gy of >4–6 cm cohort at the same time.
| Characteristic . | All patients (n = 35)* . | >2–3 cm (n = 5) . | >3–4 cm (n = 18)# . | >4–6 cm (n = 13)# . |
|---|---|---|---|---|
| Age, median (range), y | 63 (25-76) | 67 (56-72) | 63.5 (34-76) | 63 (25-72) |
| Sex | ||||
| Female | 23 (66%) | 4 | 12 | 7 |
| Male | 12 (34%) | 1 | 6 | 6 |
| Race | ||||
| White | 28 (80%) | 4 | 18 | 7 |
| Other | 7 (20%) | 1 | 0 | 6 |
| Primary cancer | ||||
| Non-small cell lung cancer | 20 (57%) | 4 | 9 | 8 |
| Breast cancer | 5 (14%) | 1 | 3 | 1 |
| Colon cancer | 3 (9%) | 0 | 1 | 2 |
| Esophageal cancer | 3 (9%) | 0 | 2 | 0 |
| Endometrial cancer | 2 (5%) | 0 | 1 | 2 |
| Melanoma | 1 (3%) | 0 | 1 | 0 |
| Prostate cancer | 1 (3%) | 0 | 1 | 0 |
| KPS | ||||
| 90 | 6 (17%) | 0 | 3 | 2 |
| 80 | 17 (49%) | 5 | 7 | 6 |
| 70 | 11 (31%) | 0 | 7 | 5 |
| 60 | 1 (3%) | 0 | 1 | 0 |
| Characteristic . | All patients (n = 35)* . | >2–3 cm (n = 5) . | >3–4 cm (n = 18)# . | >4–6 cm (n = 13)# . |
|---|---|---|---|---|
| Age, median (range), y | 63 (25-76) | 67 (56-72) | 63.5 (34-76) | 63 (25-72) |
| Sex | ||||
| Female | 23 (66%) | 4 | 12 | 7 |
| Male | 12 (34%) | 1 | 6 | 6 |
| Race | ||||
| White | 28 (80%) | 4 | 18 | 7 |
| Other | 7 (20%) | 1 | 0 | 6 |
| Primary cancer | ||||
| Non-small cell lung cancer | 20 (57%) | 4 | 9 | 8 |
| Breast cancer | 5 (14%) | 1 | 3 | 1 |
| Colon cancer | 3 (9%) | 0 | 1 | 2 |
| Esophageal cancer | 3 (9%) | 0 | 2 | 0 |
| Endometrial cancer | 2 (5%) | 0 | 1 | 2 |
| Melanoma | 1 (3%) | 0 | 1 | 0 |
| Prostate cancer | 1 (3%) | 0 | 1 | 0 |
| KPS | ||||
| 90 | 6 (17%) | 0 | 3 | 2 |
| 80 | 17 (49%) | 5 | 7 | 6 |
| 70 | 11 (31%) | 0 | 7 | 5 |
| 60 | 1 (3%) | 0 | 1 | 0 |
*Unless otherwise indicated, data were presented as number (percentage) of patients.
#1 patient with 2 lesions was enrolled in 15 Gy of >3–4 cm cohort and 12 Gy of >4–6 cm cohort at the same time.
