-
PDF
- Split View
-
Views
-
Cite
Cite
Marja Puurunen, Caroline Kurtz, Alistair Wheeler, Kyra Mulder, Kyle Wood, Anna Swenson, Gary Curhan, Twenty-four-hour urine oxalate and risk of chronic kidney disease, Nephrology Dialysis Transplantation, Volume 39, Issue 5, May 2024, Pages 788–794, https://doi.org/10.1093/ndt/gfad221
Close - Share Icon Share
ABSTRACT
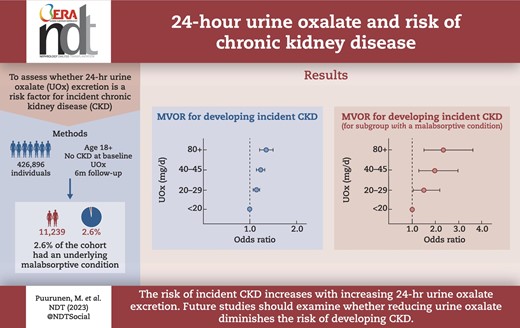
To assess whether 24-h urine oxalate (UOx) excretion is a risk factor for incident chronic kidney disease (CKD).
This longitudinal observational USA-based study included 426 896 individuals aged ≥18 years with no CKD at baseline and with at least one UOx, and at least 6 months of baseline and 6 months of follow-up data. Of these, 11 239 (2.6%) had an underlying malabsorptive condition. Incident CKD, defined by relevant International Classification of Diseases codes, was identified from a multi-source data cloud containing individual-level healthcare claims and electronic medical records data. The association between categories of UOx and incident CKD was modeled using logistic regression adjusting for age, sex, race, body mass index, baseline urine calcium, urine citrate, urine volume, tobacco use, hypertension, diabetes, malabsorption and cardiovascular disease.
Mean follow-up time was 38.9 months (standard deviation 21.7). Compared with individuals with UOx <20 mg/24 h, the odds of developing incident CKD increased for UOx 20–29 mg/24 h [multivariable-adjusted odds ratio (MVOR) 1.14 (95% CI 1.07, 1.21)] through 80+ mg/24 h [MVOR 1.35 (1.21, 1.50)] and was statistically significant for each UOx category. A similar pattern was seen in the subgroup with a malabsorptive condition though the magnitudes of association were larger, with the odds of developing incident CKD increased for UOx 20–29 mg/24 h [MVOR 1.50 (1.03, 2.20)] through 80+ mg/24 h [MVOR 2.34 (1.50, 3.63)] as compared with UOx <20 mg/24 h.
The risk of incident CKD increases with increasing 24-h UOx excretion. Future studies should examine whether reducing UOx diminishes the risk of developing CKD.
What was known:
Twenty-four-hour urine oxalate (UOx) excretion has been associated with progression of chronic kidney disease (CKD), but it is unknown whether UOx is associated with higher risk of incident CKD.
This study adds:
In this longitudinal observational USA-based cohort study of 447 958 individuals aged ≥18 years with at least one 24-h urine collection without a history of CKD, we found that higher UOx excretion was significantly associated with up to a 35% higher risk of incident CKD, and up to 134% higher in those with a malabsorptive condition.
Potential impact:
Given the substantial morbidity and mortality associated with CKD, it may be clinically helpful to measure and make recommendations to reduce UOx excretion, particularly in individuals with a malabsorptive condition.
INTRODUCTION
Chronic kidney disease (CKD) affects 37 million adults in the USA [1] and >700 million worldwide [2]. The prevalence of CKD is rising due to the aging of the general population and the increasing prevalence of the most common causes of CKD: diabetes mellitus and hypertension. Individuals with CKD are at higher risk for end-stage kidney disease (ESKD), cardiovascular events, mortality and decreased quality of life [3]. Years of life lost to CKD are expected to double globally by the year 2040, due in part to the aging population [4]. Treatment of common risk factors for CKD can reduce the risk of developing CKD [5]. Treatment of other metabolic factors, such as acid–base status, may also reduce the risk of CKD [6].
Renal damage from oxalate has been well-described in primary hyperoxaluria (an autosomal recessive disorder) [7] and in malabsorptive conditions, often referred to as enteric hyperoxaluria [8]. In addition to these high-risk conditions, urine oxalate (UOx) is increasingly being recognized as a potential important contributor in the pathogenesis of more common forms of acute kidney injury and CKD [9–13]. UOx is derived from two sources: endogenous production from the metabolism of precursor molecules in the liver and gastrointestinal absorption of dietary oxalate. UOx levels reflect a combination of dietary intake, gut absorption and secretion, endogenous production, and fecal excretion of oxalate. High UOx due to increased intestinal absorption of dietary oxalate may result from low calcium intake, inflammatory bowel disease, malabsorptive conditions and gut microbiome dysbiosis [14]. Higher UOx levels can be toxic to the kidney through a variety of mechanisms, including tubular obstruction from calcium oxalate crystal deposition, inflammation and tubular epithelial cell damage [3].
Among individuals with existing CKD, Waikar et al. found that higher UOx excretion was independently associated with an increased risk of CKD progression and ESKD [15]. However, there are no published studies of the association of UOx and incident CKD. Therefore, to fill this gap in knowledge, we examined the association between 24-h UOx and incident CKD in a large general population and also among those with a malabsorptive condition.
MATERIALS AND METHODS
Study population
This was a longitudinal, retrospectively assembled cohort study of patients in the USA who completed at least one 24-h urine collection as part of routine clinical care, analyzed by LithoLink Corporation (Labcorp, Chicago, IL, USA) between January 2013 through December 2020, and who could be linked to the OM1 Real-World Data Cloud (OM1, Inc., Boston, MA, USA). Patients with at least one 24-h UOx value during the study period, who had at least 6 months of clinical data [electronic medical record (EMR) or claims data] before and after the index date, and who were at least 18 years of age on the index date were included in the study. The index date was the date of the first 24-h UOx test during the study period. There were no exclusion criteria for this real-world study. A subgroup of patients who had evidence of a malabsorptive condition (MC) was analyzed separately. Malabsorptive conditions were defined by relevant International Classification of Diseases 9th or 10th Revision (ICD-9/10) diagnostic or Current Procedural Terminology (CPT) procedure codes for enteric disorders associated with malabsorption (Supplementary data, Table S1). The index date for patients in the MC group was the date of the first 24-h UOx test on or after their first recorded diagnosis of a malabsorptive disorder in the OM1 Real-World Data Cloud. These patients were also included in the overall cohort with an earlier index date if they had a malabsorptive condition diagnosed after their first available 24-h urine test.
