-
PDF
- Split View
-
Views
-
Cite
Cite
Fotini Iatridi, Juan Jesus Carrero, Emilie Cornec-Le Gall, Mehmet Kanbay, Valerie Luyckx, Rukshana Shroff, Charles J Ferro, KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease in Children and Adults: a commentary from the European Renal Best Practice (ERBP), Nephrology Dialysis Transplantation, Volume 40, Issue 2, February 2025, Pages 273–282, https://doi.org/10.1093/ndt/gfae209
Close - Share Icon Share
ABSTRACT
The Kidney Disease: Improving Global Outcomes (KDIGO) 2024 Guideline for Identification and Management of Chronic Kidney Disease (CKD) is a welcome development, coming 12 years after the paradigm-changing 2012 guidelines. We are living in an unprecedented era in nephrology with novel therapies, including sodium-glucose cotransporter-2 inhibitors, glucagon-like peptide-1 receptor agonists and non-steroidal mineralocorticoid receptor antagonists, now being proven in multiple randomized controlled clinical trials to reduce both the progression of CKD and cardiovascular morbidity and mortality. The KDIGO 2024 CKD Guideline is aimed at a broad audience looking after children and adults with CKD and provide practical and actionable steps to improve care. This commentary reviews the guideline sections pertaining to the evaluation and risk assessment of individuals with CKD from a European perspective. We feel that despite the last guideline being published 12 years ago, and the fact that the assessment of CKD has been emphasized by many other national/international nephrology, cardiology and diabetology guidelines and societies, the diagnosis and treatment of CKD remains poor across Europe. As such, the KDIGO 2024 CKD Guideline should be seen as an urgent call to action to improve diagnosis and care of children and adults with CKD across Europe. We know what we need to do. We now need to get on and do it.
INTRODUCTION
Chronic kidney disease (CKD) is a major global health priority affecting 10%–12% of the population (over 850 million people) with the number of affected individuals projected to increase rapidly over the next few decades [1–4]. The prevalence of CKD is higher in those over 60 years of age, as well as in individuals with increased body mass index, diabetes and hypertension [5]. Having CKD has significant negative implications for both life expectancy and quality of life, especially as a core component of the increasingly recognized cardio-kidney-metabolic multimorbidity syndrome [6–8]. The 2024 Report from the Global Burden of Diseases, Injuries, and Risk Factors Study using 2021 data shows that CKD is the 11th leading cause of death, with 1.53 million deaths [6], with a further 2.1 million cardiovascular deaths attributable to CKD [7]. CKD is also expected to rise from the 23rd leading cause of disease burden worldwide in 2022 to the 10th by 2050 [8]. However, much of the kidney disease burden globally, and its impact on outcomes of other conditions, remains uncounted, therefore these numbers are likely conservative estimates.
The new 2024 Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for the Evaluation and Management of CKD [9], 12 years on from the previous guideline in 2012 [10], are a very welcome update which emphasize both the advancements in the fields and the remaining gaps to fill. The document is an impressive text at 198 pages with 937 references, with multiple associated documents including supplementary material, executive summary and takeaway message summaries [9].
The purpose of this article is not to summarize the KDIGO 2024 CKD Guideline but, on behalf of the European Renal Best Practice (ERBP) group of the European Renal Association (ERA), to highlight some of what is new and relevant, especially in the European context. Given the rapidly evolving and expanding field of treatment for CKD [for example, the FLOW trial data on Semaglutide, a Glucagon Like Peptide-1 receptor agonist (GLP-1ra), on kidney disease progression [11] which was published after the KDIGO 2024 CKD Guideline], we chose to focus on the remaining Achilles’ heel of the global push to improve CKD care and outcomes—the first step towards effective management of the disease—evaluation and risk assessment in people with CKD.
EVALUATION OF CKD
Advances in nephroprotective and targeted treatments for CKD, along with the increased availability of precision diagnostic tools such as genetic testing, immunology panels and new biomarkers, have called for a renewed approach towards the evaluation of CKD. The CGA classification, which considers the cause of CKD (C), the glomerular filtration rate (G) and the degree of albuminuria (A), introduced by the KDIGO 2012 CKD Guideline [10], remains the cornerstone of CKD assessment.
Cause of CKD
The KDIGO 2024 CKD Guideline places specific emphasis on the often-overlooked cause (C) component of the CGA classification. While histology should be obtained whenever possible, other precision diagnostic tools, such as genetic testing, are also promoted. More than 10% of people with CKD have an underlying genetic cause, and identifying actionable genes through genetic testing can significantly impact the clinical management of these patients [12–16]. Additionally, obtaining such a diagnosis in one person can facilitate early detection and appropriate management in other family members. This approach will require a well-educated workforce with expertise in kidney genetics [17]. It should also be recognized that while costs of genetic testing are decreasing, accessibility may still be limited in some regions, and interpreting variants of uncertain significance can present challenges. Over time, the importance of making a genetic diagnosis may increase as more directly targeted therapies emerge. This is illustrated by inaxaplin, a small molecule that significantly reduces proteinuria in individuals with gain of function variants in the genes encoding two apolipoprotein L1 (APOL1) risk alleles (G1 or G2) [18].
GFR estimation with creatinine and/or cystatin C
While the creatinine-based estimated glomerular filtration rate (eGFRcr) is still suggested as the first approach in adults at risk of CKD, the new guidelines promote the use of the combination of serum creatinine and cystatin C–based eGFR (eGFRcr-cys) when more precision is required. eGFRcr-cys has been proven to be superior for distinguishing eGFR risk stages by the CKD-Prognosis Consortium (CKD-PC) [19], and more accurate against measured GFR in clinical situations of discordance between both filtration markers [20–22]. eGFRcr-cys should at minimum be considered in situations where eGFR from serum creatinine alone may be subject to error (e.g. muscle wasting/loss, special diets, severe illness) and/or when greater accuracy is needed for clinical decision-making (e.g. drugs with a narrow therapeutic index or high toxicity). However, challenges in implementation include access to cystatin C testing, costs and same-day turnaround time. Situations in which cystatin C is less reliable (e.g. high cell turnover in haematology) are still not yet fully documented.
Choice of equation to estimate GFR
The KDIGO 2024 CKD Guideline emphasises that race, which is not a biological variable but a social construct, should not be included as a covariate in an eGFR equation. However, promoting the use of one formula over another at the international level remains delicate and complex. Instead of being prescriptive, the KDIGO 2024 CKD Guideline advocates for the use of the best-fitting validated equation within geographical regions (continents, countries or regions). To that end, they provide a list of validated equations that have undergone rigorous development and validation, and that show adequate accuracy compared with measured GFR (mGFR), with at least 90% of the eGFR values within ±30% (P30) of the mGFR. This leads to a range of choices, with the possibility to use for example equations developed by the Chronic Kidney Disease Epidemiology (CKD-EPI) collaboration or equations developed by the European Kidney Function Consortium (EKFC), or their average—not to mention the additional modifications developed for specific regions.