Clinical and Dosimetric Characteristics of Lesions Treated With Preoperative SRS Followed by Surgical Resection
| Characteristic . | All patients (n = 35) . | >2–3 cm (n = 5) . | >3–4 cm (n = 18)# . | >4–6 cm (n = 13)# . |
|---|---|---|---|---|
| Location (%) | ||||
| Supratentorial | 28 (80%) | 5 (100%) | 14 (78%) | 10 (77%) |
| Infratentorial | 7 (20%) | 0 (0%) | 4 (22%) | 3 (23%) |
| Piecemeal surgery (%) | 13 (37%) | 1 (20%) | 5 (28%) | 8 (62%) |
| SRS parameters* | ||||
| Maximal dimension, cm | 3.80 (2.21–5.60) | 2.42 (2.21–2.68) | 3.69 (3.07–3.98) | 4.50 (4.10–5.60) |
| Lesion volume, cc | 18.1 (4.4–64.8) | 5.0 (4.4–9.2) | 16.6 (8.5–23.5) | 24.4 (6.6–64.8) |
| Maximum dose, Gy | 32.7 (23.1–42.1) | 38.2 (29.5–42.1) | 33.0 (28.6–41.2) | 27.8 (23.1–36.0) |
| Homogeneity index | 1.85 (1.64–2.50) | 1.82 (1.64–2.01) | 1.85 (1.64–2.50) | 1.85 (1.79–2.00) |
| Conformity index | 1.36 (1.21–1.71) | 1.45 (1.28–1.55) | 1.37 (1.26–1.71) | 1.35 (1.21–1.71) |
| Characteristic . | All patients (n = 35) . | >2–3 cm (n = 5) . | >3–4 cm (n = 18)# . | >4–6 cm (n = 13)# . |
|---|---|---|---|---|
| Location (%) | ||||
| Supratentorial | 28 (80%) | 5 (100%) | 14 (78%) | 10 (77%) |
| Infratentorial | 7 (20%) | 0 (0%) | 4 (22%) | 3 (23%) |
| Piecemeal surgery (%) | 13 (37%) | 1 (20%) | 5 (28%) | 8 (62%) |
| SRS parameters* | ||||
| Maximal dimension, cm | 3.80 (2.21–5.60) | 2.42 (2.21–2.68) | 3.69 (3.07–3.98) | 4.50 (4.10–5.60) |
| Lesion volume, cc | 18.1 (4.4–64.8) | 5.0 (4.4–9.2) | 16.6 (8.5–23.5) | 24.4 (6.6–64.8) |
| Maximum dose, Gy | 32.7 (23.1–42.1) | 38.2 (29.5–42.1) | 33.0 (28.6–41.2) | 27.8 (23.1–36.0) |
| Homogeneity index | 1.85 (1.64–2.50) | 1.82 (1.64–2.01) | 1.85 (1.64–2.50) | 1.85 (1.79–2.00) |
| Conformity index | 1.36 (1.21–1.71) | 1.45 (1.28–1.55) | 1.37 (1.26–1.71) | 1.35 (1.21–1.71) |
*Data were presented as median (range).
#1 patient with 2 lesions was enrolled in 15 Gy of > 3–4 cm cohort and 12 Gy of > 4–6 cm cohort at the same time.
Clinical and Dosimetric Characteristics of Lesions Treated With Preoperative SRS Followed by Surgical Resection
| Characteristic . | All patients (n = 35) . | >2–3 cm (n = 5) . | >3–4 cm (n = 18)# . | >4–6 cm (n = 13)# . |
|---|---|---|---|---|
| Location (%) | ||||
| Supratentorial | 28 (80%) | 5 (100%) | 14 (78%) | 10 (77%) |
| Infratentorial | 7 (20%) | 0 (0%) | 4 (22%) | 3 (23%) |
| Piecemeal surgery (%) | 13 (37%) | 1 (20%) | 5 (28%) | 8 (62%) |
| SRS parameters* | ||||
| Maximal dimension, cm | 3.80 (2.21–5.60) | 2.42 (2.21–2.68) | 3.69 (3.07–3.98) | 4.50 (4.10–5.60) |
| Lesion volume, cc | 18.1 (4.4–64.8) | 5.0 (4.4–9.2) | 16.6 (8.5–23.5) | 24.4 (6.6–64.8) |
| Maximum dose, Gy | 32.7 (23.1–42.1) | 38.2 (29.5–42.1) | 33.0 (28.6–41.2) | 27.8 (23.1–36.0) |
| Homogeneity index | 1.85 (1.64–2.50) | 1.82 (1.64–2.01) | 1.85 (1.64–2.50) | 1.85 (1.79–2.00) |
| Conformity index | 1.36 (1.21–1.71) | 1.45 (1.28–1.55) | 1.37 (1.26–1.71) | 1.35 (1.21–1.71) |
| Characteristic . | All patients (n = 35) . | >2–3 cm (n = 5) . | >3–4 cm (n = 18)# . | >4–6 cm (n = 13)# . |