Data source
This study was conducted using the OM1 Real-World Data Cloud. This dataset is derived from deterministically linked, de-identified, individual-level healthcare claims, EMR and other data from January 2013 to the present. The EMR data are from sources geographically representative of the US population and include diagnoses documented by a healthcare provider. The medical claims contain billing and coding history on inpatient and outpatient encounters from acute care facilities, ambulatory surgery centers and clinics. De-identified 24-h urine laboratory test results were provided by LithoLink and deterministically linked to the OM1 Real-World Data cloud. The dataset used for this study was fully de-identified. A description of the dataset was submitted to an Institutional Review Board for approval and was determined to be exempt.
Exposure assessment
UOx was measured using the oxalate oxidase method. For patients with more than one 24-h urine collection, only the first eligible UOx value was used. In the analysis, UOx was examined as a continuous variable and also in clinically relevant categories. Hyperoxaluria was defined as UOx ≥45 mg/24 h. In a sensitivity analysis, hyperoxaluria was defined as UOx ≥40 mg/24 h.
Outcome assessment
The primary outcome of interest was incident CKD. CKD was defined using ICD-9/10 diagnosis codes and CPT procedure codes (Supplementary data, Table S1). Incident CKD was defined as a new diagnosis of CKD in a patient who did not have a CKD code prior to or on the index date. Values for estimated glomerular filtration rate (eGFR) were not available for most patients in the dataset so CKD diagnosis was based on CKD diagnosis codes in the patient record.
Analytic approach
Demographic and baseline characteristics were described overall and for the MC group. Continuous variables were reported as mean [standard deviation (SD)] or median (interquartile range). Because the diagnosis of CKD was made in the real-world setting, we used logistic regression rather than time to event approaches to model the association between index UOx and incident CKD. Index UOx was modeled as a categorical variable as follows: <20 (reference group), 20–29, 30–39, 40–44, 45–49, 50–59, 60–69, 70–79 and ≥80 mg/24 h. Covariates were selected a priori based on their clinical relevance. Covariates included in the model were baseline 24-h urine calcium, baseline 24-h urine citrate, baseline 24-h urine volume, age, sex, race, body mass index (BMI), tobacco use, hypertension, diabetes, cardiovascular disease (CVD) and malabsorptive condition (yes/no). The model was also run separately for the MC group. SAS statistical software version 9.4 was used for all analyses (SAS, Cary, NC, USA).
RESULTS
Characteristics of study population by cohort
There were 764 860 patients who had a valid 24-h urine test and linked clinical data in the OM1 Real-World Data Cloud. Of these, 447 958 patients were at least 18 years old on the index date and had at least 6 months of clinical data available before and after the index date so were included in the final study population (Supplementary data, Table S2). The mean age at the index date was 55.4 years (SD 14.9), 49.6% were female, 92.6% were white and 32.4% of those who had ethnicity information were Hispanic or Latino. Average duration of follow-up was 38.9 (SD 21.7) months (Table 1). Of the overall cohort, 12 522 (2.8%) also met the criteria to be included in the MC group. In this group, the mean age at the index date was 54.3 years (SD 14.1), 58% were female, 93.3% were white, and 24.5% were Hispanic or Latino (Table 1).
| . | All patients (N = 447 958) . | Patients with a malabsorptive condition (N = 12 522) . |
|---|---|---|
| Age (years) | 55.4 (14.9) | 54.3 (14.1) |
| Female, n (%) | 222 255 (49.6) | 7258 (58.0) |
| Race, n (%) | ||
| White | 139 492 (92.6) | 4863 (93.3) |
| Black | 8790 (5.8) | 318 (6.1) |
| Asian | 2154 (1.4) | 26 (0.5) |
| Other | 165 (0.1) | 5 (0.0) |
| Other/unknown | ||
| Ethnicity, n (%) | ||
| Hispanic or Latino | 8768 (32.4) | 228 (24.5) |
| Not Hispanic or Latino | 18 292 (67.6) | 703 (75.5) |
| Other ethnicity | 3 (0.0) | 0 (0.0) |
| Not reported/unknown | 420 895 | 11 591 |
| BMI (kg/m2) | 30.2 (6.7) | 29.9 (7.2) |
| Duration of follow-up (months) | 38.9 (21.7) | 35.6 (20.6) |
| History of tobacco use, n (%) | 85 327 (19.0) | 3600 (28.7) |
| UOx at index (mg/24 h) | 36.1 (14.6) | 40.5 (19.5) |
| Urine calcium at index (mg/24 h) | 197.1 (113.6) | 161.3 (108.0) |
| Urine citrate at index (mg/24 h) | 595.2 (350.3) | 461.7 (353.6) |
| Urine creatinine at index (mg/24 h) | 1638.8 (553.1) | 1497.1 (524.1) |