While we think it is an advancement to embrace diversity, the resulting panorama is complex and challenging for routine care, policy makers and research. In academic discussions there is inevitable comparison of precision between equations, but in truth all these equations are excellent and perform rather well notwithstanding the considerable natural variation of the filtration markers. Clinical decisions to initiate or stop treatments would likely not vary too much by one or another equation, and when precision is needed, we are called to used better filtration markers of mGFR. Most important for the individual patient is using the same equation consistently to monitor their kidney function.
To remove the race coefficient, the USA has immediately adopted the CKD-EPI 2021 equation [23, 24]. This equation, however, has poorer performance compared with the previous version in European populations, particularly amongst whites [23–25]. Imitating our American colleagues and universally implementing the CKD-EPI 2021 in Europe may not be optimal, by introducing less accuracy among our predominantly non-Black population. Moreover, nearly all European countries were not using the race coefficient for the 2009 CKD-EPI equation, and the argument for changing to a less accurate equation to remove an unused coefficient feels unconvincing [26]. In addition, changing equations also affects disease burden and policy making. For example, in a North European health system, the prevalence of CKD stages 3–5 decreased from 5.1% to 3.8% when using this new equation [25].
More recently, the EKFC equations were developed and validated mainly in European individuals and based on creatinine [27], or on cystatin C without considering sex or race [28]. To have a smorgasbord of equations creates the problem of choice, which calls for a consensus across the diversity of European countries. Having European countries use either CKD-EPI 2009 or EFKC may complicate epidemiological studies by impairing comparability and would present a challenge for clinical trials (with recruitment based on different eGFR estimates across countries), and hinder patient follow-up for those moving between countries.
Point of care testing
The KDIGO 2024 CKD Guideline endorses point of care testing (POCT) for creatinine measurements given the convenience associated with this in some settings, and place less value on the diagnostic accuracy. Given the need to improve the rate of detection of CKD in the community, even in Europe where most health systems are relatively robust, POCT may be an important tool to improve the rates of case finding. POCT for urinary albumin-to-creatinine ratio (uACR) may be similarly convenient and would permit more complete CKD staging in appropriately selected individuals [29].
The key practice points from the KDIGO 2024 CKD Guideline are summarized in Table 1. A detailed discussion of the accuracy of POCT devices for creatinine and uACR is beyond the scope of this article, but as discussed in the KDIGO 2024 CKD Guideline, it is important to highlight the potential drawbacks especially in regions where resources may permit more accurate testing. Given the relevant concerns regarding accuracy of the various devices for both creatinine and albuminuria, the potential benefits and harms should be considered and individualized. As with any test it is important that the test is performed when indicated, in patients at risk of CKD. Importantly, a diagnosis of CKD cannot be made on single testing and must be confirmed through nephrology consultation of suspicion of advanced CKD or repeat testing in individuals with higher eGFR and/or lower levels of albuminuria.
| . | KDIGO practice point . | Sensitivity (%) . | Specificity (%) . | Comments . |
|---|---|---|---|---|
| Practice point 1.4.1 | Whenever a POCT device is used for creatinine and urine albumin testing, ensure that the same pre-analytical, analytical and post-analytical quality criteria relating to the specimen collection and performance of the device, including external quality assessment, and the interpretation of the result is used | 73.9 to 86.1 across the 3 best-performing devices for eGFR <30 mL/min/1.73 m2 [29] | 98.9 to 99.2, across the 3 best performing devices for eGFR <30 mL/min/1.73 m2 [29] | Accuracy of the POCT devices has in general been deemed ‘acceptable’ but is variable. The accuracy appears greater at an eGFR <30 mL/min/1.73 m2 but is lower at higher eGFR POCT creatinine can be utilized to rapidly assess eGFR, in some cases generated automatically by the device. The caveat of accuracy must be considered, and the risk of inaccuracy should be carefully considered in the individual patient Benefits and harms must be considered in the contexts of the available resources and the individual patient circumstances Single values for creatinine and albuminuria should not be utilized to diagnose CKD, repeat testing is require for confirmation Cost-effectiveness is largely unknown |
| Practice point 1.4.2 | Where a POCT device for creatinine testing is being used, generate an estimate of GFR. Use the equation consistent with that used within the region | |||
| Practice point 1.4.3 | Where a POCT device is being used for albuminuria testing, the capability of also analysing creatinine and producing an ACR is important. Assess the ability of the POCT ACR devices to produce a positive result in 85% of people with significant albuminuria (ACR ≥30 mg/g or ≥3 mg/mmol), as part of the evaluation and consideration of using the device | Semiquantitative test: 76 (95% CI 63–86); quantitative test: 96 (95% CI 78–99) [28] | For uACR ≥30 mg/g (≥3 mg/mmol): 17.5–99.5 For uACR ≥30 mg/g (≥3 mg/mmol): 30–98.7 For uPCR >200 mg/g (>20 mg/mmol): 80.8–96.9 For uPCR >500 mg/g (>50 mg/mmol) 75.6–95.2 Semiquantitative test: 93 (95% CI 84–97) Quantitative test: 99 (95% CI 93–99) [28] |
| . | KDIGO practice point . | Sensitivity (%) . | Specificity (%) . | Comments . |
|---|---|---|---|---|
| Practice point 1.4.1 | Whenever a POCT device is used for creatinine and urine albumin testing, ensure that the same pre-analytical, analytical and post-analytical quality criteria relating to the specimen collection and performance of the device, including external quality assessment, and the interpretation of the result is used | 73.9 to 86.1 across the 3 best-performing devices for eGFR <30 mL/min/1.73 m2 [29] | 98.9 to 99.2, across the 3 best performing devices for eGFR <30 mL/min/1.73 m2 [29] | Accuracy of the POCT devices has in general been deemed ‘acceptable’ but is variable. The accuracy appears greater at an eGFR <30 mL/min/1.73 m2 but is lower at higher eGFR POCT creatinine can be utilized to rapidly assess eGFR, in some cases generated automatically by the device. The caveat of accuracy must be considered, and the risk of inaccuracy should be carefully considered in the individual patient Benefits and harms must be considered in the contexts of the available resources and the individual patient circumstances Single values for creatinine and albuminuria should not be utilized to diagnose CKD, repeat testing is require for confirmation Cost-effectiveness is largely unknown |
| Practice point 1.4.2 | Where a POCT device for creatinine testing is being used, generate an estimate of GFR. Use the equation consistent with that used within the region | |||
| Practice point 1.4.3 | Where a POCT device is being used for albuminuria testing, the capability of also analysing creatinine and producing an ACR is important. Assess the ability of the POCT ACR devices to produce a positive result in 85% of people with significant albuminuria (ACR ≥30 mg/g or ≥3 mg/mmol), as part of the evaluation and consideration of using the device | Semiquantitative test: 76 (95% CI 63–86); quantitative test: 96 (95% CI 78–99) [28] | For uACR ≥30 mg/g (≥3 mg/mmol): 17.5–99.5 For uACR ≥30 mg/g (≥3 mg/mmol): 30–98.7 For uPCR >200 mg/g (>20 mg/mmol): 80.8–96.9 For uPCR >500 mg/g (>50 mg/mmol) 75.6–95.2 Semiquantitative test: 93 (95% CI 84–97) Quantitative test: 99 (95% CI 93–99) [28] |
CI, confidence intervals.
