-
PDF
- Split View
-
Views
-
Cite
Cite
Masatoshi Nishimoto, Miho Murashima, Maiko Kokubu, Masaru Matsui, Masahiro Eriguchi, Ken-Ichi Samejima, Yasuhiro Akai, Kazuhiko Tsuruya, Kidney function at 3 months after acute kidney injury is an unreliable indicator of subsequent kidney dysfunction: the NARA-AKI Cohort Study, Nephrology Dialysis Transplantation, Volume 38, Issue 3, March 2023, Pages 664–670, https://doi.org/10.1093/ndt/gfac172
Close - Share Icon Share
ABSTRACT
The relationship between kidney function at 3 months after acute kidney injury (AKI) and kidney function prognosis has not been characterized.
This retrospective cohort study included adults who underwent noncardiac surgery under general anesthesia. Exclusion criteria included obstetric or urological surgery, missing data and preoperative dialysis. Linear mixed-effects models were used to compare estimated glomerular filtration rate (eGFR) slopes in patients with and without AKI. Multivariable Cox proportional hazard models were used to examine the associations of AKI with incident chronic kidney disease (CKD) and decline in eGFR ≥30%.
Among 5272 patients, 316 (6.0%) developed AKI. Among 1194 patients with follow-up creatinine values, eGFR was stable or increased in patients with and without AKI at 3 months postoperatively and declined thereafter. eGFR decline after 3 months postoperatively was faster among patients with AKI than among patients without AKI (P = .09). Among 938 patients without CKD—both at baseline and at 3 months postoperatively—226 and 161 developed incident CKD and a decline in eGFR ≥30%, respectively. Despite adjustment for eGFR at 3 months, AKI was associated with incident CKD {hazard ratio [HR] 1.73 [95% confidence interval (CI) 1.06–2.84]} and a decline in eGFR ≥30% [HR 2.41 (95% CI 1.51–3.84)].
AKI was associated with worse kidney outcomes, regardless of eGFR at 3 months after surgery. Creatinine-based eGFR values at 3 months after AKI might be affected by acute illness-induced loss of muscle mass. Kidney function might be more accurately evaluated much later after surgery or using cystatin C values.
What is already known about this subject?
The Kidney Disease: Improving Global Outcomes guideline recommends that patients should be evaluated at 3 months after acute kidney injury (AKI) to identify the resolution, new onset or worsening of pre-existing chronic kidney disease.
However, this recommendation is not evidence-based; additional research is warranted to determine whether preserved kidney function at 3 months after AKI is indicative of good kidney function prognosis.
What this study adds?
The creatinine-based estimated glomerular filtration rate (eGFR) was stable or increased among patients with and without AKI at 3 months postoperatively and declined thereafter.
Among patients without chronic kidney disease—both at baseline and at 3 months postoperatively—AKI was independently associated with a subsequent decline in kidney function despite adjustment for the eGFR at 3 months.
What impact this may have on practice or policy?
The creatinine-based eGFR at 3 months after AKI might be affected by acute illness–induced loss of muscle mass, leading to unreliable values.
Preservation of the eGFR at 3 months after AKI does not guarantee subsequent preservation of kidney function. Kidney function might be more accurately evaluated much later after surgery or using cystatin C values.
INTRODUCTION
Postoperative acute kidney injury (AKI) is a serious complication of surgical procedures. It is now well recognized that the presence of AKI is predictive of incident chronic kidney disease (CKD) or end-stage kidney disease, regardless of whether kidney function recovers to near baseline [1–7]. The importance of long-term follow-up after AKI has been suggested, however, there has been little evidence to support appropriate follow-up timing.
The Kidney Disease: Improving Global Outcomes (KDIGO) guideline recommends that patients should be evaluated at 3 months after AKI to identify the resolution, new onset or worsening of pre-existing CKD [8]. This recommendation is ‘not graded’, which indicates that it is not evidence-based. Thus this study was performed to determine whether preserved kidney function at 3 months after AKI is indicative of good kidney function prognosis.