|---|---|---|---|---|
| Location (%) | ||||
| Supratentorial | 28 (80%) | 5 (100%) | 14 (78%) | 10 (77%) |
| Infratentorial | 7 (20%) | 0 (0%) | 4 (22%) | 3 (23%) |
| Piecemeal surgery (%) | 13 (37%) | 1 (20%) | 5 (28%) | 8 (62%) |
| SRS parameters* | ||||
| Maximal dimension, cm | 3.80 (2.21–5.60) | 2.42 (2.21–2.68) | 3.69 (3.07–3.98) | 4.50 (4.10–5.60) |
| Lesion volume, cc | 18.1 (4.4–64.8) | 5.0 (4.4–9.2) | 16.6 (8.5–23.5) | 24.4 (6.6–64.8) |
| Maximum dose, Gy | 32.7 (23.1–42.1) | 38.2 (29.5–42.1) | 33.0 (28.6–41.2) | 27.8 (23.1–36.0) |
| Homogeneity index | 1.85 (1.64–2.50) | 1.82 (1.64–2.01) | 1.85 (1.64–2.50) | 1.85 (1.79–2.00) |
| Conformity index | 1.36 (1.21–1.71) | 1.45 (1.28–1.55) | 1.37 (1.26–1.71) | 1.35 (1.21–1.71) |
*Data were presented as median (range).
#1 patient with 2 lesions was enrolled in 15 Gy of > 3–4 cm cohort and 12 Gy of > 4–6 cm cohort at the same time.
For the >2–3 cm group, 2 patients were treated with the starting SRS dose of 18 Gy, and 3 additional patients were treated with dose-escalated SRS to 21 Gy. For the >3–4 cm group, 4 patients were treated with SRS at the starting dose of 15 Gy while awaiting completion of the predetermined follow-up period. Nine subsequent patients were escalated to 18 Gy and an additional 5 patients were further escalated to 21 Gy. For the >4–6 cm group, 2 patients were treated at the starting dose of 12 Gy, 7 patients were escalated to 15 Gy, and 4 additional patients were further escalated to 18 Gy.
Overall, patients tolerated the dose-escalated SRS followed by surgical resection well, with a total of 3 DLTs reported in the trial. There were 2 DLTs in the >3–4 cm arm at the highest dose of 21 Gy, one with grade 3 wound dehiscence and the other with grade 3 wound infection (Table 4, additional details are provided in a Supplementary Table 1). The first patient was a 56-year-old with metastatic colon cancer who underwent surgical resection 1 day after SRS, and she developed grade 3 wound dehiscence approximately 1 month after surgery and received surgical management. The second patient was a 48-year-old female with metastatic breast cancer who underwent surgical resection 2 days after SRS, and she developed a grade 3 wound infection at 2 months after surgery. She then underwent surgical incision and debridement, followed by long-term antibiotics. The MTD of the >3–4 cm arm was therefore determined to be 18 Gy. There was 1 DLT in the >4–6 cm arm at the dose of 15 Gy, and this patient had grade 4 hematoma (Table 4). This patient was a 63-year-old female with metastatic breast cancer, managed with SRS followed by surgical resection of the 4.3 cm left cerebellar index lesion 1 day later. She developed grade 4 hematoma with hydrocephalus at 2 days after surgery, requiring admission to the neurosurgical intensive care unit and placement of an external ventricular drain, and at 3 days after surgery required emergent surgical decompression. Of note, this patient had thrombocytopenia that was not responsive to multiple transfusions and intravenous immunoglobulin.