| . | KDIGO practice point . | Sensitivity (%) . | Specificity (%) . | Comments . |
|---|---|---|---|---|
| Practice point 1.4.1 | Whenever a POCT device is used for creatinine and urine albumin testing, ensure that the same pre-analytical, analytical and post-analytical quality criteria relating to the specimen collection and performance of the device, including external quality assessment, and the interpretation of the result is used | 73.9 to 86.1 across the 3 best-performing devices for eGFR <30 mL/min/1.73 m2 [29] | 98.9 to 99.2, across the 3 best performing devices for eGFR <30 mL/min/1.73 m2 [29] | Accuracy of the POCT devices has in general been deemed ‘acceptable’ but is variable. The accuracy appears greater at an eGFR <30 mL/min/1.73 m2 but is lower at higher eGFR POCT creatinine can be utilized to rapidly assess eGFR, in some cases generated automatically by the device. The caveat of accuracy must be considered, and the risk of inaccuracy should be carefully considered in the individual patient Benefits and harms must be considered in the contexts of the available resources and the individual patient circumstances Single values for creatinine and albuminuria should not be utilized to diagnose CKD, repeat testing is require for confirmation Cost-effectiveness is largely unknown |
| Practice point 1.4.2 | Where a POCT device for creatinine testing is being used, generate an estimate of GFR. Use the equation consistent with that used within the region | |||
| Practice point 1.4.3 | Where a POCT device is being used for albuminuria testing, the capability of also analysing creatinine and producing an ACR is important. Assess the ability of the POCT ACR devices to produce a positive result in 85% of people with significant albuminuria (ACR ≥30 mg/g or ≥3 mg/mmol), as part of the evaluation and consideration of using the device | Semiquantitative test: 76 (95% CI 63–86); quantitative test: 96 (95% CI 78–99) [28] | For uACR ≥30 mg/g (≥3 mg/mmol): 17.5–99.5 For uACR ≥30 mg/g (≥3 mg/mmol): 30–98.7 For uPCR >200 mg/g (>20 mg/mmol): 80.8–96.9 For uPCR >500 mg/g (>50 mg/mmol) 75.6–95.2 Semiquantitative test: 93 (95% CI 84–97) Quantitative test: 99 (95% CI 93–99) [28] |
| . | KDIGO practice point . | Sensitivity (%) . | Specificity (%) . | Comments . |
|---|---|---|---|---|
| Practice point 1.4.1 | Whenever a POCT device is used for creatinine and urine albumin testing, ensure that the same pre-analytical, analytical and post-analytical quality criteria relating to the specimen collection and performance of the device, including external quality assessment, and the interpretation of the result is used | 73.9 to 86.1 across the 3 best-performing devices for eGFR <30 mL/min/1.73 m2 [29] | 98.9 to 99.2, across the 3 best performing devices for eGFR <30 mL/min/1.73 m2 [29] | Accuracy of the POCT devices has in general been deemed ‘acceptable’ but is variable. The accuracy appears greater at an eGFR <30 mL/min/1.73 m2 but is lower at higher eGFR POCT creatinine can be utilized to rapidly assess eGFR, in some cases generated automatically by the device. The caveat of accuracy must be considered, and the risk of inaccuracy should be carefully considered in the individual patient Benefits and harms must be considered in the contexts of the available resources and the individual patient circumstances Single values for creatinine and albuminuria should not be utilized to diagnose CKD, repeat testing is require for confirmation Cost-effectiveness is largely unknown |
| Practice point 1.4.2 | Where a POCT device for creatinine testing is being used, generate an estimate of GFR. Use the equation consistent with that used within the region | |||
| Practice point 1.4.3 | Where a POCT device is being used for albuminuria testing, the capability of also analysing creatinine and producing an ACR is important. Assess the ability of the POCT ACR devices to produce a positive result in 85% of people with significant albuminuria (ACR ≥30 mg/g or ≥3 mg/mmol), as part of the evaluation and consideration of using the device | Semiquantitative test: 76 (95% CI 63–86); quantitative test: 96 (95% CI 78–99) [28] | For uACR ≥30 mg/g (≥3 mg/mmol): 17.5–99.5 For uACR ≥30 mg/g (≥3 mg/mmol): 30–98.7 For uPCR >200 mg/g (>20 mg/mmol): 80.8–96.9 For uPCR >500 mg/g (>50 mg/mmol) 75.6–95.2 Semiquantitative test: 93 (95% CI 84–97) Quantitative test: 99 (95% CI 93–99) [28] |
CI, confidence intervals.
The potential benefits of POCT, as outlined in the KDIGO 2024 CKD Guideline, include the convenience for the patient and the rapidity of obtaining the test result, which permit testing in non-clinical environments (nursing homes, remote areas). For individuals, the convenience may outweigh the concerns about accuracy. In children, the tiny volume of blood required may be an additional advantage, especially if used for follow-up testing once a diagnosis is established. The improved accuracy below an eGFR of 30 mL/min/1.73 m2 improved the value of POCT for those who most require a rapid diagnosis [30].
POCT may, however, be associated with potential harms, including overdiagnosis and underdiagnosis related to the accuracy concerns. These may differ between devices. The tendency of POCT for creatinine to ‘overdiagnose’ CKD stage [30] may be beneficial from a clinical perspective in terms of fewer missed cases, however overdiagnosis may cause unnecessary anxiety in some patients. It is important that clinical guidelines and/or referral pathways are in place to ensure appropriate follow-up and management.
RISK ASSESSMENT IN PEOPLE WITH CKD
A summary of the KDIGO 2024 CKD Guideline recommendation statements and practice points on the risk assessment of people with CKD is presented in Table 2.