To examine this clinical question, we chose the NARA-AKI cohort on postoperative AKI in noncardiac surgery. Compared with AKI after cardiac surgery or in the intensive care setting, AKI after noncardiac surgery has not been extensively investigated, presumably because the incidence of such AKI is lower; however, the annual number of patients who undergo noncardiac surgery is much larger than the annual number of patients who undergo cardiac surgery or receive critical care. Moreover, the mortality rate after noncardiac surgery–related AKI is lower than the mortality rate after cardiac surgery–related AKI or after critical care–related AKI. Thus we examined long-term kidney outcomes after postoperative AKI in patients who underwent noncardiac surgery; such patients survive for long periods after AKI and require kidney function preservation.
MATERIALS AND METHODS
Study design and patients
The NARA-AKI Cohort Study is a single-center, retrospective cohort study [9–14]. Inclusion criteria were age ≥18 years and treatment involving noncardiac surgery under general anesthesia from April 2007 (when our hospital began to use electronic medical records) to December 2011 at Nara Medical University Hospital. Exclusion criteria were treatment involving obstetric surgery (because hemodilution causes decreased serum creatinine levels during pregnancy and because criteria for AKI are not validated in pregnant women) or urological surgery (because nephrectomy or ureteral manipulation–related increases in creatinine could involve causative mechanisms distinct from other postoperative AKI), receipt of preoperative dialysis treatment and the absence of analysis data (e.g. serum creatinine within 1 month before and 1 week after surgery). If patients underwent multiple surgeries during the study period, then only the first eligible surgery was considered. Observation ended on 31 December 2015 or at the final recorded follow-up. The study protocol and waiver of consent were approved by the Nara Medical University Ethics Committee [approval numbers 1208 and 1208-2 (amendment)]. The requirement for written informed consent was waived because of the retrospective nature of this study. Furthermore, patients were able to opt out using a method described on our department's web page (http://nephrology.naramed-u.ac.jp/research/clinical.html). The study was conducted in accordance with the Declaration of Helsinki and registered in the University Hospital Medical Information Network (UMIN000037141).
Exposures of interest and outcomes
The exposure of interest was postoperative AKI within 7 days after noncardiac surgery. The outcome variables were a change in estimated glomerular filtration rate (eGFR) within and after 3 months postoperatively, incident CKD (i.e. eGFR <60 mL/min/1.73 m2) and a decline in eGFR ≥30% after 3 months postoperatively. Concerning the change in eGFR, differences in the slopes of eGFR within and after 3 months postoperatively were both tested in patients with and without AKI. Additionally, the slopes of eGFR after 3 months postoperatively were compared between patients with and without AKI. Concerning incident CKD, the first instance of incident CKD after 3 months postoperatively was examined; with respect to decline in eGFR ≥30%, the first instance of a decline in eGFR ≥30% from baseline after 3 months postoperatively was examined. Decline in eGFR ≥30% was selected as an outcome because it is regarded as a surrogate marker for CKD progression [15] and has been used in multiple clinical studies [16–18].
Data acquisition and definition
The list of patients who underwent noncardiac surgery under general anesthesia was automatically extracted from electronic medical records, collecting data concerning their age, sex, date of surgery and laboratory findings. Information regarding comorbidities, medications and outcomes was manually extracted from electronic medical records by investigators. These data were obtained from detailed chart reviews that included inquiries about medications prescribed by other medical facilities. Chart reviews were performed by M.N., M.M. and M.K. using the same data collection sheet and definitions of each variable. One hundred randomly selected samples were cross-checked to confirm the absence of discrepancies among data collectors.
In accordance with KDIGO criteria, AKI was defined as an increase in serum creatinine ≥0.3 mg/dL or 150% compared with the preoperative baseline value or urine output of <0.5 mL/kg/h for ≥6 h [8]. Baseline laboratory data, including serum creatinine, were defined as values within 1 month preoperatively and closest to the date of surgery. Cerebrovascular diseases were defined as either hemorrhagic or ischemic stroke. The presence of cardiovascular diseases included one or more of the following: ischemic heart disease, congestive heart failure, peripheral artery disease or atrial fibrillation. Noncardiac surgeries were classified into four categories: intrathoracic, intra-abdominal, pelvic, major joint and other. eGFR was calculated using the equation developed for Japanese populations by the Japanese Society of Nephrology, based on the baseline serum creatinine value [19]. Proteinuria was defined as a dipstick test result of + or greater. To maximize the data available for analysis, serum creatinine values at 3, 6 and 12 months after surgery were defined as values measured at 3 months ± 15 days, 6 months ± 15 days and 12 months ± 3 months, respectively. After 12 months postoperatively, serum creatinine values were measured annually when available at 24, 36, 48, 60, 72, 84 and 96 months (all ±3 months).
