-
PDF
- Split View
-
Views
-
Cite
Cite
Sivaramakrishnan Ramanarayanan, Shivani Sharma, Oscar Swift, Keith R Laws, Hamza Umar, Ken Farrington, Systematic review and meta-analysis of preoperative interventions to support the maturation of arteriovenous fistulae in patients with advanced kidney disease, Nephrology Dialysis Transplantation, Volume 38, Issue 10, October 2023, Pages 2330–2339, https://doi.org/10.1093/ndt/gfad040
Close - Share Icon Share
ABSTRACT
There is great potential to improve outcomes of arteriovenous fistulas (AVFs) by focusing more on the preoperative period of AVF creation. We aim to systematically review the evidence on safety and efficacy of various preoperative interventions that have been tried to improve AVF maturation and success rate.
We searched five databases: PubMed, Embase, CINAHL, Cochrane Library and King's Fund Library. Experimental studies that investigated the effect of various preoperative interventions to improve AVF outcomes among advanced chronic kidney disease (CKD) patients were searched. The effect size for primary outcome was calculated as the weighted mean difference in the final vessel calibre, rate of AVF maturation or primary failure between the intervention and control arm. We also assessed adverse effects and dropout rates. This review was preregistered in the International Prospective Register of Systematic Reviews (CRD42020193257).
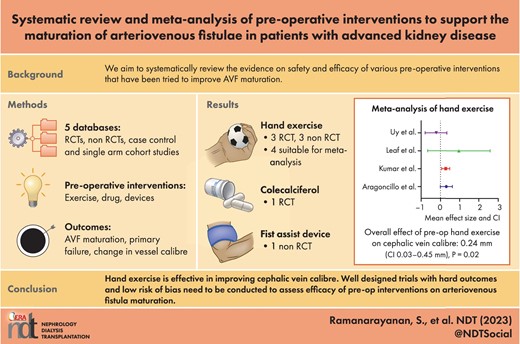
Eight eligible studies were identified involving three types of intervention: hand exercise (n = 6), cholecalciferol supplementation (n = 1) and pneumatic compression of the arm using a Fist Assist device (n = 1). The overall effect size of hand exercise on distal cephalic vein calibre was 0.24 mm [95% confidence interval (CI) 0.03–0.45] on meta-analysis of hand exercise studies. On restricting analysis to two randomized controlled trials (RCTs) that had independent control groups, the effect size was higher, at 0.29 mm (95% CI 0.11–0.47). Hand exercise was a well-tolerated intervention, especially when confined to the first 4 weeks.
Hand exercise is the predominant intervention tried in the preoperative period of AVF creation, although there is methodological heterogeneity. Intermittent pneumatic compression using a Fist Assist device is a novel intervention that has shown some promise. Well-designed prospective RCTs are needed on preoperative interventions among advanced CKD patients, aimed at improving AVF outcomes.
What is already known about this subject?
The rate of non-maturation of arteriovenous fistulas (AVFs) remains high.
There is a paucity of data on the relative efficacy of interventions applied in the preoperative period of AVF creation that can improve AVF success rate.
What this study adds?
This study provides the most up-to-date data on various preoperative interventions that have been applied to improve AVF outcomes.
This study provides a meta-analysis of effect size of preoperative hand exercise on vessel calibre.
The paucity of preoperative studies with hard outcomes such as AVF maturation and primary failure rate and various limitations of study designs are highlighted.
What impact this may have on practice or policy?
The study points towards positive effects of hand exercise and intermittent pneumatic compression of arm veins on vessel calibre.
Readers are advised on choosing the exercise regime that gives the best effect size in the preoperative period.
This study will also hopefully encourage researchers to design studies on preoperative interventions with more hard endpoints.
INTRODUCTION
Arteriovenous fistulas (AVFs) are the preferred form of vascular access (VA). They have the lowest rate of infection, stenosis and thrombosis among all types of VA [1, 2]. However, despite the creation of AVFs well before dialysis initiation, a significant proportion fail and patients need to start haemodialysis (HD) through a catheter, putting them at high risk of catheter-related complications. Globally, the rate of primary AVF failure has been estimated at 30–70%, with a 1-year patency rate of 40–70% [3]. Variations in the estimates of AVF failure are likely to reflect operational definitions of what constitutes ‘failure’: e.g. whether the AVF is unsuitable for HD and requires a ‘salvage’ intervention or failure is defined by other problematic outcomes such as thrombosis alone [4]. Bylsma et al. [5], in their systematic review and meta-analysis of AVFs for dialysis, stated that there is ambiguity in how patency is reported across studies, notably that definitions used often do not align to clinical utility.