Outcome of Local Progression and Radiation Necrosis for Each Study Cohort Stratified by Maximal Dimension of Index Lesion
| Study cohort* . | Preoperative SRS dose (Gy) . | No. of patients enrolled . | No. with local progression . | No. with radiation necrosis . | No. with DLT . | Description of DLT . | Maximum tolerated dose . |
|---|---|---|---|---|---|---|---|
| >2–3 cm | 18 | 2 | 0 | 0 | 0 | Not reached | |
| 21 | 3 | 0 | 1 | 0 | |||
| >3–4 cm | 15 | 4 | 2 | 0 | 0 | 18 | |
| 18 | 9 | 0 | 0 | 0 | |||
| 21 | 5 | 1 | 1 | 2 | Grade 3 wound dehiscence and grade 3 wound infection | ||
| >4–6 cm | 12 | 2 | 2 | 0 | 0 | 18 | |
| 15 | 7 | 0 | 2 | 1 | Grade 4 hematoma | ||
| 18 | 4 | 1 | 0 | 0 |
| Study cohort* . | Preoperative SRS dose (Gy) . | No. of patients enrolled . | No. with local progression . | No. with radiation necrosis . | No. with DLT . | Description of DLT . | Maximum tolerated dose . |
|---|---|---|---|---|---|---|---|
| >2–3 cm | 18 | 2 | 0 | 0 | 0 | Not reached | |
| 21 | 3 | 0 | 1 | 0 | |||
| >3–4 cm | 15 | 4 | 2 | 0 | 0 | 18 | |
| 18 | 9 | 0 | 0 | 0 | |||
| 21 | 5 | 1 | 1 | 2 | Grade 3 wound dehiscence and grade 3 wound infection | ||
| >4–6 cm | 12 | 2 | 2 | 0 | 0 | 18 | |
| 15 | 7 | 0 | 2 | 1 | Grade 4 hematoma | ||
| 18 | 4 | 1 | 0 | 0 |
*Grouped by maximal dimension of index lesion.
Outcome of Local Progression and Radiation Necrosis for Each Study Cohort Stratified by Maximal Dimension of Index Lesion
| Study cohort* . | Preoperative SRS dose (Gy) . | No. of patients enrolled . | No. with local progression . | No. with radiation necrosis . | No. with DLT . | Description of DLT . | Maximum tolerated dose . |
|---|---|---|---|---|---|---|---|
| >2–3 cm | 18 | 2 | 0 | 0 | 0 | Not reached | |
| 21 | 3 | 0 | 1 | 0 | |||
| >3–4 cm | 15 | 4 | 2 | 0 | 0 | 18 | |
| 18 | 9 | 0 | 0 | 0 | |||
| 21 | 5 | 1 | 1 | 2 | Grade 3 wound dehiscence and grade 3 wound infection | ||
| >4–6 cm | 12 | 2 | 2 | 0 | 0 | 18 | |
| 15 | 7 | 0 | 2 | 1 | Grade 4 hematoma | ||
| 18 | 4 | 1 | 0 | 0 |
| Study cohort* . | Preoperative SRS dose (Gy) . | No. of patients enrolled . | No. with local progression . | No. with radiation necrosis . | No. with DLT . | Description of DLT . | Maximum tolerated dose . |
|---|---|---|---|---|---|---|---|
| >2–3 cm | 18 | 2 | 0 | 0 | 0 | Not reached | |
| 21 | 3 | 0 | 1 | 0 | |||
| >3–4 cm | 15 | 4 | 2 | 0 | 0 | 18 | |
| 18 | 9 | 0 | 0 | 0 | |||
| 21 | 5 | 1 | 1 | 2 | Grade 3 wound dehiscence and grade 3 wound infection | ||
| >4–6 cm | 12 | 2 | 2 | 0 | 0 | 18 | |
| 15 | 7 | 0 | 2 | 1 | Grade 4 hematoma | ||
| 18 | 4 | 1 | 0 | 0 |
*Grouped by maximal dimension of index lesion.