Summary of recommendation statements and practice points relevant to risk assessment in people with CKD in the 2024 KDIGO Guideline and differences from the 2012 KDIGO Guideline.
| 2.1 Overview on monitoring for progression of CKD based upon GFR and ACR categories | |
| Practice point 2.1.1: Assess albuminuria in adults, or albuminuria/proteinuria in children, and GFR at least annually in people with CKD | In agreement with 2012 KDIGO CKD Guideline |
| Practice point 2.1.2: Assess albuminuria and GFR more often for individuals at higher risk of CKD progression when measurement will impact therapeutic decisions. | |
| Practice point 2.1.3: For people with CKD, a change in eGFR of >20% on a subsequent test exceeds the expected variability and warrants evaluation | Introducing cutoffs for eGFR change, in general and in patients initiating haemodynamically active therapies, which should prompt further evaluation |
| Practice point 2.1.4: Among people with CKD who initiate haemodynamically active therapies, GFR reductions of >30% on subsequent testing exceed the expected variability and warrant evaluation | |
| Practice point 2.1.5: For albuminuria monitoring of people with CKD, a doubling of the ACR on a subsequent test exceeds laboratory variability and warrants evaluation | Defining a doubling in albuminuria or more as exceeding the expected variability and warranting evaluation |
| 2.2 Risk prediction in people with CKD | |
| Recommendation 2.2.1: In people with CKD G3–G5, we recommend using an externally validated risk equation to estimate the absolute risk of kidney failure (1A) | Highlights the need and potential benefits on the use of validated risk equations to estimate the absolute risk of outcomes for each individual and enable a personalized care plan for people with CKD |
| Practice point 2.2.1: A 5-year kidney failure risk of 3%–5% can be used to determine need for nephrology referral in addition to criteria based on eGFR or urine ACR, and other clinical considerations | |
| Practice point 2.2.2: A 2-year kidney failure risk of >10% can be used to determine the timing of multidisciplinary care in addition to eGFR-based criteria and other clinical considerations | |
| Practice point 2.2.3: A 2-year kidney failure risk threshold of >40% can be used to determine the modality education, timing of preparation for KRT including vascular access planning or referral for transplantation, in addition to eGFR-based criteria and other clinical considerations | |
| Practice point 2.2.4: Note that risk prediction equations developed for use in people with CKD G3–G5, may not be valid for use in those with CKD G1–G2 | |
| Practice point 2.2.5: Use disease-specific, externally validated prediction equations in people with immunoglobulin A nephropathy and autosomal dominant polycystic kidney disease | |
| Practice point 2.2.6: Consider the use of eGFRcys in some specific circumstances | |
| 2.3 Prediction of cardiovascular risk in people with CKD | |
| Practice point 2.3.1: For cardiovascular risk prediction to guide preventive therapies in people with CKD, use externally validated models that are either developed within CKD populations or that incorporate eGFR and albuminuria | Highlighting the potential benefits of the use of cardiovascular and mortality risk equations in the CKD population |
| Practice point 2.3.2: For mortality risk prediction to guide discussions about goals of care, use externally validated models that predict all-cause mortality specifically developed in the CKD population | |
| 2.1 Overview on monitoring for progression of CKD based upon GFR and ACR categories | |
| Practice point 2.1.1: Assess albuminuria in adults, or albuminuria/proteinuria in children, and GFR at least annually in people with CKD | In agreement with 2012 KDIGO CKD Guideline |
| Practice point 2.1.2: Assess albuminuria and GFR more often for individuals at higher risk of CKD progression when measurement will impact therapeutic decisions. | |
| Practice point 2.1.3: For people with CKD, a change in eGFR of >20% on a subsequent test exceeds the expected variability and warrants evaluation | Introducing cutoffs for eGFR change, in general and in patients initiating haemodynamically active therapies, which should prompt further evaluation |
| Practice point 2.1.4: Among people with CKD who initiate haemodynamically active therapies, GFR reductions of >30% on subsequent testing exceed the expected variability and warrant evaluation | |
| Practice point 2.1.5: For albuminuria monitoring of people with CKD, a doubling of the ACR on a subsequent test exceeds laboratory variability and warrants evaluation | Defining a doubling in albuminuria or more as exceeding the expected variability and warranting evaluation |
| 2.2 Risk prediction in people with CKD | |
| Recommendation 2.2.1: In people with CKD G3–G5, we recommend using an externally validated risk equation to estimate the absolute risk of kidney failure (1A) | Highlights the need and potential benefits on the use of validated risk equations to estimate the absolute risk of outcomes for each individual and enable a personalized care plan for people with CKD |
| Practice point 2.2.1: A 5-year kidney failure risk of 3%–5% can be used to determine need for nephrology referral in addition to criteria based on eGFR or urine ACR, and other clinical considerations | |
| Practice point 2.2.2: A 2-year kidney failure risk of >10% can be used to determine the timing of multidisciplinary care in addition to eGFR-based criteria and other clinical considerations | |
| Practice point 2.2.3: A 2-year kidney failure risk threshold of >40% can be used to determine the modality education, timing of preparation for KRT including vascular access planning or referral for transplantation, in addition to eGFR-based criteria and other clinical considerations | |
| Practice point 2.2.4: Note that risk prediction equations developed for use in people with CKD G3–G5, may not be valid for use in those with CKD G1–G2 | |
| Practice point 2.2.5: Use disease-specific, externally validated prediction equations in people with immunoglobulin A nephropathy and autosomal dominant polycystic kidney disease | |
| Practice point 2.2.6: Consider the use of eGFRcys in some specific circumstances | |
| 2.3 Prediction of cardiovascular risk in people with CKD | |
| Practice point 2.3.1: For cardiovascular risk prediction to guide preventive therapies in people with CKD, use externally validated models that are either developed within CKD populations or that incorporate eGFR and albuminuria | Highlighting the potential benefits of the use of cardiovascular and mortality risk equations in the CKD population |
| Practice point 2.3.2: For mortality risk prediction to guide discussions about goals of care, use externally validated models that predict all-cause mortality specifically developed in the CKD population | |
Summary of recommendation statements and practice points relevant to risk assessment in people with CKD in the 2024 KDIGO Guideline and differences from the 2012 KDIGO Guideline.