The use of prediction tools to estimate the risk of failure based on a mix of clinical and patient demographic factors [6] as well as preoperative vessel mapping [7] has been suggested to improve AVF maturation, but the rate of primary failure and non-maturation remain at unacceptably high levels. Therefore, interventions aimed at reducing early failure will be advantageous economically, help facilitate patient decision making around VA choices and improve their overall health-related quality of life [8].
Most patients with advanced kidney disease are under the care of a nephrologist long before AVF creation is required. This offers a window of opportunity for interventions to improve success rates of AVF maturation. Although there are individual systematic reviews that summarize the outcomes of individual intervention types (hand exercises, pharmacological measures, infrared therapy etc.) on AVF, these have not focused on teasing whether the preoperative period specifically is an important factor in intervention success. For example, Bashar et al. [9] looked at the impact of far infrared therapy in AVF maturation, but the randomized controlled trials (RCTs) included in their review had data from patients who were already receiving HD through an AVF.
The aim of this systemic review and meta-analysis is to summarize the types of preoperative interventions that have been implemented to support AVF maturation, to pool data across RCTs specifically to estimate the impact of interventions on outcomes of interest and to undertake subgroup and mediator analyses to understand if the interventions work better for specific patient groups.
MATERIALS AND METHODS
This review was preregistered in the International Prospective Register of Systematic Reviews (PROSPERO; CRD42020193257).
Review process and search strategy
The systematic review and meta-analyses were undertaken following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [10]. The following electronic databases were used to perform searches of published articles without any restrictions and all articles were captured up to 15 May 2022: PubMed, Embase, CINAHL, Cochrane Library and King's Fund Library. The search strategy can be found on https://www.crd.york.ac.uk/PROSPEROFILES/193257_STRATEGY_20200619.pdf.
In brief, a combination of terms for advanced kidney disease or end-stage kidney disease (ESKD), haemodialysis, AVF maturation or patency and medical or surgical interventions (including exercises) were combined through the PICO format. Broad terms were used in the search strategy to capture as many studies as possible.
The results of searches were then transferred to Endnote citation manager (version X9.3.3; Clarivate, Philadelphia, PA, USA). After removing duplicate publications, titles and abstracts were screened.
Study selection criteria
S.R. screened the titles and abstracts against the following inclusion criteria: the study involved adult patients with advanced CKD including patients with ESKD; the study explored the efficacy and/or safety of a preoperative intervention aimed at improving the rate of AVF creation or outcome of AVF surgery (using preoperative endpoints such as vein calibre, brachial artery flow etc. or postoperative endpoints such as AVF maturation, primary failure rate); the interventions are confined to the preoperative period of AVF creation; and the study was published in English.
We excluded studies that looked at outcomes based on synthetic AV grafts and also studies that were purely investigative, such as aids in vessel selection for AVF creation (e.g. vein mapping) and computational fluid dynamics.
The full texts of potentially relevant citations were accessed and assessed using the inclusion/exclusion criteria by two authors (S.R. and O.S.) independently. Any disagreement or discrepancies were discussed and resolved with other members of the study team (K.F. and S.S.).
We included RCTs, non-RCTs, case–control studies and cohort studies, including single-arm prospective studies that used baseline values before intervention as a control. We did not include reviews, although they were screened for potentially eligible studies. The primary outcome of interest was maturation of the AVF, assessed by various direct or indirect ways including the proportion of patients achieving the criteria of AVF maturation within a specified time frame in the intervention versus control group; the incidence of primary failure of the AVF in the intervention and control groups; preoperative endpoints such as venous diameter, venous distensibility, arterial diameter or flow and an increase in vessels available for AVF creation, especially in the distal forearm. Additional outcomes of interest included adverse events, dropout rate and adherence.
Data extraction
The data from eligible studies were extracted by one author (S.R.). The data extracted included author details, country of origin, year published, population studied, eligibility criteria, sample size, study design, baseline demographic and clinical characteristics, intervention characteristics (such as nature, dose and duration, supervised or unsupervised, adherence rate), follow-up time, outcome parameters and adverse events.
Where adequate data were not available, attempts were made to contact the principal investigator to obtain the necessary information.
Quality and risk of bias assessments
The quality and risk of bias assessments were performed by two independent reviewers (S.R. and H.U.). The Cochrane Risk of Bias in Non-randomized Studies of Interventions (ROBINS I) tool [11] was used for risk of bias assessment of non-randomized study designs (Supplemental Table 1) while the Cochrane Risk of Bias 2 (ROB-2) tool [12] was used to determine the quality and risk of bias for randomized study designs (Supplemental Table 2). After independent risk of bias analysis, the two independent reviewers met and discussed their results and discrepancies, if any, were resolved.