Statistical methods
Data are shown as numbers with percentages, means with standard deviations (SDs) or medians with interquartile ranges (IQRs). Categorical variables were compared using the chi-squared test or Fisher's exact test and continuous variables were compared using Student's t-test or the Mann–Whitney U-test, as appropriate. First, eGFR slopes within and after 3 months postoperatively were compared among patients with and without AKI who had eGFR measurements at 3 months and at 6 and/or 12 months postoperatively. Linear mixed-effects models were used with adjustments for age, sex, body mass index (BMI), hypertension, diabetes, cerebrovascular disease, cardiovascular disease, angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs), other antihypertensives, diuretics, insulin therapy, malignancy surgery and proteinuria. The time point at 3 months after surgery was treated as the inflection point in the model because raw data suggested the presence of an inflection point in the eGFR slope at 3 months (Supplementary data, Figure S1). Because there were sparse data regarding eGFR measurements after 12 months postoperatively, the estimation of eGFR slope was unreliable, thus eGFR values after 12 months were excluded from the analyses of eGFR slope. The day of surgery was regarded as time 0. Furthermore, the slopes of eGFR after 3 months were compared between patients with AKI and without AKI. Second, multivariable Cox proportional hazard models and cumulative incidence curves were used to examine the associations of AKI with incident CKD and a decline in eGFR ≥30% with adjustments for age, sex, BMI, hypertension, diabetes, cerebrovascular disease, cardiovascular disease, ACEIs or ARBs, other antihypertensives, diuretics, insulin therapy, malignancy surgery, proteinuria and eGFR at 3 months postoperatively. These analyses were performed in patients without CKD—both at baseline and at 3 months postoperatively; 3 months postoperatively was regarded as time 0. Competing risk regression analyses (Fine and Gray models) using the same potential confounders were also performed, with all-cause death treated as a competing risk [20]. Because eGFR values were collected annually and some patients died several years after the last eGFR measurements, it was unclear whether those patients had developed incident CKD or a ≥30% decline in eGFR before death. For competing risk regression, patients were assumed to have died without developing outcomes of interest if they died within 6 months after the last eGFR measurement. Otherwise, data were censored at the last eGFR measurement. P-values <.05 were considered statistically significant except for P for interaction, for which P-values <.10 were considered statistically significant. Statistical analyses were performed using Stata version 15 (Stata Corp, College Station, TX, USA).
RESULTS
Patient characteristics
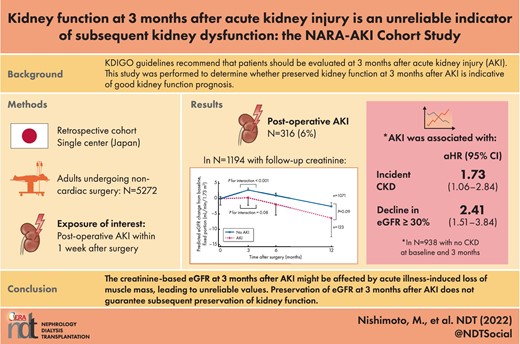
During the study period, 12 771 patients underwent noncardiac surgeries under general anesthesia at Nara Medical University Hospital. After the application of exclusion criteria, 5272 patients were eligible for inclusion in the study (Fig. 1) and 316 patients (6.0%) developed postoperative AKI. Compared with patients without AKI, patients with AKI were older, had a higher BMI and had a lower baseline eGFR (Table 1). The proportions of patients with comorbidities and proteinuria, regular use of ACEIs or ARBs, other antihypertensive agents and diuretics were higher among patients with AKI than among patients without AKI.