| 2.1 Overview on monitoring for progression of CKD based upon GFR and ACR categories | |
| Practice point 2.1.1: Assess albuminuria in adults, or albuminuria/proteinuria in children, and GFR at least annually in people with CKD | In agreement with 2012 KDIGO CKD Guideline |
| Practice point 2.1.2: Assess albuminuria and GFR more often for individuals at higher risk of CKD progression when measurement will impact therapeutic decisions. | |
| Practice point 2.1.3: For people with CKD, a change in eGFR of >20% on a subsequent test exceeds the expected variability and warrants evaluation | Introducing cutoffs for eGFR change, in general and in patients initiating haemodynamically active therapies, which should prompt further evaluation |
| Practice point 2.1.4: Among people with CKD who initiate haemodynamically active therapies, GFR reductions of >30% on subsequent testing exceed the expected variability and warrant evaluation | |
| Practice point 2.1.5: For albuminuria monitoring of people with CKD, a doubling of the ACR on a subsequent test exceeds laboratory variability and warrants evaluation | Defining a doubling in albuminuria or more as exceeding the expected variability and warranting evaluation |
| 2.2 Risk prediction in people with CKD | |
| Recommendation 2.2.1: In people with CKD G3–G5, we recommend using an externally validated risk equation to estimate the absolute risk of kidney failure (1A) | Highlights the need and potential benefits on the use of validated risk equations to estimate the absolute risk of outcomes for each individual and enable a personalized care plan for people with CKD |
| Practice point 2.2.1: A 5-year kidney failure risk of 3%–5% can be used to determine need for nephrology referral in addition to criteria based on eGFR or urine ACR, and other clinical considerations | |
| Practice point 2.2.2: A 2-year kidney failure risk of >10% can be used to determine the timing of multidisciplinary care in addition to eGFR-based criteria and other clinical considerations | |
| Practice point 2.2.3: A 2-year kidney failure risk threshold of >40% can be used to determine the modality education, timing of preparation for KRT including vascular access planning or referral for transplantation, in addition to eGFR-based criteria and other clinical considerations | |
| Practice point 2.2.4: Note that risk prediction equations developed for use in people with CKD G3–G5, may not be valid for use in those with CKD G1–G2 | |
| Practice point 2.2.5: Use disease-specific, externally validated prediction equations in people with immunoglobulin A nephropathy and autosomal dominant polycystic kidney disease | |
| Practice point 2.2.6: Consider the use of eGFRcys in some specific circumstances | |
| 2.3 Prediction of cardiovascular risk in people with CKD | |
| Practice point 2.3.1: For cardiovascular risk prediction to guide preventive therapies in people with CKD, use externally validated models that are either developed within CKD populations or that incorporate eGFR and albuminuria | Highlighting the potential benefits of the use of cardiovascular and mortality risk equations in the CKD population |
| Practice point 2.3.2: For mortality risk prediction to guide discussions about goals of care, use externally validated models that predict all-cause mortality specifically developed in the CKD population | |
| 2.1 Overview on monitoring for progression of CKD based upon GFR and ACR categories | |
| Practice point 2.1.1: Assess albuminuria in adults, or albuminuria/proteinuria in children, and GFR at least annually in people with CKD | In agreement with 2012 KDIGO CKD Guideline |
| Practice point 2.1.2: Assess albuminuria and GFR more often for individuals at higher risk of CKD progression when measurement will impact therapeutic decisions. | |
| Practice point 2.1.3: For people with CKD, a change in eGFR of >20% on a subsequent test exceeds the expected variability and warrants evaluation | Introducing cutoffs for eGFR change, in general and in patients initiating haemodynamically active therapies, which should prompt further evaluation |
| Practice point 2.1.4: Among people with CKD who initiate haemodynamically active therapies, GFR reductions of >30% on subsequent testing exceed the expected variability and warrant evaluation | |
| Practice point 2.1.5: For albuminuria monitoring of people with CKD, a doubling of the ACR on a subsequent test exceeds laboratory variability and warrants evaluation | Defining a doubling in albuminuria or more as exceeding the expected variability and warranting evaluation |
| 2.2 Risk prediction in people with CKD | |
| Recommendation 2.2.1: In people with CKD G3–G5, we recommend using an externally validated risk equation to estimate the absolute risk of kidney failure (1A) | Highlights the need and potential benefits on the use of validated risk equations to estimate the absolute risk of outcomes for each individual and enable a personalized care plan for people with CKD |
| Practice point 2.2.1: A 5-year kidney failure risk of 3%–5% can be used to determine need for nephrology referral in addition to criteria based on eGFR or urine ACR, and other clinical considerations | |
| Practice point 2.2.2: A 2-year kidney failure risk of >10% can be used to determine the timing of multidisciplinary care in addition to eGFR-based criteria and other clinical considerations | |
| Practice point 2.2.3: A 2-year kidney failure risk threshold of >40% can be used to determine the modality education, timing of preparation for KRT including vascular access planning or referral for transplantation, in addition to eGFR-based criteria and other clinical considerations | |
| Practice point 2.2.4: Note that risk prediction equations developed for use in people with CKD G3–G5, may not be valid for use in those with CKD G1–G2 | |
| Practice point 2.2.5: Use disease-specific, externally validated prediction equations in people with immunoglobulin A nephropathy and autosomal dominant polycystic kidney disease | |
| Practice point 2.2.6: Consider the use of eGFRcys in some specific circumstances | |
| 2.3 Prediction of cardiovascular risk in people with CKD | |
| Practice point 2.3.1: For cardiovascular risk prediction to guide preventive therapies in people with CKD, use externally validated models that are either developed within CKD populations or that incorporate eGFR and albuminuria | Highlighting the potential benefits of the use of cardiovascular and mortality risk equations in the CKD population |
| Practice point 2.3.2: For mortality risk prediction to guide discussions about goals of care, use externally validated models that predict all-cause mortality specifically developed in the CKD population | |
Monitoring of CKD
Monitoring eGFR and albuminuria in both adults and children with CKD is important not only to update staging for prognosis but also to guide clinical decisions. Because the KDIGO CKD staging classification based on eGFR and albuminuria is based on future kidney failure risk, their recommendation is to monitor those at higher risk of progression more frequently (e.g. they suggest monitoring kidney function and albuminuria once annually to people with CKD G1A2, but thrice annually to people with CKD G1A3, and consider even increasing this depending on the underlying aetiology of CKD). While the recommendation makes sense, the challenge lies in its implementation. European healthcare systems, as in other parts of the world, are affected by a severe under-recognition of CKD [5, 31–34]. While creatinine is measured in healthcare for many indications (and in most European healthcare systems eGFR is automatically reported), albuminuria screening and monitoring is still the bottleneck [35]. For example, in a North European evaluation of processes of CKD care, only about a third of patients with incident CKD stages 3–5 received albuminuria monitoring within the following 18 months [36]. Strategies to improve monitoring rates could involve educational campaigns to primary care and general physicians as well as individuals at high risk of CKD. Indeed, the KDIGO 2024 CKD Guideline helpfully provides a list of at-risk individuals (Table 3). Economic incentives have been successfully shown in the UK to improve monitoring rates [37]. In settings with less access to laboratory services, utilisation of novel and accurate POCT tools could also be potentially useful.