| Stage 3 or higher CKD in baseline period, n (%) | 12 214 (2.7) | 735 (5.9) |
| Malabsorption in baseline period, n (%) | ||
| Any malabsorption | 11 637 (2.6) | 12 522 (100.0) |
| Malabsorptive condition | 10 360 (2.3) | 11 141 (89.0) |
| Malabsorptive surgical procedure | 1747 (0.4) | 1875 (15.0) |
| Bariatric surgery | 1265 (0.3) | 1365 (10.9) |
| . | All patients (N = 447 958) . | Patients with a malabsorptive condition (N = 12 522) . |
|---|---|---|
| Age (years) | 55.4 (14.9) | 54.3 (14.1) |
| Female, n (%) | 222 255 (49.6) | 7258 (58.0) |
| Race, n (%) | ||
| White | 139 492 (92.6) | 4863 (93.3) |
| Black | 8790 (5.8) | 318 (6.1) |
| Asian | 2154 (1.4) | 26 (0.5) |
| Other | 165 (0.1) | 5 (0.0) |
| Other/unknown | ||
| Ethnicity, n (%) | ||
| Hispanic or Latino | 8768 (32.4) | 228 (24.5) |
| Not Hispanic or Latino | 18 292 (67.6) | 703 (75.5) |
| Other ethnicity | 3 (0.0) | 0 (0.0) |
| Not reported/unknown | 420 895 | 11 591 |
| BMI (kg/m2) | 30.2 (6.7) | 29.9 (7.2) |
| Duration of follow-up (months) | 38.9 (21.7) | 35.6 (20.6) |
| History of tobacco use, n (%) | 85 327 (19.0) | 3600 (28.7) |
| UOx at index (mg/24 h) | 36.1 (14.6) | 40.5 (19.5) |
| Urine calcium at index (mg/24 h) | 197.1 (113.6) | 161.3 (108.0) |
| Urine citrate at index (mg/24 h) | 595.2 (350.3) | 461.7 (353.6) |
| Urine creatinine at index (mg/24 h) | 1638.8 (553.1) | 1497.1 (524.1) |
| Stage 3 or higher CKD in baseline period, n (%) | 12 214 (2.7) | 735 (5.9) |
| Malabsorption in baseline period, n (%) | ||
| Any malabsorption | 11 637 (2.6) | 12 522 (100.0) |
| Malabsorptive condition | 10 360 (2.3) | 11 141 (89.0) |
| Malabsorptive surgical procedure | 1747 (0.4) | 1875 (15.0) |
| Bariatric surgery | 1265 (0.3) | 1365 (10.9) |
Values are mean (SD) or n (%). Percentages calculated after removing “unknown” category.
| . | All patients (N = 447 958) . | Patients with a malabsorptive condition (N = 12 522) . |
|---|---|---|
| Age (years) | 55.4 (14.9) | 54.3 (14.1) |
| Female, n (%) | 222 255 (49.6) | 7258 (58.0) |
| Race, n (%) | ||
| White | 139 492 (92.6) | 4863 (93.3) |
| Black | 8790 (5.8) | 318 (6.1) |
| Asian | 2154 (1.4) | 26 (0.5) |
| Other | 165 (0.1) | 5 (0.0) |
| Other/unknown | ||
| Ethnicity, n (%) | ||
| Hispanic or Latino | 8768 (32.4) | 228 (24.5) |
| Not Hispanic or Latino | 18 292 (67.6) | 703 (75.5) |
| Other ethnicity | 3 (0.0) | 0 (0.0) |
| Not reported/unknown | 420 895 | 11 591 |
| BMI (kg/m2) | 30.2 (6.7) | 29.9 (7.2) |
| Duration of follow-up (months) | 38.9 (21.7) | 35.6 (20.6) |
| History of tobacco use, n (%) | 85 327 (19.0) | 3600 (28.7) |
| UOx at index (mg/24 h) | 36.1 (14.6) | 40.5 (19.5) |
| Urine calcium at index (mg/24 h) | 197.1 (113.6) | 161.3 (108.0) |
| Urine citrate at index (mg/24 h) | 595.2 (350.3) | 461.7 (353.6) |
| Urine creatinine at index (mg/24 h) | 1638.8 (553.1) | 1497.1 (524.1) |
| Stage 3 or higher CKD in baseline period, n (%) | 12 214 (2.7) | 735 (5.9) |
| Malabsorption in baseline period, n (%) | ||
| Any malabsorption | 11 637 (2.6) | 12 522 (100.0) |
| Malabsorptive condition | 10 360 (2.3) | 11 141 (89.0) |
| Malabsorptive surgical procedure | 1747 (0.4) | 1875 (15.0) |
| Bariatric surgery | 1265 (0.3) | 1365 (10.9) |
| . | All patients (N = 447 958) . | Patients with a malabsorptive condition (N = 12 522) . |
|---|---|---|
| Age (years) | 55.4 (14.9) | 54.3 (14.1) |
| Female, n (%) | 222 255 (49.6) | 7258 (58.0) |
| Race, n (%) | ||
| White | 139 492 (92.6) | 4863 (93.3) |
| Black | 8790 (5.8) | 318 (6.1) |
| Asian | 2154 (1.4) | 26 (0.5) |
| Other | 165 (0.1) | 5 (0.0) |
| Other/unknown | ||
| Ethnicity, n (%) | ||
| Hispanic or Latino | 8768 (32.4) | 228 (24.5) |
| Not Hispanic or Latino | 18 292 (67.6) | 703 (75.5) |
| Other ethnicity | 3 (0.0) | 0 (0.0) |
| Not reported/unknown | 420 895 | 11 591 |
| BMI (kg/m2) | 30.2 (6.7) | 29.9 (7.2) |
| Duration of follow-up (months) | 38.9 (21.7) | 35.6 (20.6) |
| History of tobacco use, n (%) | 85 327 (19.0) | 3600 (28.7) |
| UOx at index (mg/24 h) | 36.1 (14.6) | 40.5 (19.5) |
| Urine calcium at index (mg/24 h) | 197.1 (113.6) | 161.3 (108.0) |
| Urine citrate at index (mg/24 h) | 595.2 (350.3) | 461.7 (353.6) |
| Urine creatinine at index (mg/24 h) | 1638.8 (553.1) | 1497.1 (524.1) |
| Stage 3 or higher CKD in baseline period, n (%) | 12 214 (2.7) | 735 (5.9) |
| Malabsorption in baseline period, n (%) | ||
| Any malabsorption | 11 637 (2.6) | 12 522 (100.0) |
| Malabsorptive condition | 10 360 (2.3) | 11 141 (89.0) |
| Malabsorptive surgical procedure | 1747 (0.4) | 1875 (15.0) |
| Bariatric surgery | 1265 (0.3) | 1365 (10.9) |
Values are mean (SD) or n (%). Percentages calculated after removing “unknown” category.