| Characteristics . | No AKI (n = 4956) . | AKI (n = 316) . | P-value . |
|---|---|---|---|
| Age (years), median (IQR) | 63 (49–72) | 67 (56–74) | <.001 |
| Male, n (%) | 2268 (45.8) | 177 (56.0) | <.001 |
| BMI (kg/m2) | 22.8 (3.7) | 23.8 (4.4) | <.001 |
| Hypertension, n (%) | 1714 (34.6) | 148 (46.8) | <.001 |
| Diabetes, n (%) | 751 (15.2) | 77 (24.4) | <.001 |
| Cerebrovascular disease, n (%) | |||
| Hemorrhagic stroke | 157 (3.2) | 10 (3.2) | 1.00 |
| Ischemic stroke | 277 (5.6) | 13 (4.1) | .31 |
| Cardiovascular disease, n (%) | |||
| Ischemic heart disease | 221 (4.5) | 24 (7.6) | .02 |
| Congestive heart failure | 61 (1.2) | 16 (5.1) | <.001 |
| Peripheral artery disease | 52 (1.0) | 5 (1.6) | .39 |
| Atrial fibrillation | 118 (2.4) | 17 (5.4) | .003 |
| ACEIs or ARBs, n (%) | 897 (18.1) | 91 (28.8) | <.001 |
| Other antihypertensives, n (%) | 1166 (23.5) | 111 (35.1) | <.001 |
| Diuretics, n (%) | 369 (7.4) | 53 (16.8) | <.001 |
| Insulin therapy, n (%) | 176 (3.6) | 28 (8.9) | <.001 |
| Type of surgery, n (%) | |||
| Intrathoracic | 455 (9.2) | 38 (12.0) | <.001 |
| Intra-abdominal | 922 (18.6) | 104 (32.9) | |
| Pelvic or major joint | 766 (15.5) | 67 (21.2) | |
| Other | 2813 (56.8) | 107 (33.9) | |
| Malignancy surgery, n (%) | 1630 (32.9) | 152 (48.1) | <.001 |
| eGFR (mL/min/1.73 m2), mean (SD) | 80.3 (22.8) | 71.9 (35.9) | <.001 |
| Dipstick proteinuria (≥+), n (%) | 422 (8.5) | 76 (24.1) | <.001 |
| Characteristics . | No AKI (n = 4956) . | AKI (n = 316) . | P-value . |
|---|---|---|---|
| Age (years), median (IQR) | 63 (49–72) | 67 (56–74) | <.001 |
| Male, n (%) | 2268 (45.8) | 177 (56.0) | <.001 |
| BMI (kg/m2) | 22.8 (3.7) | 23.8 (4.4) | <.001 |
| Hypertension, n (%) | 1714 (34.6) | 148 (46.8) | <.001 |
| Diabetes, n (%) | 751 (15.2) | 77 (24.4) | <.001 |
| Cerebrovascular disease, n (%) | |||
| Hemorrhagic stroke | 157 (3.2) | 10 (3.2) | 1.00 |
| Ischemic stroke | 277 (5.6) | 13 (4.1) | .31 |
| Cardiovascular disease, n (%) | |||
| Ischemic heart disease | 221 (4.5) | 24 (7.6) | .02 |
| Congestive heart failure | 61 (1.2) | 16 (5.1) | <.001 |
| Peripheral artery disease | 52 (1.0) | 5 (1.6) | .39 |
| Atrial fibrillation | 118 (2.4) | 17 (5.4) | .003 |
| ACEIs or ARBs, n (%) | 897 (18.1) | 91 (28.8) | <.001 |
| Other antihypertensives, n (%) | 1166 (23.5) | 111 (35.1) | <.001 |
| Diuretics, n (%) | 369 (7.4) | 53 (16.8) | <.001 |
| Insulin therapy, n (%) | 176 (3.6) | 28 (8.9) | <.001 |
| Type of surgery, n (%) | |||
| Intrathoracic | 455 (9.2) | 38 (12.0) | <.001 |
| Intra-abdominal | 922 (18.6) | 104 (32.9) | |
| Pelvic or major joint | 766 (15.5) | 67 (21.2) | |
| Other | 2813 (56.8) | 107 (33.9) | |
| Malignancy surgery, n (%) | 1630 (32.9) | 152 (48.1) | <.001 |
| eGFR (mL/min/1.73 m2), mean (SD) | 80.3 (22.8) | 71.9 (35.9) | <.001 |
| Dipstick proteinuria (≥+), n (%) | 422 (8.5) | 76 (24.1) | <.001 |
P-values were determined using Student's t-test, Mann–Whitney U-test, chi-squared test or Fisher's exact test, as appropriate.