Statistical analysis
The outcome data were grouped according to the type of intervention. For example, studies implementing hand exercise as the intervention were grouped together in order to pool the outcome data. Where it was not possible to pool the data due to significant heterogeneity, the data were summarized narratively. Binary outcomes were expressed as odds ratios (ORs) and 95% confidence intervals (CIs). Only one type of intervention, namely hand exercise, was suitable for meta-analysis, as the relevant studies had common outcome variables, namely vessel diameter. As all hand exercise studies consistently reported cephalic vein diameter in the distal forearm at baseline and end of follow-up (6–8 weeks), this parameter was pooled and meta-analysis was performed using ReviewManager version 5.4 software (https://training.cochrane.org/online-learning/core-software/revman) [13]. The effect size was expressed as the weighted mean difference in the final diameter between the intervention and the control arm. A random effects model was applied for meta-analysis of the effect size for hand exercise on vessel diameters.
Heterogeneity was assessed using the I2 statistic, and for interpretation we followed Cochrane guidance [14], where I2 values of 0–40% identified as might not be important, 30–60% as moderate heterogeneity, 50–90% as substantial heterogeneity and 75–100% as considerable heterogeneity. The presence of any significant publication bias was assessed by examining funnel plots for asymmetry and through the Egger's test.
RESULTS
Study and participant characteristics
The steps of study selection and exclusion are summarized in the PRISMA diagram (Fig. 1). The reason for study exclusion at each step was recorded.
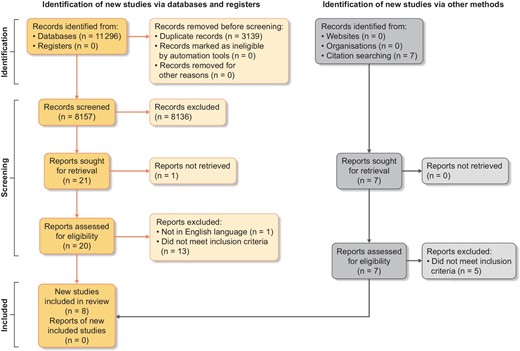
Eight studies, conducted between 2003 and 2021, were eligible for inclusion in the systematic review (for study characteristics, see Table 1). The number of participants ranged from 5 patients to a maximum of 138 patients, with total participants across the eight studies being 323.
| Study and year . | Intervention . | Study design . | Mean follow-up . | Population source . | Study size . | Mean age . | Gender (% Males) . | Diabetics (%) . | Outcome measure . |
|---|---|---|---|---|---|---|---|---|---|
| Sauco et al., 2019 [15] | IA: isometric exercise, hand grip contractions; elastic band, arm flexion and extension CA: no exercise | RCT, open label | AVF surgery after 8 weeks of exercise and follow-up until 3 months after AVF creation | 4 centres in Madrid, Spain, CKD stage 4/5 | 167 screened,138 randomized, 68 in intervention and 70 in control | IA: 64.7 (21.1) CA: 67.9 (55.3) | IA: 79.2% CA:72.1% | IA: 52.8% CA: 55.7% | Primary: percentage PF by 12 weeks; secondary: mean increase in venous calibre, mean increase in PSV, % increase in distal AVF creation |
| Barbosa et al., 2017 [16] | IA: exercise (isometric and isotonic) BFR training CA: exercise without BFR | RCT, double blind | 8 weeks from recruitment | Nephrology outpatients, CKD 4/5 | 35 screened, 26 randomized, 12 in BFR and 14 in CA | IA: 61.33 ± 7.82 CA: 60.14 ± 10.67 | IA: 66.7% CA: 71.4% | IA: 75% CA: 21.4% | Primary outcome: increased CV diameter (mm) of at least 0.22 mm; secondary: increased CV distensibility (mm), increased RA diameter, increased PSV (cm/s) and mean velocity (cm/s) in the upper limbs, increased forearm circumference (cm) and increased HGS |
| Kumar et al., 2020 [17] | IA: isometric hand grip exercise CA: no exercise | RCT, open label | 8 weeks | Nephrology outpatients, CKD 4/5 | 36 patients, 18 intervention, 18 control. 2 in exercise arm lost to FU | IA: 48.6 ± 3.4 CA: 43.4 ± 3.7 | IA:75% CA: 77.8% | IA: 18.8% CA: 16.7% | Primary: mean change in the distal forearm CV diameter between the intervention and control group at weeks 4 and 8; secondary: HGS |
| Uy et al., 2012 [21] | IA: isometric hand grip exercise in one arm CA: opposite limb of same patient | Split-body trial | 8 weeks | Nephrology outpatients, CKD 4/5 | 15 patients (28 limbs examined, as no detectable distal veins in 2 non-exercise limbs) | 68.7 ± 4.2 | 46% | 20% | Primary: mean change in venous diameter between the control and study arm at weeks 4 and 8; secondary: proportion of subjects that achieved at least one CV diameter >2.5 mm, proportion of subjects who had a successful AVF placement |