Compared with the overall population, the mean baseline 24-h urine calcium was lower in the MC group (161.3 vs 197.1 mg/24 h), as was the mean 24-h urine citrate (461.7 vs 595.2 mg/24 h). In the MC group, 89% of patients had a diagnosis code for a malabsorptive condition, and 15% had a record of enteric surgery, of which 73% were bariatric surgery.
Characteristics of cohorts according to 24-h UOx excretion
Mean baseline 24-h UOx was 36.1 mg/24 h (SD 14.6) in the overall population and 40.5 mg/24 h (SD 19.5) in the MC group (Table 2). Using the definition of UOx ≥45 mg/24 h, 21.0% of the overall population and 30.7% of the MC group were classified as having hyperoxaluria at baseline. The distribution of 24-h UOx followed an inverted “U” shape with the highest proportion of patients found in the 20–29 mg/24 h (30.5% overall) and 30–39 mg/24 h categories (30.6% overall). The MC group followed a similar pattern but was skewed towards a higher proportion of patients in the higher categories of UOx (Table 2).
Distribution of 24-h UOx overall and among patients with a malabsorptive condition.
| . | All patients (N = 447 958) . | Patients with a malabsorptive condition (N = 12 522) . |
|---|---|---|
| Urine oxalate (mg/24 h), n (%) | ||
| <20 | 35 246 (7.9) | 991 (7.9) |
| 20–29 | 136 608 (30.5) | 3320 (26.5) |
| 30–39 | 137 148 (30.6) | 3265 (26.1) |
| 40–44 | 45 040 (10.1) | 1102 (8.8) |
| 45–49 | 31 216 (7.0) | 902 (7.2) |
| 50–59 | 33 868 (7.6) | 1154 (9.2) |
| 60–69 | 14 766 (3.3) | 684 (5.5) |
| 70–79 | 6863 (1.5) | 437 (3.5) |
| 80+ | 7203 (1.6) | 667 (5.3) |
| Urine oxalate 40+ mg/24 h, n (%) | 138 956 (31.0) | 4946 (39.5) |
| Urine oxalate 45+ mg/24 h, n (%) | 93 916 (21.0) | 3844 (30.7) |
| Urine oxalate (mg/24 h) | ||
| Mean (SD) | 36.1 (14.6) | 40.5 (19.5) |
| Min, max | 11.0, 117.8 | 11.0, 117.5 |
| Median (Q1–Q3) | 33.4 (26.2–42.8) | 35.4 (27.0–48.7) |
| . | All patients (N = 447 958) . | Patients with a malabsorptive condition (N = 12 522) . |
|---|---|---|
| Urine oxalate (mg/24 h), n (%) | ||
| <20 | 35 246 (7.9) | 991 (7.9) |
| 20–29 | 136 608 (30.5) | 3320 (26.5) |
| 30–39 | 137 148 (30.6) | 3265 (26.1) |
| 40–44 | 45 040 (10.1) | 1102 (8.8) |
| 45–49 | 31 216 (7.0) | 902 (7.2) |
| 50–59 | 33 868 (7.6) | 1154 (9.2) |
| 60–69 | 14 766 (3.3) | 684 (5.5) |
| 70–79 | 6863 (1.5) | 437 (3.5) |
| 80+ | 7203 (1.6) | 667 (5.3) |
| Urine oxalate 40+ mg/24 h, n (%) | 138 956 (31.0) | 4946 (39.5) |
| Urine oxalate 45+ mg/24 h, n (%) | 93 916 (21.0) | 3844 (30.7) |
| Urine oxalate (mg/24 h) | ||
| Mean (SD) | 36.1 (14.6) | 40.5 (19.5) |
| Min, max | 11.0, 117.8 | 11.0, 117.5 |
| Median (Q1–Q3) | 33.4 (26.2–42.8) | 35.4 (27.0–48.7) |
All values are from the index urine test.
Distribution of 24-h UOx overall and among patients with a malabsorptive condition.
| . | All patients (N = 447 958) . | Patients with a malabsorptive condition (N = 12 522) . |
|---|---|---|
| Urine oxalate (mg/24 h), n (%) | ||
| <20 | 35 246 (7.9) | 991 (7.9) |
| 20–29 | 136 608 (30.5) | 3320 (26.5) |
| 30–39 | 137 148 (30.6) | 3265 (26.1) |
| 40–44 | 45 040 (10.1) | 1102 (8.8) |
| 45–49 | 31 216 (7.0) | 902 (7.2) |
| 50–59 | 33 868 (7.6) | 1154 (9.2) |
| 60–69 | 14 766 (3.3) | 684 (5.5) |
| 70–79 | 6863 (1.5) | 437 (3.5) |
| 80+ | 7203 (1.6) | 667 (5.3) |
| Urine oxalate 40+ mg/24 h, n (%) | 138 956 (31.0) | 4946 (39.5) |
| Urine oxalate 45+ mg/24 h, n (%) | 93 916 (21.0) | 3844 (30.7) |
| Urine oxalate (mg/24 h) | ||
| Mean (SD) | 36.1 (14.6) | 40.5 (19.5) |
| Min, max | 11.0, 117.8 | 11.0, 117.5 |
| Median (Q1–Q3) | 33.4 (26.2–42.8) | 35.4 (27.0–48.7) |
| . | All patients (N = 447 958) . | Patients with a malabsorptive condition (N = 12 522) . |
|---|---|---|
| Urine oxalate (mg/24 h), n (%) | ||
| <20 | 35 246 (7.9) | 991 (7.9) |
| 20–29 | 136 608 (30.5) | 3320 (26.5) |
| 30–39 | 137 148 (30.6) | 3265 (26.1) |
| 40–44 | 45 040 (10.1) | 1102 (8.8) |
| 45–49 | 31 216 (7.0) | 902 (7.2) |
| 50–59 | 33 868 (7.6) | 1154 (9.2) |
| 60–69 | 14 766 (3.3) | 684 (5.5) |
| 70–79 | 6863 (1.5) | 437 (3.5) |
| 80+ | 7203 (1.6) | 667 (5.3) |
| Urine oxalate 40+ mg/24 h, n (%) | 138 956 (31.0) | 4946 (39.5) |
| Urine oxalate 45+ mg/24 h, n (%) | 93 916 (21.0) | 3844 (30.7) |
| Urine oxalate (mg/24 h) | ||
| Mean (SD) | 36.1 (14.6) | 40.5 (19.5) |
| Min, max | 11.0, 117.8 | 11.0, 117.5 |
| Median (Q1–Q3) | 33.4 (26.2–42.8) | 35.4 (27.0–48.7) |
All values are from the index urine test.