| Characteristics . | No AKI (n = 4956) . | AKI (n = 316) . | P-value . |
|---|---|---|---|
| Age (years), median (IQR) | 63 (49–72) | 67 (56–74) | <.001 |
| Male, n (%) | 2268 (45.8) | 177 (56.0) | <.001 |
| BMI (kg/m2) | 22.8 (3.7) | 23.8 (4.4) | <.001 |
| Hypertension, n (%) | 1714 (34.6) | 148 (46.8) | <.001 |
| Diabetes, n (%) | 751 (15.2) | 77 (24.4) | <.001 |
| Cerebrovascular disease, n (%) | |||
| Hemorrhagic stroke | 157 (3.2) | 10 (3.2) | 1.00 |
| Ischemic stroke | 277 (5.6) | 13 (4.1) | .31 |
| Cardiovascular disease, n (%) | |||
| Ischemic heart disease | 221 (4.5) | 24 (7.6) | .02 |
| Congestive heart failure | 61 (1.2) | 16 (5.1) | <.001 |
| Peripheral artery disease | 52 (1.0) | 5 (1.6) | .39 |
| Atrial fibrillation | 118 (2.4) | 17 (5.4) | .003 |
| ACEIs or ARBs, n (%) | 897 (18.1) | 91 (28.8) | <.001 |
| Other antihypertensives, n (%) | 1166 (23.5) | 111 (35.1) | <.001 |
| Diuretics, n (%) | 369 (7.4) | 53 (16.8) | <.001 |
| Insulin therapy, n (%) | 176 (3.6) | 28 (8.9) | <.001 |
| Type of surgery, n (%) | |||
| Intrathoracic | 455 (9.2) | 38 (12.0) | <.001 |
| Intra-abdominal | 922 (18.6) | 104 (32.9) | |
| Pelvic or major joint | 766 (15.5) | 67 (21.2) | |
| Other | 2813 (56.8) | 107 (33.9) | |
| Malignancy surgery, n (%) | 1630 (32.9) | 152 (48.1) | <.001 |
| eGFR (mL/min/1.73 m2), mean (SD) | 80.3 (22.8) | 71.9 (35.9) | <.001 |
| Dipstick proteinuria (≥+), n (%) | 422 (8.5) | 76 (24.1) | <.001 |
| Characteristics . | No AKI (n = 4956) . | AKI (n = 316) . | P-value . |
|---|---|---|---|
| Age (years), median (IQR) | 63 (49–72) | 67 (56–74) | <.001 |
| Male, n (%) | 2268 (45.8) | 177 (56.0) | <.001 |
| BMI (kg/m2) | 22.8 (3.7) | 23.8 (4.4) | <.001 |
| Hypertension, n (%) | 1714 (34.6) | 148 (46.8) | <.001 |
| Diabetes, n (%) | 751 (15.2) | 77 (24.4) | <.001 |
| Cerebrovascular disease, n (%) | |||
| Hemorrhagic stroke | 157 (3.2) | 10 (3.2) | 1.00 |
| Ischemic stroke | 277 (5.6) | 13 (4.1) | .31 |
| Cardiovascular disease, n (%) | |||
| Ischemic heart disease | 221 (4.5) | 24 (7.6) | .02 |
| Congestive heart failure | 61 (1.2) | 16 (5.1) | <.001 |
| Peripheral artery disease | 52 (1.0) | 5 (1.6) | .39 |
| Atrial fibrillation | 118 (2.4) | 17 (5.4) | .003 |
| ACEIs or ARBs, n (%) | 897 (18.1) | 91 (28.8) | <.001 |
| Other antihypertensives, n (%) | 1166 (23.5) | 111 (35.1) | <.001 |
| Diuretics, n (%) | 369 (7.4) | 53 (16.8) | <.001 |
| Insulin therapy, n (%) | 176 (3.6) | 28 (8.9) | <.001 |
| Type of surgery, n (%) | |||
| Intrathoracic | 455 (9.2) | 38 (12.0) | <.001 |
| Intra-abdominal | 922 (18.6) | 104 (32.9) | |
| Pelvic or major joint | 766 (15.5) | 67 (21.2) | |
| Other | 2813 (56.8) | 107 (33.9) | |
| Malignancy surgery, n (%) | 1630 (32.9) | 152 (48.1) | <.001 |
| eGFR (mL/min/1.73 m2), mean (SD) | 80.3 (22.8) | 71.9 (35.9) | <.001 |
| Dipstick proteinuria (≥+), n (%) | 422 (8.5) | 76 (24.1) | <.001 |
P-values were determined using Student's t-test, Mann–Whitney U-test, chi-squared test or Fisher's exact test, as appropriate.