Externally validated risk equations for predicting kidney failure in patients with CKD G3–G5.
| Equation . | Variables . | Population . | Outcome . | Discrimination . | Website . |
|---|---|---|---|---|---|
| KFRE [41] | Age, sex, eGFR, ACR for 4-variable equation + calcium, phosphate, bicarbonate, albumin for 8-variable equation | >1 million patients with >10 000 events from more than 30 countries | Treated kidney failure at 2 and 5 years | 0.88–0.91 | www.kidneyfailurerisk.com; www.ckdpc.org/risk-models.html |
| KPNW [42] | Age, sex, eGFR, albuminuria, systolic BP, antihypertensive use, diabetes, diabetic complications | 39 103 patients with 1097 events from the Kaiser Permanente Health System (USA) | Kidney failure at 5 years | 0.95 | |
| Landray et al. [43] | Sex, SCr, albuminuria, phosphate | 595 patients from the CRIB and East Kent cohorts (UK) | Kidney failure | 0.91 | |
| Z6 score [44] | SCr, albumin, cystatin C, urea, haemoglobin, uACR | 7978 patients with 870 events from the German CKD study and validated in 3 additional European cohorts | Kidney failure at 5 years | 0.89–0.92 | |
| KDpredict [45] | Age, sex, eGFR, uACR/uPCR, for 4-variable equation + diabetes, cardiovascular disease for 6-variable model | 67 492 patients from Alberta Canada and validated in 17 528 patients from Denmark and 7740 patients from Scotland | Kidney failure at 2 and 5 years (and all-cause death) | 0.88–0.94 | http://kdpredict.com |
| Equation . | Variables . | Population . | Outcome . | Discrimination . | Website . |
|---|---|---|---|---|---|
| KFRE [41] | Age, sex, eGFR, ACR for 4-variable equation + calcium, phosphate, bicarbonate, albumin for 8-variable equation | >1 million patients with >10 000 events from more than 30 countries | Treated kidney failure at 2 and 5 years | 0.88–0.91 | www.kidneyfailurerisk.com; www.ckdpc.org/risk-models.html |
| KPNW [42] | Age, sex, eGFR, albuminuria, systolic BP, antihypertensive use, diabetes, diabetic complications | 39 103 patients with 1097 events from the Kaiser Permanente Health System (USA) | Kidney failure at 5 years | 0.95 | |
| Landray et al. [43] | Sex, SCr, albuminuria, phosphate | 595 patients from the CRIB and East Kent cohorts (UK) | Kidney failure | 0.91 | |
| Z6 score [44] | SCr, albumin, cystatin C, urea, haemoglobin, uACR | 7978 patients with 870 events from the German CKD study and validated in 3 additional European cohorts | Kidney failure at 5 years | 0.89–0.92 | |
| KDpredict [45] | Age, sex, eGFR, uACR/uPCR, for 4-variable equation + diabetes, cardiovascular disease for 6-variable model | 67 492 patients from Alberta Canada and validated in 17 528 patients from Denmark and 7740 patients from Scotland | Kidney failure at 2 and 5 years (and all-cause death) | 0.88–0.94 | http://kdpredict.com |
BP, blood pressure; CRIB, Chronic Renal Impairment in Birmingham; KPMW, Kaiser Permanente Northwest; SCr, serum creatinine.
Externally validated risk equations for predicting kidney failure in patients with CKD G3–G5.
| Equation . | Variables . | Population . | Outcome . | Discrimination . | Website . |
|---|---|---|---|---|---|
| KFRE [41] | Age, sex, eGFR, ACR for 4-variable equation + calcium, phosphate, bicarbonate, albumin for 8-variable equation | >1 million patients with >10 000 events from more than 30 countries | Treated kidney failure at 2 and 5 years | 0.88–0.91 | www.kidneyfailurerisk.com; www.ckdpc.org/risk-models.html |
| KPNW [42] | Age, sex, eGFR, albuminuria, systolic BP, antihypertensive use, diabetes, diabetic complications | 39 103 patients with 1097 events from the Kaiser Permanente Health System (USA) | Kidney failure at 5 years | 0.95 | |
| Landray et al. [43] | Sex, SCr, albuminuria, phosphate | 595 patients from the CRIB and East Kent cohorts (UK) | Kidney failure | 0.91 | |
| Z6 score [44] | SCr, albumin, cystatin C, urea, haemoglobin, uACR | 7978 patients with 870 events from the German CKD study and validated in 3 additional European cohorts | Kidney failure at 5 years | 0.89–0.92 | |
| KDpredict [45] | Age, sex, eGFR, uACR/uPCR, for 4-variable equation + diabetes, cardiovascular disease for 6-variable model | 67 492 patients from Alberta Canada and validated in 17 528 patients from Denmark and 7740 patients from Scotland | Kidney failure at 2 and 5 years (and all-cause death) | 0.88–0.94 | http://kdpredict.com |
| Equation . | Variables . | Population . | Outcome . | Discrimination . | Website . |
|---|---|---|---|---|---|
| KFRE [41] | Age, sex, eGFR, ACR for 4-variable equation + calcium, phosphate, bicarbonate, albumin for 8-variable equation | >1 million patients with >10 000 events from more than 30 countries | Treated kidney failure at 2 and 5 years | 0.88–0.91 | www.kidneyfailurerisk.com; www.ckdpc.org/risk-models.html |
| KPNW [42] | Age, sex, eGFR, albuminuria, systolic BP, antihypertensive use, diabetes, diabetic complications | 39 103 patients with 1097 events from the Kaiser Permanente Health System (USA) | Kidney failure at 5 years | 0.95 | |
| Landray et al. [43] | Sex, SCr, albuminuria, phosphate | 595 patients from the CRIB and East Kent cohorts (UK) | Kidney failure | 0.91 | |
| Z6 score [44] | SCr, albumin, cystatin C, urea, haemoglobin, uACR | 7978 patients with 870 events from the German CKD study and validated in 3 additional European cohorts | Kidney failure at 5 years | 0.89–0.92 | |
| KDpredict [45] | Age, sex, eGFR, uACR/uPCR, for 4-variable equation + diabetes, cardiovascular disease for 6-variable model | 67 492 patients from Alberta Canada and validated in 17 528 patients from Denmark and 7740 patients from Scotland | Kidney failure at 2 and 5 years (and all-cause death) | 0.88–0.94 | http://kdpredict.com |
BP, blood pressure; CRIB, Chronic Renal Impairment in Birmingham; KPMW, Kaiser Permanente Northwest; SCr, serum creatinine.