Patients with higher index UOx were older, less likely to be female and had lower eGFR (Supplementary data, Table S3). Patients with higher UOx also had higher BMI, 24-h urine creatinine and Charlson Comorbidity score, and were more likely to have CKD at baseline. Urine calcium and urine citrate rose then fell, whereas urine volume consistently increased across UOx categories. Among patients in the MC group, similar patterns were seen though in the highest oxalate category, urine calcium (MC group 101.4 mg; overall 150.7 mg) and urine citrate (MC group 357.0 mg; overall 607.8 mg) were even lower and the proportion with baseline CKD was even higher (MC group 10.9%; overall 5.2%) (Supplementary data, Tables S3 and S4).
UOx and prevalent CKD
For each category of index UOx, the proportion of patients with prevalent CKD in the overall population increased in a monotonic fashion, rising from 4.0% of patients in the <20 mg/24 h category to 8.2% in the ≥80 mg/24 h category. A similar pattern of an increased proportion of patients with CKD for each increase in category of UOx (<20 mg/24 h 7.3%; ≥80 mg/24 h 17.1%) was seen in the MC group, but the proportion of patients in each category was roughly double that in the overall population. Thus, a higher proportion of patients in the MC group had prevalent CKD than in the overall population (10.2% vs 4.7%; Table 3).
Baseline prevalence of CKD by category of 24-h UOx overall and among patients with a malabsorptive condition.
| . | 24-h urine oxalate (mg/24 h) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| . | <20 . | 20–29 . | 30–39 . | 40–44 . | 45–49 . | 50–59 . | 60–69 . | 70–79 . | 80+ mg . |
| All patients, n | 35 246 | 136 608 | 137 148 | 45 040 | 31 216 | 33 868 | 14 766 | 6863 | 7203 |
| Baseline CKD, n (%) | 1395 (4.0) | 5813 (4.3) | 6294 (4.6) | 2229 (4.9) | 1586 (5.1) | 1775 (5.2) | 905 (6.1) | 472 (6.9) | 593 (8.2) |
| Patients with a malabsorptive condition, n | 991 | 3320 | 3265 | 1102 | 902 | 1154 | 684 | 437 | 667 |
| Baseline CKD, n (%) | 72 (7.3) | 286 (8.6) | 295 (9.0) | 105 (9.5) | 116 (12.9) | 139 (12.0) | 91 (13.3) | 65 (14.9) | 114 (17.1) |
| . | 24-h urine oxalate (mg/24 h) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| . | <20 . | 20–29 . | 30–39 . | 40–44 . | 45–49 . | 50–59 . | 60–69 . | 70–79 . | 80+ mg . |
| All patients, n | 35 246 | 136 608 | 137 148 | 45 040 | 31 216 | 33 868 | 14 766 | 6863 | 7203 |
| Baseline CKD, n (%) | 1395 (4.0) | 5813 (4.3) | 6294 (4.6) | 2229 (4.9) | 1586 (5.1) | 1775 (5.2) | 905 (6.1) | 472 (6.9) | 593 (8.2) |
| Patients with a malabsorptive condition, n | 991 | 3320 | 3265 | 1102 | 902 | 1154 | 684 | 437 | 667 |
| Baseline CKD, n (%) | 72 (7.3) | 286 (8.6) | 295 (9.0) | 105 (9.5) | 116 (12.9) | 139 (12.0) | 91 (13.3) | 65 (14.9) | 114 (17.1) |
Baseline prevalence of CKD by category of 24-h UOx overall and among patients with a malabsorptive condition.
| . | 24-h urine oxalate (mg/24 h) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| . | <20 . | 20–29 . | 30–39 . | 40–44 . | 45–49 . | 50–59 . | 60–69 . | 70–79 . | 80+ mg . |
| All patients, n | 35 246 | 136 608 | 137 148 | 45 040 | 31 216 | 33 868 | 14 766 | 6863 | 7203 |
| Baseline CKD, n (%) | 1395 (4.0) | 5813 (4.3) | 6294 (4.6) | 2229 (4.9) | 1586 (5.1) | 1775 (5.2) | 905 (6.1) | 472 (6.9) | 593 (8.2) |
| Patients with a malabsorptive condition, n | 991 | 3320 | 3265 | 1102 | 902 | 1154 | 684 | 437 | 667 |
| Baseline CKD, n (%) | 72 (7.3) | 286 (8.6) | 295 (9.0) | 105 (9.5) | 116 (12.9) | 139 (12.0) | 91 (13.3) | 65 (14.9) | 114 (17.1) |
| . | 24-h urine oxalate (mg/24 h) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| . | <20 . | 20–29 . | 30–39 . | 40–44 . | 45–49 . | 50–59 . | 60–69 . | 70–79 . | 80+ mg . |
| All patients, n | 35 246 | 136 608 | 137 148 | 45 040 | 31 216 | 33 868 | 14 766 | 6863 | 7203 |
| Baseline CKD, n (%) | 1395 (4.0) | 5813 (4.3) | 6294 (4.6) | 2229 (4.9) | 1586 (5.1) | 1775 (5.2) | 905 (6.1) | 472 (6.9) | 593 (8.2) |
| Patients with a malabsorptive condition, n | 991 | 3320 | 3265 | 1102 | 902 | 1154 | 684 | 437 | 667 |
| Baseline CKD, n (%) | 72 (7.3) | 286 (8.6) | 295 (9.0) | 105 (9.5) | 116 (12.9) | 139 (12.0) | 91 (13.3) | 65 (14.9) | 114 (17.1) |
UOx and incident CKD
After excluding patients with prevalent CKD at baseline, there were 426 896 patients who were eligible to be included in the analysis of incident CKD. After adjusting for baseline urine calcium, urine citrate, urine volume, age, sex, race, BMI, tobacco use, hypertension, diabetes, malabsorption and CVD, baseline UOx was significantly associated with the development of incident CKD. Compared with patients with UOx <20 mg/24 h, the odds of incident CKD increased for 20–29 mg/24 h [multivariable odds ratio (MVOR) 1.14, 95% confidence interval (CI) 1.07, 1.21] through 80+ mg/24 h (MVOR 1.35, 95% CI 1.21, 1.50), and was statistically significant for each UOx category (Table 4A).