Change in eGFR among patients with and without AKI
Among 5272 patients in the study, 1194 (including 123 with AKI) had eGFR measurements at 3 months and at 6 and/or 12 months postoperatively (Fig. 1 and Supplementary data, Table S1). Creatinine-based eGFR increased at 3 months postoperatively in patients without AKI [mean eGFR slope 11.5 mL/min/1.73 m2/year (SD 63.9)], while it was stable for patients with AKI [mean eGFR slope 1.0 mL/min/1.73 m2/year (SD 84.5)] and declined thereafter (Fig. 2). Linear mixed-effects model analysis indicated that eGFR slopes were different within and after 3 months postoperatively for patients with and without AKI (P for interaction = .08 and <.0001, respectively). Furthermore, the decline in eGFR after 3 months postoperatively was faster among patients with AKI [mean eGFR slope −8.7 mL/min/1.73 m2/year (SD 7.6)] than among patients without AKI [mean eGFR slope −7.5 mL/min/1.73 m2/year (SD 7.6)] (P = .09) (Fig. 2).
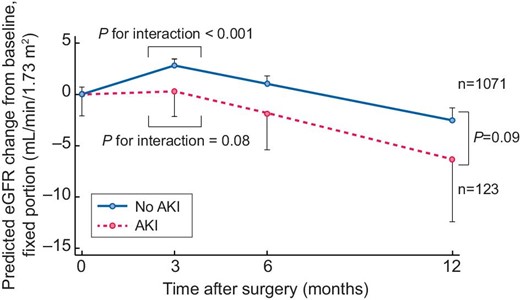
eGFR changes from baseline in patients with and without AKI. Time 0 indicates the day of surgery. Time at 3 months is regarded as the inflection point. The slopes of eGFR were compared at surgery and 3 months postoperatively among patients with AKI and among patients without AKI. Additionally, the slopes of eGFR after 3 months were compared between patients with AKI and without AKI.
Association between AKI and incident CKD
Among 5272 patients in the study, 938 (including 69 with AKI) did not have CKD—both at baseline and at 3 months postoperatively (Fig. 1 and Supplementary data, Table S2). During a median follow-up interval of 2.75 years, 226 of the 938 patients developed incident CKD after 3 months (7.77 events/100 person-years). eGFR at 3 months postoperatively tended to be higher among patients with AKI [mean eGFR 91.2 mL/min/1.73 m2 (SD 17.8)] than among patients without AKI [mean eGFR 87.8 mL/min/1.73 m2 (SD 19.6)]. Nevertheless, the adjusted cumulative incidence curve and multivariable Cox proportional hazard model confirmed that AKI was independently associated with incident CKD after 3 months postoperatively, despite adjustment for eGFR at 3 months {hazard ratio (HR) 1.73 [95% confidence interval (CI) 1.06–2.84]} (Fig. 3). Competing risk regression analysis detected 76 competing events; however, the analysis yielded a result similar to the multivariable Cox proportional hazard model findings [subhazard ratio (SHR) 1.73 (95% CI 1.06–2.84)]. This association remained despite additional adjustment for baseline eGFR [HR 2.04 (95% CI 1.24–3.36); SHR 2.02 (95% CI 1.21–3.35)].