Local control by lesion was 85.9% (95% CI: 66.5%–94.5%) and 76.6% (95% CI: 54.6%–88.9%) at 6 months and 1 year, respectively, for the index lesion managed with preoperative SRS followed by surgical resection. Stratified by the maximal dimension for the lesion, local control at 1 year was 100%, 73.8% (95% CI: 36.2%–91.4%), and 68.2% (95% CI: 29.7%–88.6%) for >2–3, >3–4, and >4–6 cm groups, respectively (Figure 1A–D and Table 4). Freedom from distant brain metastasis at the patient level was 63.1% (95% CI: 43.0%–77.8%) and 55.3% (95% CI: 35.2%–71.4%) at 6 months and 1 year, respectively (Figure 2A–B). Out of the 17 patients who developed distant brain metastases, 12 patients received SRS for the new lesions and 2 received WBRT. Overall survival was 82.9% (95% CI: 65.8%–91.9%) and 59.0% (95% CI: 40.7%–73.4%) at 6 months and 1 year, respectively.
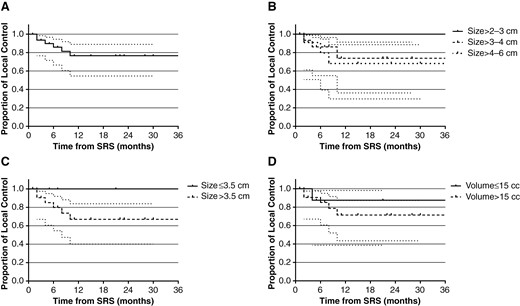
Local control (with 95% CI) after dose-escalated SRS followed by surgical resection. (A) Local control of all patients. (B) Local control stratified by size group of maximal dimension. (C) Local control stratified by the maximal dimension of the index lesion (≤3.5 vs. >3.5 cm). (D) Local control stratified by volume of the index lesion (≤15 vs. >15 cc).
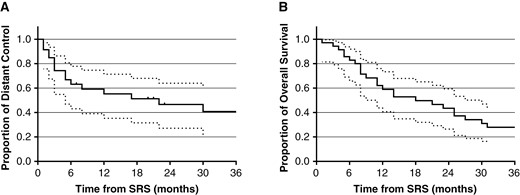
Clinical outcomes after dose-escalated SRS followed by surgical resection. (A) Freedom from distant brain failure (with 95% CI). (B) Overall survival (with 95% CI).
Only 1 patient with a tumor having the maximal dimension of 4.3 cm receiving 18 Gy developed LMD at approximately 30 months after preoperative SRS. Of note, this patient developed widespread intracranial and spinal metastases, in addition to multifocal LMD that was believed to be related to natural history of their disease. Four patients developed radiation necrosis: 1 patient of >2–3 cm group treated at 21 Gy developed grade 2 radiation necrosis at 47 months after preoperative SRS, 1 patient of >3–4 cm group treated at 21 Gy developed grade 3 radiation necrosis at 5 months after preoperative SRS and eventually required steroids and bevacizumab for management, and 2 patients of >4–6 cm group treated at 15 Gy developed grade 2 (at 8 months after preoperative SRS) and grade 1 (at 10 months after preoperative SRS) radiation necrosis, respectively (Table 4).
Discussion
Since local control for large intracranial metastasis (>2 cm) remains challenging and the paucity of prospective studies that examine the dose–response relationship of preoperative SRS for brain metastases, we proceeded with this phase I study. A retrospective study found that single fraction SRS, when performed with the use of the RTOG 90-05 standard dosing criteria, which were established in the context of re-irradiation, results in local control of about 50%.4 Preoperative SRS has emerged as a promising management approach given the advantage of target delineation and lower rates of LMD.2,12 We hypothesized that since the lesion is surgically removed after preoperative SRS, a higher radiation dose might be achievable to enhance local control without triggering DLT. To address this clinical challenge, we conducted this prospective phase I clinical trial to evaluate the safety and efficacy of dose-escalated preoperative SRS followed by surgical resection for brain metastases >2 cm in maximal dimension.