Category of index 24-h UOx and multivariable-adjusted OR of incident CKD in all patients.
| Index UOx (mg/24 h) . | Age-adjusted, OR (95% CI) . | Multivariable-adjusteda, odds ratio (95% CI) . |
|---|---|---|
| <20 | 1.00 (ref) | 1.00 (ref) |
| 20–29 | 1.07 (1.00–1.13) | 1.14 (1.07, 1.21) |
| 30–39 | 1.08 (1.02–1.14) | 1.17 (1.10, 1.24) |
| 40–44 | 1.14 (1.07–1.22) | 1.23 (1.14, 1.32) |
| 45–49 | 1.14 (1.06–1.23) | 1.20 (1.11, 1.30) |
| 50–59 | 1.20 (1.12–1.29) | 1.26 (1.17, 1.36) |
| 60–69 | 1.20 (1.19–1.42) | 1.26 (1.14, 1.38) |
| 70–79 | 1.41 (1.26–1.57) | 1.24 (1.11, 1.40) |
| 80+ | 1.87 (1.69–2.06) | 1.35 (1.21, 1.50) |
| Index UOx (mg/24 h) . | Age-adjusted, OR (95% CI) . | Multivariable-adjusteda, odds ratio (95% CI) . |
|---|---|---|
| <20 | 1.00 (ref) | 1.00 (ref) |
| 20–29 | 1.07 (1.00–1.13) | 1.14 (1.07, 1.21) |
| 30–39 | 1.08 (1.02–1.14) | 1.17 (1.10, 1.24) |
| 40–44 | 1.14 (1.07–1.22) | 1.23 (1.14, 1.32) |
| 45–49 | 1.14 (1.06–1.23) | 1.20 (1.11, 1.30) |
| 50–59 | 1.20 (1.12–1.29) | 1.26 (1.17, 1.36) |
| 60–69 | 1.20 (1.19–1.42) | 1.26 (1.14, 1.38) |
| 70–79 | 1.41 (1.26–1.57) | 1.24 (1.11, 1.40) |
| 80+ | 1.87 (1.69–2.06) | 1.35 (1.21, 1.50) |
aModel was adjusted for age, sex, race, BMI, tobacco use, baseline urine citrate, baseline urine calcium, baseline urine volume, history of hypertension, diabetes, CVD and malabsorptive condition.
Patients with CKD during baseline were excluded from this table, leaving 426 896 patients included in this analysis.
CKD status was defined using only diagnosis codes.
Category of index 24-h UOx and multivariable-adjusted OR of incident CKD in all patients.
| Index UOx (mg/24 h) . | Age-adjusted, OR (95% CI) . | Multivariable-adjusteda, odds ratio (95% CI) . |
|---|---|---|
| <20 | 1.00 (ref) | 1.00 (ref) |
| 20–29 | 1.07 (1.00–1.13) | 1.14 (1.07, 1.21) |
| 30–39 | 1.08 (1.02–1.14) | 1.17 (1.10, 1.24) |
| 40–44 | 1.14 (1.07–1.22) | 1.23 (1.14, 1.32) |
| 45–49 | 1.14 (1.06–1.23) | 1.20 (1.11, 1.30) |
| 50–59 | 1.20 (1.12–1.29) | 1.26 (1.17, 1.36) |
| 60–69 | 1.20 (1.19–1.42) | 1.26 (1.14, 1.38) |
| 70–79 | 1.41 (1.26–1.57) | 1.24 (1.11, 1.40) |
| 80+ | 1.87 (1.69–2.06) | 1.35 (1.21, 1.50) |
| Index UOx (mg/24 h) . | Age-adjusted, OR (95% CI) . | Multivariable-adjusteda, odds ratio (95% CI) . |
|---|---|---|
| <20 | 1.00 (ref) | 1.00 (ref) |
| 20–29 | 1.07 (1.00–1.13) | 1.14 (1.07, 1.21) |
| 30–39 | 1.08 (1.02–1.14) | 1.17 (1.10, 1.24) |
| 40–44 | 1.14 (1.07–1.22) | 1.23 (1.14, 1.32) |
| 45–49 | 1.14 (1.06–1.23) | 1.20 (1.11, 1.30) |
| 50–59 | 1.20 (1.12–1.29) | 1.26 (1.17, 1.36) |
| 60–69 | 1.20 (1.19–1.42) | 1.26 (1.14, 1.38) |
| 70–79 | 1.41 (1.26–1.57) | 1.24 (1.11, 1.40) |
| 80+ | 1.87 (1.69–2.06) | 1.35 (1.21, 1.50) |
aModel was adjusted for age, sex, race, BMI, tobacco use, baseline urine citrate, baseline urine calcium, baseline urine volume, history of hypertension, diabetes, CVD and malabsorptive condition.
Patients with CKD during baseline were excluded from this table, leaving 426 896 patients included in this analysis.
CKD status was defined using only diagnosis codes.