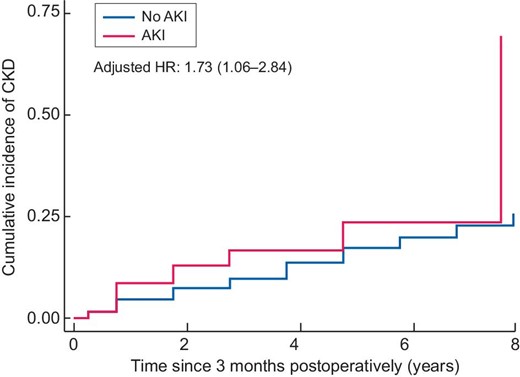
Adjusted cumulative incidence curves and HRs for incident CKD. Time 0 indicates 3 months postoperatively.
Association between AKI and decline in eGFR ≥30%
Of the 938 patients without CKD—both at baseline and at 3 months postoperatively—161 developed a decline in eGFR ≥30% after 3 months (5.36 events/100 person-years) during a median follow-up interval of 3.75 years. Despite adjustment for eGFR at 3 months postoperatively, the adjusted cumulative incidence curve and multivariable Cox proportional hazard model confirmed that AKI was independently associated with a decline in eGFR ≥30% [HR 2.41 (95% CI 1.51–3.84)] (Fig. 4). Competing risk regression analysis detected 88 competing events; however, the analysis yielded a result similar to the multivariable Cox proportional hazard model findings [SHR 2.39 (95% CI 1.49–3.84)]. This association remained despite additional adjustment for baseline eGFR [HR 2.06 (95% CI 1.28–3.34; SHR 2.08 (95% CI 1.28–3.38)].
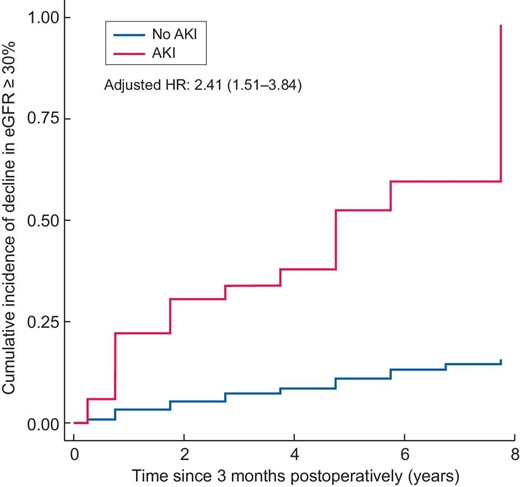
Adjusted cumulative incidence curves and HRs for decline in eGFR ≥30%. Time 0 indicates 3 months postoperatively.
DISCUSSION
This cohort study demonstrated that, compared with baseline, creatinine-based eGFR at 3 months postoperatively was stable even in patients with AKI and eGFR declined thereafter. Linear mixed-effects model analysis confirmed that eGFR slopes differed within and after 3 months postoperatively for patients with and without AKI. Furthermore, the decline in eGFR after 3 months was faster among patients with AKI than among patients without AKI. Among patients who did not have CKD—both at baseline and at 3 months postoperatively—AKI was independently associated with incident CKD or a decline in eGFR ≥30% after 3 months despite adjustment for eGFR at 3 months postoperatively.
