-
PDF
- Split View
-
Views
-
Cite
Cite
Paris J Baptiste, Angel Y S Wong, Anna Schultze, Catherine M Clase, Clémence Leyrat, Elizabeth Williamson, Emma Powell, Johannes F E Mann, Marianne Cunnington, Koon Teo, Kevin Wing, Laurie Tomlinson, #530 Cardiorenal effects of dual blockade with ACE inhibitors and ARBs among people with CKD: emulation of a reference trial (ONTARGET) using CPRD, Nephrology Dialysis Transplantation, Volume 39, Issue Supplement_1, May 2024, gfae069–0562–530, https://doi.org/10.1093/ndt/gfae069.562
Close - Share Icon Share
Abstract
Cardiovascular disease (CVD) is a leading cause of death globally, and individuals with chronic kidney disease (CKD) are at increased risk. Results from the ONTARGET trial, in conjunction with the ALTITUDE and VA-Nephron-D studies, led to an end of recommendations for dual ACE inhibitor and ARB therapy due to an increase in acute kidney injury. However, these studies had low power to address long-term kidney outcomes and there remains uncertainty about whether dual therapy could be effective at reducing adverse cardiovascular and renal outcomes in patients with CKD. Observational data provides an opportunity to explore such hypotheses in subgroups underrepresented in trials and with power and follow-up enabling estimation of risk of rare outcomes. We aimed to use ONTARGET to perform a reference trial emulation analysis, before extending analysis to explore treatment effectiveness of dual ARB and ACEi use vs ACEi alone in preventing cardiovascular and renal outcomes among those with CKD.
Using routinely-collected data from the UK Clinical Practice Research Datalink (CPRD) Aurum linked with Hospital Episode Statistics secondary care data, we applied the ONTARGET trial eligibility criteria to patients prescribed an ARB/ACE inhibitor between 1/1/2001-31/7/2019. As the number of patients receiving prescriptions for both medications on the same day was likely to be small in routine care, we used an operational definition to capture dual users. Outcomes included ONTARGET primary cardiovascular composite outcome of cardiovascular-related death, myocardial infarction, stroke, or hospitalisation for heart failure and a composite renal outcome of ≥50% reduction in GFR or end-stage kidney disease (ESKD). Within the trial-eligible cohort, outcomes of interest were compared between groups prescribed dual therapy vs ACE inhibitors alone using a propensity-score—weighted time-to-event analysis using a Cox proportional hazards model. Conditional on successfully benchmarking results against the ONTARGET trial, we explored treatment effect heterogeneity by chronic kidney disease (CKD) at baseline, with CKD defined as estimated glomerular rate (eGFR) < 60 ml/min/1.73 m2.
412,406 trial-eligible patients in CPRD were included in analysis. Among those with non-missing eGFR at baseline, 37% had CKD (Table 1). We found similar effectiveness of dual therapy and ACE inhibitors in reducing the risk of the primary composite cardiovascular outcome (HR 0.98 (95% CI: 0.93, 1.03), consistent with the ONTARGET trial results (HR 0.99 (95% CI: 0.92, 1.07), with no evidence of heterogeneity by CKD (P-value for interaction = 0.14). However, dual therapy use was associated with a greater risk for the composite renal outcome compared to ACE inhibitor, HR 1.24 (95% CI: 1.14, 1.35), with no evidence of heterogeneity by CKD (P = 0.93) (Fig. 1). Analysing components of the composite renal outcome separately gave consistent results (≥50% reduction in GFR: HR 1.22 (95% CI: 1.12, 1.33); ESKD: HR 1.34 (95% CI: 1.24, 1.57)), as did a post-hoc sensitivity analysis by proteinuria status (no proteinuria: HR 1.28 (95% CI: 1.03, 1.59); proteinuria: 1.27 (95% CI: 1.08, 1.49)).
We found evidence that dual therapy use was associated with increased risk of renal outcomes in both those with and without CKD at baseline. Applying a reference trial emulation approach and successfully benchmarking findings against ONTARGET, where confounding was not present due to randomisation, provides confidence in the validity of these results.
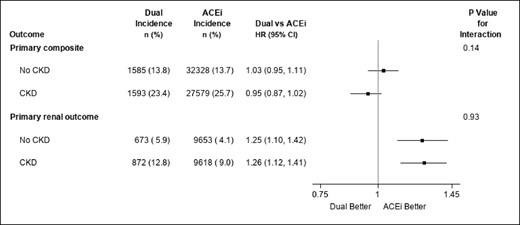
Forest plot of test for treatment effect heterogeneity by CKD status at baseline. Primary composite outcome: cardiovascular-related death, myocardial infarction, stroke, or hospitalisation for heart failure. Primary renal outcome: 50% reduction in GFR or start of kidney replacement therapy or development of GFR<15ml/min/1.73 m2. Dual therapy users are patients with overlapping prescriptions for ARB/ACE inhibitor who received a subsequent prescription for the 1st agent within 90 days of the 2nd prescription for the 2nd agent. Follow-up started from the date this condition was met.
- angiotensin-converting enzyme inhibitors
- myocardial infarction
- proteinuria
- cardiovascular diseases
- cerebrovascular accident
- ischemic stroke
- angiotensin receptor antagonists
- kidney failure, chronic
- heart failure
- renal failure, acute
- glomerular filtration rate
- heterogeneity
- altitude
- benchmarking
- cardiovascular system
- cause of death
- follow-up
- kidney glomerulus
- nephrons
- renal replacement therapy
- kidney
- risk reduction
- propensity score method
- sensitivity analysis
- ontarget trial
- secondary care
- treatment effectiveness
- composite outcomes
- underrepresented groups
- treatment effect heterogeneity






Comments