Defining CKD progression
Classically, rapid CKD progression has been defined as a loss of >5 mL/min/year. The KDIGO 2024 CKD Guideline avoids defining any thresholds, arguing that any worsening could reflect deteriorating kidney health. Instead, advice is provided to better appreciate the intraindividual random biological variation in eGFR and albuminuria: a change in eGFR of >20% or a doubling of uACR on a subsequent test exceeds the expected variability and warrants evaluation. While this definition focuses more broadly on how to interpret the changes in kidney function during patient monitoring, we may miss the denominator of time. Without considering the time between both subsequent tests, we do not have an impression on the speed of this decline.
The initiation of haemodynamically active therapies, such as angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, non-steroidal mineralocorticoid receptor antagonists (nsMRA), GLP-1ra and sodium-glucose cotransporter 2 inhibitors (SGLT2i) results in transient short-term ‘dips’ in eGFR of a magnitude of 10%–30%. The KDIGO 2024 CKD Guideline recommends review of underlying causes and close monitoring in patients with eGFR reductions of >30% during the first 3 months of therapy with these agents. However, providers should avoid the urge to stop these kidney-protective agents, particularly since these earlier ‘dips’ are typically reversible and not an indication of drug toxicity [38, 39], especially as the potential clinical benefits are considerable [40].
Risk scores
A significant innovation of the KDIGO 2024 CKD Guideline refers to the introduction of risk scores for personalization of care. While the traditional KDIGO staging heat map illustrates risks at a population level, the true individual risk of a given patient is determined not only by his/her eGFR or albuminuria, but also the underlying aetiology of CKD, demographic characteristics, comorbid conditions (especially cardiovascular disease) and other factors, including lifestyle, socioeconomic status, nutritional status and intercurrent events. Individual risk prediction in nephrology using accurate and externally validated risk equations have the potential to help inform key clinical decisions and improve the patient–healthcare provider dialogue.
We reflect that the panorama of risk scores in nephrology is changing rapidly. The KDIGO 2024 CKD Guideline mentions four externally validated risk scores for kidney failure [41–44], but since the publication of the guidelines new ones have emerged [45] (Table 3). Academic discussions may revolve around small improvements in prediction of one score versus another. From a clinical point of view all of them tend to offer excellent precision, and differences in performance must be evaluated within the context of the alternative, which frequently is not to use any. After all, features like old age, hypertension, low eGFR and albuminuria, which are common to all risk scores, largely capture the patient’s future kidney risks. Amongst available validated equations, the Kidney Failure Risk Equation (KFRE) has so far had the largest penetration in routine care: many North American health systems [46, 47] and the National Health Service in the UK [48] have integrated KFRE reporting in electronic medical records. However, we are not aware of these tools been used much in the routine care practice of other European countries yet, and we hope that this guideline statement contributes to a paradigm change.
When attempting implementation, two things should be kept in mind. First, validation and recalibration of risk scores is ideally necessary to better adapt to each country/region/setting's background risks. Thus, we encourage this type of research across European countries to ensure best predictive performance. However, as for choosing which eGFR equation to use, it makes sense to ensure some uniformity across Europe to facilitate epidemiological research, trials and public health planning. Second, risk equations are developed within a specific population (e.g. CKD stages 4–5) and for predicting a specific outcome (e.g. kidney replacement therapy; KRT). Applying these scores in other populations (e.g. CKD stages 1–2) or outcomes (e.g. referral to nephrologist care) should be avoided. Although an obvious statement, automatic reporting in health systems without consideration of this detail may provide incorrect risk estimates and prompt incorrect clinical decisions.
Risk scores are meant as an aid to support clinical decisions and patient discussions, but not as a substitute for clinical judgement. The 2024 KDIGO CKD Guideline provides a series of practice points suggesting KFRE thresholds to motivate decisions like referral to nephrologist or interprofessional care, or vascular access planning. Although well intended, these thresholds are suggestions that require validation. In a Swedish validation study, the utility of using KFRE >40% and KFRE >50% for vascular access planning was considerably higher compared with the more traditionally used eGFR threshold <15 mL/min/1.73 m2 [49]. More work along these lines may increase our confidence and experience in integrating risk scores in our European routine clinical practice. However, at present the evidence that the use of risk-based equations leads to better outcomes or patient experience is lacking and further studies into this are required. The 2024 KDIGO CKD Guideline ends this section encouraging us to use also cardiovascular disease risk prediction scores to help inform cardiovascular prognostication and further communication with cardiologists.
OPTIMAL MODELS OF CARE
The components of an optimal care model for patients with CKD includes timely referral and evaluation of such patients by a specialized healthcare team, recognition and addressing of CKD-related signs and symptoms promptly, improving access to healthcare systems, optimizing best available therapeutic options and providing supportive care. The KDIGO 2024 CKD Guideline is significant in pointing out the need for special attention to optimize models of care based on assessment of risk and local contexts/work force capacity. Early referral is most important as when followed by appropriate management it leads to slower progression of CKD, better blood pressure control, higher likelihood of receiving permanent vascular access before KRT, pre-emptive transplantation, fewer healthcare-related costs, shorter hospital stays and lower mortality rates [50, 51]. Physicians should focus more on underrecognized uraemia-related symptoms of CKD which may impact quality of life and exacerbate morbidity, including bone and/or joint pain, restricted mobility, fatigue, poor sleep quality, pruritus, sexual dysfunction and decreased appetite including screening tools for malnutrition. Although nephrologists are at the centre of specialized care for such patients, multidisciplinary care teams consisting of nurses, social workers, pharmacists, nutritional specialists, endocrinology and cardiology specialists, and psychologists have led to superior outcomes [52, 53].
SPECIAL CONSIDERATIONS BASED ON ADVANCED AGE AND REPRODUCTIVE EXPECTATIONS
In a separate section, in contrast to the 2012 guidelines, the 2024 CKD Guideline discusses several special populations which must be considered, especially those at advanced age [10]. This reflects the important challenges in managing advanced CKD in this demographic (Fig. 1). The evaluation of eGFR may be misleading as creatinine-based equations may be inaccurate due to sarcopenia [54] and quality of life may be prioritized over survival advantage [55], therefore appropriate diagnosis and shared decision-making are key.