In the MC group, there were 11 239 (2.6%) patients with no CKD at baseline who were eligible to be included in the analysis of incident CKD. A similar pattern seen as in the overall cohort though the magnitudes of association were larger, with the odds of developing incident CKD increased for 20–29 mg/24 h (MVOR 1.50, 95% CI 1.03, 2.20) through 80+ mg/24 h (MVOR 2.34, 95% CI 1.50, 3.63) compared with patients with UOx <20 mg/24 h (Table 4B).
Category of baseline 24-h UOx and multivariable-adjusted OR of incident CKD among patients with a malabsorptive condition.
| Index UOx (mg/24 h) . | Age-adjusted, OR (95% CI) . | Multivariable-adjusteda, OR (95% CI) . |
|---|---|---|
| <20 | 1.00 (ref) | 1.00 (ref) |
| 20–29 | 1.32 (0.91, 1.92) | 1.50 (1.03, 2.20) |
| 30–39 | 1.38 (0.95, 2.00) | 1.60 (1.09, 2.34) |
| 40–44 | 1.72 (1.13, 2.60) | 1.95 (1.27, 2.99) |
| 45–49 | 1.53 (0.98, 2.37) | 1.72 (1.09, 2.72) |
| 50–59 | 1.50 (0.99, 2.28) | 1.55 (1.00, 2.40) |
| 60–69 | 1.84 (1.18, 2.88) | 1.72 (1.08, 2.73) |
| 70–79 | 1.70 (1.03, 2.82) | 1.42 (0.84, 2.39) |
| 80+ | 3.35 (2.21, 5.08) | 2.34 (1.50, 3.63) |
| Index UOx (mg/24 h) . | Age-adjusted, OR (95% CI) . | Multivariable-adjusteda, OR (95% CI) . |
|---|---|---|
| <20 | 1.00 (ref) | 1.00 (ref) |
| 20–29 | 1.32 (0.91, 1.92) | 1.50 (1.03, 2.20) |
| 30–39 | 1.38 (0.95, 2.00) | 1.60 (1.09, 2.34) |
| 40–44 | 1.72 (1.13, 2.60) | 1.95 (1.27, 2.99) |
| 45–49 | 1.53 (0.98, 2.37) | 1.72 (1.09, 2.72) |
| 50–59 | 1.50 (0.99, 2.28) | 1.55 (1.00, 2.40) |
| 60–69 | 1.84 (1.18, 2.88) | 1.72 (1.08, 2.73) |
| 70–79 | 1.70 (1.03, 2.82) | 1.42 (0.84, 2.39) |
| 80+ | 3.35 (2.21, 5.08) | 2.34 (1.50, 3.63) |
aModel was adjusted for age, sex, race, BMI, tobacco use, baseline urine citrate, baseline urine calcium, baseline urine volume, history of hypertension, diabetes and CVD.
Patients with CKD during baseline were excluded, leaving 11 239 patients included in this table.
CKD status was defined using only diagnosis codes.
Category of baseline 24-h UOx and multivariable-adjusted OR of incident CKD among patients with a malabsorptive condition.
| Index UOx (mg/24 h) . | Age-adjusted, OR (95% CI) . | Multivariable-adjusteda, OR (95% CI) . |
|---|---|---|
| <20 | 1.00 (ref) | 1.00 (ref) |
| 20–29 | 1.32 (0.91, 1.92) | 1.50 (1.03, 2.20) |
| 30–39 | 1.38 (0.95, 2.00) | 1.60 (1.09, 2.34) |
| 40–44 | 1.72 (1.13, 2.60) | 1.95 (1.27, 2.99) |
| 45–49 | 1.53 (0.98, 2.37) | 1.72 (1.09, 2.72) |
| 50–59 | 1.50 (0.99, 2.28) | 1.55 (1.00, 2.40) |
| 60–69 | 1.84 (1.18, 2.88) | 1.72 (1.08, 2.73) |
| 70–79 | 1.70 (1.03, 2.82) | 1.42 (0.84, 2.39) |
| 80+ | 3.35 (2.21, 5.08) | 2.34 (1.50, 3.63) |
| Index UOx (mg/24 h) . | Age-adjusted, OR (95% CI) . | Multivariable-adjusteda, OR (95% CI) . |
|---|---|---|
| <20 | 1.00 (ref) | 1.00 (ref) |
| 20–29 | 1.32 (0.91, 1.92) | 1.50 (1.03, 2.20) |
| 30–39 | 1.38 (0.95, 2.00) | 1.60 (1.09, 2.34) |
| 40–44 | 1.72 (1.13, 2.60) | 1.95 (1.27, 2.99) |
| 45–49 | 1.53 (0.98, 2.37) | 1.72 (1.09, 2.72) |
| 50–59 | 1.50 (0.99, 2.28) | 1.55 (1.00, 2.40) |
| 60–69 | 1.84 (1.18, 2.88) | 1.72 (1.08, 2.73) |
| 70–79 | 1.70 (1.03, 2.82) | 1.42 (0.84, 2.39) |
| 80+ | 3.35 (2.21, 5.08) | 2.34 (1.50, 3.63) |
aModel was adjusted for age, sex, race, BMI, tobacco use, baseline urine citrate, baseline urine calcium, baseline urine volume, history of hypertension, diabetes and CVD.
Patients with CKD during baseline were excluded, leaving 11 239 patients included in this table.
CKD status was defined using only diagnosis codes.
In both the overall and MC group, well-recognized risk factors for CKD were in the expected direction, including higher odds of incident CKD for hypertension and diabetes and lower risk for women. When further adjusting for the Charlson Comorbidity Index score, the results were not materially changed. Because CVD could be along the causal pathway between UOx and CKD, we ran a model that did not adjust for CVD, and the results were unchanged (data not shown).
DISCUSSION
This is the first study to examine the association between 24-h UOx excretion and incident CKD. This real-world analysis found that the odds of incident CKD increased across UOx categories, even after adjusting for known risk factors for CKD such as diabetes and hypertension. These findings were even more pronounced in the group with a malabsorptive condition. It is reassuring that well-recognized risk factors for CKD, such as hypertension, diabetes and sex, were independently associated with CKD in the multivariable models. The results of this study support the premise that higher UOx is an independent risk factor for development of CKD.