The inflection point in the eGFR slope at 3 months postoperatively may have been caused by the hospitalization-related loss of muscle mass at 3 months in patients who underwent surgery or had acute illness. Among patients with critical illness, muscle wasting can rapidly occur because of disuse atrophy and systemic inflammation [21]. A recent study suggested that substantial leg muscle atrophy occurred in patients who were hospitalized for 6 days after elective surgery [22]. Thus the use of creatinine-based eGFR might lead to an overestimation of the true GFR [23]. Although the KDIGO guideline recommends the evaluation of kidney function at 3 months after AKI [8], our results suggest that the use of creatinine measurement for this evaluation has important limitations and measured creatinine clearance or cystatin C–based eGFR might be more appropriate. Recently, one prospective cohort study demonstrated that the associations of AKI with incident heart failure and death after 3 months were not significant after adjustment for cystatin C and creatinine-based eGFR at 3 months after AKI [24]. Those results suggested that excess risks of heart failure and death among patients with AKI are mediated by persistent kidney dysfunction at 3 months. However, the authors did not examine whether such excess risks were present for incident CKD or the progression of CKD. Furthermore, it was unclear whether the results would have differed if the analysis had used creatinine-based eGFR without cystatin C.
Here we demonstrated that regardless of normal creatinine-based eGFR values at 3 months after AKI, AKI remains associated with incident CKD and a decline in eGFR ≥30%. Thus recovery of serum creatinine to baseline values does not preclude the potential for permanent damage to the kidneys after AKI. We previously reported an association between AKI and anemia during long-term follow-up [13]. In that study, postoperative AKI was independently associated with lower hematocrit at 3 months after surgery, despite adjustment for eGFR at 3 months. This result was interpreted as the impairment of erythropoietin production because of interstitial damage after AKI; the damage was not reflected by eGFR at 3 months. We presume that tissue damage progresses and becomes apparent as a decline in kidney function during long-term follow-up. Our results suggest that the preservation of creatine-based eGFR at 3 months after AKI does not guarantee the preservation of kidney function thereafter.
There were a few notable strengths in this study. To our knowledge, this is the first study to examine whether creatinine-based kidney function at 3 months after AKI is useful as an indicator of future kidney dysfunction. Additionally, because the maximum follow-up interval was 8 years, the overall follow-up interval in this study is among the longest in published studies of AKI.
Nevertheless, there were some limitations to this study. First, patients with AKI might have more comorbidities and thus might undergo more frequent measurements of creatinine and this might have increased the likelihood of detecting kidney dysfunction. We attempted to minimize this potential bias by limiting the number of time points for eGFR data collection. However, the bias could not be completely precluded and the numbers of analyzed patients decreased in the mixed-effects models. Second, we could not determine whether patients with and without AKI lost similar amounts of muscle mass after surgery. Because of differences in their backgrounds and acute illness severities, patients with AKI might be more susceptible to muscle mass reduction at 3 months, thus their eGFR values at 3 months could be overestimated. We could not rule out the possibility that the difference in eGFR slope after 3 months might reflect a severe overestimation of eGFR at 3 months among patients with AKI. Third, data concerning changes in kidney function before surgery were not available.
In conclusion, AKI was associated with worse kidney outcomes regardless of eGFR at 3 months postoperatively. The evaluation of creatinine-based eGFR at 3 months after AKI might have limited value because of acute illness–related muscle mass reduction; the use of measured creatinine clearance or cystatin C–based eGFR might provide greater clinical benefit. Finally, the preservation of creatinine-based eGFR at 3 months after AKI does not guarantee subsequent preservation of kidney function.
ACKNOWLEDGEMENTS
We thank Ryan Chastain-Gross, from Edanz (https://jp.edanz.com/ac), for editing a draft of this manuscript. This study was supported by the Nara Medical University Grant-in-Aid for Young Scientists.
AUTHORS’ CONTRIBUTIONS
M.N.,M.Mu.,M.K. and K.T. were responsible for conception or design, analysis and interpretation of data. M.N., M.Mu., M.K., M.Ma., M.E., K.S., Y.A. and K.T. were responsible for drafting or revision of the article. M.Mu., M.Ma., M.E., K.S., Y.A. and K.T. were responsible for provision of important intellectual content. M.N., M.Mu., M.K., M.Ma., M.E., K.S., Y.A. and K.T. were responsible for final approval of the version to be published.
CONFLICT OF INTEREST STATEMENT
The authors have nothing to disclose.
DATA AVAILABILITY STATEMENT
The study data are available from the corresponding author upon reasonable request.





Comments