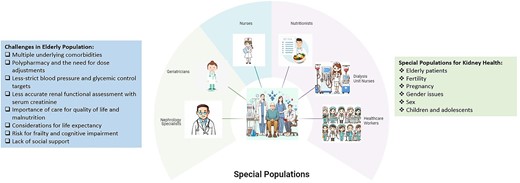
The special populations in terms of kidney health with important clinical considerations.
The deleterious effects of CKD on reproductive health and pregnancy outcomes are well-established [56], with a clear need for discussion with individuals considering pregnancy. Although the KDIGO 2024 CKD Guideline has taken a step forward to identify multiple special populations potentially differing in terms of CKD management, it is important to emphasize that the current knowledge of optimal approaches to care in these specific patient groups is limited and evidence-based recommendations are weak, highlighting the definitive need for future studies.
CKD IN MEN AND WOMEN
Although it is becoming increasingly clear that CKD is more common among women than men, at least in part to their longer life expectancy [57, 58], there still appear to be major differences in terms of detection, monitoring, referral and therapy-related adverse event rates [36]. In a North European study, women with newly detected CKD were less likely to be monitored for their kidney function, receive a clinical diagnosis, be referred to nephrologist care or initiate antiproteinuric treatment compared with equal men [36]. Such differences are worrisome and should be further investigated (and if present corrected) in other countries.
SPECIAL CONSIDERATIONS IN CHILDREN AND YOUNG ADULTS
The KDIGO 2024 CKD Guideline provides an evidence-informed approach to the evaluation, prevention, treatment and holistic care of children and young adults with CKD, promoting individualized care across the lifespan. The underlying aetiology of CKD, CKD trajectories, CKD-related complications and disease burden, as well as the care delivery differs between childhood and adult-onset CKD. Paediatric recommendations are embedded throughout the guideline. As in adults, here we summarize the main recommendations in the evaluation and risk assessment in children and young adults with CKD. In general, the same considerations as in adults apply to children in terms of the European context.
Estimating GFR
A validated equation such as the CKD-EPI, or the Chronic Kidney Disease in Children under 25 (CKiD U25)] equation [59], should be used to estimate GFR. This latter equation was developed and validated in paediatric and young adult CKD populations, including white, Hispanic and Black people, and in European populations. Another commonly used paediatric eGFR equation, the EKFC equation [27], was developed in a European cohort, and has the advantage of not requiring height measurements, but included only a small proportion of children with CKD. The validated equations are all based on a combination of creatinine and/or cystatin C.
In children with low muscle mass (such as those with metabolic disorders, neuromuscular diseases or severe malnutrition), a cystatin C–based eGFR estimating equation should be used as the creatinine-based equations may give falsely high values.
What is a normal GFR
Although nephrogenesis is completed by 36 weeks of intrauterine life, kidney function increases through infancy to early childhood. Age- and gestation-appropriate normal ranges for eGFR must be used in children under 2 years of age [60].
A new recommendation has been added to the guideline which emphasizes that children and adolescents should have excellent kidney function. It states that an eGFR below 90 mL/min/1.73 m2 can be flagged as ‘low’ in children and adolescents over the age of 2 years. It is based on a study showing the trajectory of eGFR decline [61], and stresses the need for careful assessment and treatment to attenuate the progression of CKD across the lifespan. This is a key point that cannot be overemphasized.
Monitoring GFR
Frequent monitoring of kidney function is required during periods of rapid growth in puberty, as kidney function may decline rapidly in those with low eGFR [62]. A trajectory of preserved eGFR or even hyperfiltration is common during childhood because of excellent renal reserves, but these reserves may be exhausted during adolescence or young adulthood.
Testing for albuminuria/proteinuria
Testing for both urinary protein-to-creatinine ratio (uPCR) and uACR in initial screening is recommended for children, because children are more likely to have tubular proteinuria (e.g. β2-microglobulin). For subsequent screening, the most appropriate test can be used, keeping in mind that uPCR varies with age and body size [63].
Diagnosing the cause of CKD
Children and young people with kidney failure, especially those from consanguineous families, are more likely to have a genetic cause of their kidney disease than adults. Where genetic testing is feasible a kidney biopsy may be avoided in some circumstances.
In summary, children with CKD are a ‘rare disease’ cohort. High-quality observational and clinical trial data remain scarce, with management decisions often extrapolated from adult data. Trials in childhood CKD cohorts are essential, including clinical trials of the new medications slowing CKD progression such as the SGLT2i, GLP-1ra and nsMRA.
CONCLUDING REMARKS
It is now over 12 years since the launch of the KDIGO 2012 CKD Guideline and so the KDIGO 2024 CKD Guideline is a very welcome authoritative update. However, despite the timescale involved it is regrettable that awareness, diagnosis and coding of CKD remains very poor across European countries and globally. Reasons for this lack of awareness of the need for CKD diagnosis and monitoring, despite estimation of GFR and uACR being enshrined not only in all CKD guidelines but also in multiple other guidelines including those for diabetes [64, 65], hypertension [66, 67] and cardiovascular disease [68] for many years, are not really understood. However, it is likely that multiple barriers in combination conspire to maintain the current situation [69]. We live in an era where we now have multiple treatments that can reduce the progression of CKD and modify the risk of the most important associated risk of cardiovascular morbidity and mortality. The importance of early and accurate identification of individuals with CKD is now more important than ever.
This apparent lack of awareness within healthcare systems of the importance of CKD needs to be tackled as an urgent health priority across Europe. At the same time as addressing the lack of awareness of CKD amongst healthcare practitioners in general, it would seem sensible that people with concerns about their kidneys for whatever reason, and especially those considered at risk of CKD, should be empowered to seek clinical advice and be tested for kidney health. The KDIGO 2024 CKD Guideline helpfully provides specific guidance on this (Table 3). Overall, the KDIGO 2024 CKD Guideline should be seen as much more than just another document. It should be seen as a call to action across Europe to improve the prevention, detection and treatment of CKD. The guidelines are much more than just words.
ACKNOWLEDGEMENTS
European Renal Best Practice (ERBP) is an official body of the European Renal Association (ERA).
FUNDING
No specific funding was received for the writing of this article.
AUTHORS’ CONTRIBUTIONS
All authors have contributed sections of the manuscript and critically reviewed and approved the whole manuscript before submission.
DATA AVAILABILITY STATEMENT
Not applicable.
CONFLICT OF INTEREST STATEMENT
C.J.F. has received speaker and consulting fees from Bayer and CSL Vifor. J.J.C. reports funding to Karolinska Institutet by AstraZeneca, Astellas, Vifor Pharma, MSD, Boehringer Ingelheim and NovoNordisk; and speaker and consulting fees from Fresenius Kabi and AstraZeneca. V.L. has received royalties as editor of The Kidney.





Comments