Our analysis demonstrates a 1-year local control rate of 76.6% among all patients. For the >3–4 cm cohort, 1-year local control was 73.8% which is a clinically meaningful improvement compared to our previously published data demonstrating a 1-year local control rate of 45% for intact brain metastases treated at RTOG 90-05 standard of 15 Gy.4 For the largest size cohort of >4–6 cm, the 1-year local control rate was 68.2% and typically tumors of this size would be considered too large to be safely treated with single session SRS. Only 1 patient in the >4–6 cm group developed LMD more than 2 years after treatment on trial with widespread metastases, which may reflect the natural history of disease progression and not be directly related to the prior SRS and surgical treatment of one metastasis.
The previously reported series of single-session preoperative SRS utilized a dose de-escalation approach with approximately 20% less SRS dose per size cohort compared to RTOG 90-05 dosing.15 They reported an excellent 1-year local control rate of 86% with no LMD or wound complications, though there was a higher rate of local failure for lesions >10 cc (by volume) and >3.4 cm (by maximal dimension), in addition to a trend of failure for lesions at the surface and those in eloquent areas. Our local control rates at the time of this analysis do not reflect the impact of the highest dose level as there are 0 treated at the 3rd level for >2–3 cm and 5 treated for >3–4 cm and 4 for the >4–6 cm dose level. Therefore, the ultimate impact of dose escalation on local control is not yet known and may be most beneficial for the larger tumor cohorts. Two of our patients had grade 3 wound toxicities in the 3rd dose level (21 Gy) for the >3–4 cm arm, resulting in the previous dose level (18 Gy) being the MTD. These patients had resection 1 and 2 days following radiosurgery. The impact of tumor location and the interval between radiosurgery and surgical resection on toxicity and control rates was not able to be determined as the majority of targets were at the surface and underwent surgery the day after radiosurgery. Certainly, resection type (en bloc vs. piecemeal) may impact local control outcomes as well and will be further evaluated in the Phase II component of this trial in which all lesions >3 and up to 6 cm in maximal diameter will receive the same radiosurgery dose.
Strategies for dose escalation have been found necessary to improve local control of larger brain metastases. A review of the available data reporting local control of radiosurgery alone for brain metastases was performed utilizing an α/β ratio of 12.16 The authors found a BED of at least 40 Gy, corresponding to 3 × 8.5 Gy, 2 × 11.6 Gy, or 20 Gy in a single fraction, was necessary to achieve a 1-year LC rate of ≥70%. Unfortunately, their analysis did not include a breakdown by lesion size. Minniti et al. evaluated 2 approaches of radiosurgery for brain metastases >2 cm in size and found that a hypofractionated regimen of 9 Gy × 3 improved local control compared to single fraction doses of 16–18 Gy.17 They found the most significant advantage was for brain metastases >3 cm in size with 1-year local control of 54% and 73% after a single fraction and their hypofractionated regimen, respectively. Kim et al. performed a dose-escalation fractionated radiosurgery trial for brain metastases >3cm in size and found that dose escalation beyond 9 Gy × 3 resulted in unacceptable toxicities.18 Other approaches of radiotherapy dose escalation have included a study of permanent brachytherapy implants in the resection cavity of resected metastases with a relatively large median metastasis volume of 13.5 cc.19 This study evaluated the efficacy and toxicity of surgery and iodine-125 brachytherapy for brain metastases and although they reported a crude local control of 90%, they had a 15% rate of radiation necrosis.