Renal damage due to high UOx has been well-described in patients with enteric hyperoxaluria [8] and primary hyperoxaluria [7, 16], but data are limited in patients without these conditions. Although there are no other published studies of 24-h UOx and incident CKD, our findings are in line with those reported by previous studies of the relation between oxalate and kidney function decline. Most relevant is the study by Waikar et al. which found that 24-h UOx was associated with faster kidney function decline in individuals with reduced kidney function at baseline [15]. In that study of over 3100 participants, higher UOx excretion was independently associated with >30% higher risk of subsequent CKD progression and development of ESKD. However, in contrast to the Waikar study, we did not observe a threshold for UOx. In our study, the odds of CKD increased across UOx categories in the overall cohort; in the MC cohort, the category-specific ORs tended to, but did not always, increase as UOx increased, though the highest OR was in the highest UOx category.
UOx may cause renal toxicity through a variety of mechanisms. Precipitation of calcium oxalate crystals in renal tubules can cause tubular obstruction. Oxalate can activate the NLRP3 inflammasome, which has been shown to cause renal damage in mice [13]. In another mouse model of CKD induced by high oxalate intake, the histologic changes found in the kidney, including tubular damage, interstitial inflammation and fibrosis, as well other systemic effects mirrored those seen in human CKD [17].
Potential clinical implications
Given the strong association of UOx with incident CKD and the progression of CKD [15] along with readily available approaches to reduce UOx, clinicians should consider measuring 24-h UOx in their patients with CKD, as well as patients with known risk factors for CKD. Measuring 24-h UOx in patients with a malabsorptive condition, such as inflammatory bowel disease or bariatric surgery, should also be considered. Patients identified to have higher than desired UOx should be counseled to reduce dietary intake of oxalate and to ensure adequate dietary intake of calcium. Although malabsorptive bariatric surgical procedures are a risk factor for developing hyperoxaluria, it is important to note that both animal [18] and human studies [19] have found that reducing dietary oxalate post-surgery is not adequate to reduce UOx levels to desired levels. These patients might require oxalate binders or other measures to reduce UOx. Several new treatments aimed at decreasing absorption of dietary oxalates or reducing production of endogenous oxalate are in development [3]. If approved, these treatments may provide an important additional avenue to lower UOx levels in patients for whom dietary modification is not adequate.
Certain limitations are inherent to the secondary use of data. The exposure was UOx from a 24-h urine collection, which is primarily ordered in patients with or suspected of having kidney stones. Therefore, although we did not impose any limitations on the population aside from occurrence of the urine collection and adequate baseline and follow-up data, the majority of patients included in this study are assumed to be stone-formers. While there is no reason to believe that the findings of this study would not apply in a broader population, additional studies should be conducted to confirm the relevance of these findings in a non-stone-former population. The urine collections were performed at home and the patients were provided with written and visual instructions, but there was likely variability in how closely the instructions were followed. Due to the open nature of the claims in the OM1 Real-World Data Cloud and the use of EMR data, continuous enrollment is approximated by patterns of encounters. In addition, because data were obtained from multiple sources, it is possible that not all relevant diagnoses were captured. Since it may take many years for CKD to develop and then be diagnosed, not all oxalate related CKD may have been captured in this study despite all patients having had at least 6 months of follow-up clinical data. Early-stage CKD may not be recognized or coded, so prevalent cases of CKD may not have been identified, and therefore excluded, at baseline. We did not have eGFR values on most patients. While a time-to-event analysis was considered, we chose logistic regression for several reasons including concern about potential earlier detection in patients with higher UOx because the study population was not being screened for kidney function on a pre-specified schedule. Many of the urine collections are likely to have come from recurrent stone-formers. Those individuals are more likely to have had recurrent episodes of ureteral obstruction or stone removal procedures, which could damage the kidney. In the current data set, we cannot separate out the direct (e.g. tubular toxicity) from the indirect (e.g. prolonged ureteral obstruction, surgical procedures) adverse effects of higher UOx. It is possible that some patients with primary hyperoxaluria may have been in the higher UOx categories; other urine metabolites that may point to primary hyperoxaluria are not routinely measured. However, the proportion of patients with hyperoxaluria from either diet or enteric hyperoxaluria is much greater than primary hyperoxaluria based on what is known about the genetics and epidemiology. Thus, it is unlikely that the few patients with primary hyperoxaluria would be enough to skew the results. There is day-to-day variability in UOx excretion, so averaging multiple collections performed relatively close in time would provide more precise estimates. However, in the real-world setting, most patients do not perform two 24-h collections close in time, so we did not have a sufficient number of patients to examine this issue.
CONCLUSIONS
In this very large study, 24-h UOx was strongly associated with the development of incident CKD. Future studies should examine whether reducing UOx diminishes the risk of developing CKD. In the meantime, given the potential clinical importance as well as the ability to reduce UOx through non-invasive approaches, clinicians should consider measuring 24-h UOx in patients with CKD or known risk factors for CKD and advising patients with higher UOx to reduce their levels, with the possibility that this could reduce the risk of onset or progression of CKD.
FUNDING
Synlogic, Inc. engaged and provided funding to OM1 to conduct this study.
AUTHORS’ CONTRIBUTIONS
Conception/design of the study: M.P., C.K., K.M. and G.C. Acquisition/analysis of the data: M.P., C.K., K.M. and G.C. Interpretation of the data: M.P., C.K., A.W., K.M., K.W., A.S. and G.C. Drafting of the paper or revising it critically for intellectual content: M.P., C.K., A.W., K.M., K.W., A.S. and G.C. Final approval of the version to be published: M.P., C.K., A.W., K.M., K.W., A.S. and G.C. All authors agree to be accountable for all aspects of the work.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
M.P., C.K. and A.W. are employees of Synlogic. K.W. acts as a consultant at Synlogic. K.M. and A.S. are employees of OM1. G.C. is an employee of OM1; and has received royalties from UpToDate.





Comments