The advantages of preoperative SRS include ease of target delineation, no need for margin of normal tissue, and decreased risk for LMD. As shown from the phase III trial randomizing patients after surgical resection of brain metastasis to either WBRT or SRS, those treated with WBRT had significantly better local control of 81% compared to 61% for those treated with postoperative SRS.9 Most would have assumed the opposite results could have been seen because of the higher biologic effective dose of SRS. This has been speculated to be related to the difficulty of delineating the resection cavity due to the irregular shape and altered brain anatomy after surgery. Also, SRS has an unforgiving sharp dose fall-off, unlike the comprehensive coverage from WBRT. Although this trial had a low rate of LMD associated with postoperative SRS, most series utilizing this approach have resulted in LMD rates up to 30%.10,13,20–23 These series showed an increased rate of LMD failure associated with breast cancer histology, posterior fossa location, multiple metastases, piecemeal resection, and hemorrhagic or cystic metastases. The Stanford group demonstrated a local control advantage when a margin of 2 mm was added to their resection cavity targets.24 A consensus guideline for postoperative target delineation was developed in 2017, which recommended a normal tissue margin of up to 5 to 10 mm in specific situations.25 In our preoperative trial, we observed a low rate of LMD failure. Primary cancer type is known to affect the propensity to develop LMD after SRS, for example, brain metastases from breast cancer and melanoma tend to portend a higher rate of LMD. In our cohort, the majority of patients had non-small cell lung cancer (57%), and a relatively small proportion of breast cancer (14%) and melanoma (3%) cases were included. Adding normal tissue to the postoperative SRS target increases the risk for radiation necrosis compared to SRS alone or preoperative SRS as shown in the analysis of management of large brain metastases by Prabhu et al.26 In contrast, the rate of radiation necrosis in previous retrospective series of preoperative SRS was low (~5%), likely due to the immediate surgical resection and no need for a normal tissue margin.26 Longer follow-up from our clinical trial may identify benefits and/or potential late toxicity associated with dose escalation in the phase II component of this trial, which will be conducted at the MTDs for each cohort.
Conclusions
Preoperative SRS with dose escalation followed by surgical resection for brain metastases greater than 2 cm in size demonstrated an acceptable safety profile in our institutional prospective phase I clinical trial.
Supplementary material
Supplementary material is available online at Neuro-Oncology (https://dbpia.nl.go.kr/neuro-oncology).
Funding
This study was supported by internal funds from Taussig Cancer Center of Cleveland Clinic. KY was supported by the Computational Genomic Epidemiology of Cancer (CoGEC) Program at Case Comprehensive Cancer Center (T32CA094186), Young Investigator Award from ASCO Conquer Cancer Foundation, RSNA Research Resident Grant, and Cleveland Clinic VeloSano Impact Award.
Acknowledgment
We thank Paul Elson for help with statistical design, and Teresa Allison and Megan Firman for their assistance with research coordination and data collection.
Conflict of interest statement
MSA is a consultant for Bayer, Novocure, Kiyatec, Insightec, GSK, Xoft, Nuvation, Cellularity, SDP Oncology, Apollomics, Prelude, Janssen, Tocagen, Voyager Therapeutics, Viewray, Caris Lifesciences, Pyramid Biosciences, Anheart Therapeutics, Varian Medical Systems, Cairn Therapeutics, and Theraguix; receives research funding from Seagen; serves on the Scientific Advisory Board for Cairn Therapeutics, Pyramid Biosciences, and ModifiBio; and reports holding stock share of Mimivax, Cytodyn, and MedInnovate Advisors LLC. GN is a consultant for Elekta AB. STC reports honorarium from Varian Medical Systems and Blue Earth Diagnostics, and research support from Blue Earth Diagnostics. E.S.M., K.Y., J.H.S., J.S.Y., G.S., L.A., M.A.V., G.H.B., and A.M.M. report no relevant conflict of interest.
Authorship statement
Literature search: E.S.M. and K.Y.. Study design: E.S.M., S.T.C., and G.B.. Patient enrollment: E.S.M., K.Y., J.H.S., J.S.Y., G.S., L.A., M.A.V., G.H.B., M.S.A., G.N., A.M.M., and S.T.C.. Data collection: E.S.M. and K.Y.. Data analysis: E.S.M., K.Y., and S.T.C.. Data interpretation: E.S.M., K.Y., J.H.S., L.A., G.H.B., A.M.M., S.T.C.. Manuscript writing: E.S.M. and K.Y.. Manuscript review and approval: all authors.
Data availability
This trial was registered at ClinicalTrials.gov with the accession number NCT01891318. Deidentified patient data will be made available upon request with approved data use agreement from Cleveland Clinic.