| Rus et al., 2003 [19] | Isometric hand grip exercise in non-AVF arm | Single-arm prospective study | 8 weeks | HD patients | 14 patients, 7 male and 7 female | 49 ± 11 | 50% | 35.7% | Change/increase in forearm circumference, venous diameter with and without tourniquet, arterial diameter and flow, HGS, EDV at 8 weeks |
| Leaf et al., 2003 [20] | IA: isometric hand grip exercise in one arm CA: opposite limb of the same patient | Split-body trial | 6 weeks | Nephrology outpatient, CKD with SCr >1.5 | 5 patients | 57 ± 9 | 100% | 60% | Change/increase in distal CV capacitance as measured by CSA with and without a tourniquet, increase in HGS |
| Wasse et al., 2011 [18] | IA: high-dose cholecalciferol 200 000 U once weekly for 3 weeks CA: placebo | RCT, double blind | 6 months after AVF creation | Adult ESRD patients receiving in-centre MHD and planned for AVF creation | 52 randomized, 25 to cholecalciferol, 27 to placebo. Final analysis: 44: 20 cholecalciferol and 24 placebo | IA: 49.9 ± 10.9 CA: 52.1 ± 14.9 | IA: 75% CA: 62.5% | IA: 45% CA: 50% | AVF maturation, defined as the ability to cannulate the AVF with two large-bore needles at ≥6 consecutive dialysis sessions and achievement of an AVF blood flow >300 ml/min assessed at 6 months after AVF creation |
| Hammes et al., 2021 [22] | Intermittent pneumatic compression of the arm using Fist Assist device | Single-arm prospective study | 84 hours/day for 3 months | CKD stage 4 and 5 patients anticipated to start HD | 37 patients (46 enrolled) | 62.1 ± 12.61 | 56.8% | 59.5% | Increase in upper arm and forearm cephalic vein diameter with and without blood pressure cuff, proportion of veins achieving threshold for suitability for AVF |
| Study and year . | Intervention . | Study design . | Mean follow-up . | Population source . | Study size . | Mean age . | Gender (% Males) . | Diabetics (%) . | Outcome measure . |
|---|---|---|---|---|---|---|---|---|---|
| Sauco et al., 2019 [15] | IA: isometric exercise, hand grip contractions; elastic band, arm flexion and extension CA: no exercise | RCT, open label | AVF surgery after 8 weeks of exercise and follow-up until 3 months after AVF creation | 4 centres in Madrid, Spain, CKD stage 4/5 | 167 screened,138 randomized, 68 in intervention and 70 in control | IA: 64.7 (21.1) CA: 67.9 (55.3) | IA: 79.2% CA:72.1% | IA: 52.8% CA: 55.7% | Primary: percentage PF by 12 weeks; secondary: mean increase in venous calibre, mean increase in PSV, % increase in distal AVF creation |
| Barbosa et al., 2017 [16] | IA: exercise (isometric and isotonic) BFR training CA: exercise without BFR | RCT, double blind | 8 weeks from recruitment | Nephrology outpatients, CKD 4/5 | 35 screened, 26 randomized, 12 in BFR and 14 in CA | IA: 61.33 ± 7.82 CA: 60.14 ± 10.67 | IA: 66.7% CA: 71.4% | IA: 75% CA: 21.4% | Primary outcome: increased CV diameter (mm) of at least 0.22 mm; secondary: increased CV distensibility (mm), increased RA diameter, increased PSV (cm/s) and mean velocity (cm/s) in the upper limbs, increased forearm circumference (cm) and increased HGS |
| Kumar et al., 2020 [17] | IA: isometric hand grip exercise CA: no exercise | RCT, open label | 8 weeks | Nephrology outpatients, CKD 4/5 | 36 patients, 18 intervention, 18 control. 2 in exercise arm lost to FU | IA: 48.6 ± 3.4 CA: 43.4 ± 3.7 | IA:75% CA: 77.8% | IA: 18.8% CA: 16.7% | Primary: mean change in the distal forearm CV diameter between the intervention and control group at weeks 4 and 8; secondary: HGS |
| Uy et al., 2012 [21] | IA: isometric hand grip exercise in one arm CA: opposite limb of same patient | Split-body trial | 8 weeks | Nephrology outpatients, CKD 4/5 | 15 patients (28 limbs examined, as no detectable distal veins in 2 non-exercise limbs) | 68.7 ± 4.2 | 46% | 20% | Primary: mean change in venous diameter between the control and study arm at weeks 4 and 8; secondary: proportion of subjects that achieved at least one CV diameter >2.5 mm, proportion of subjects who had a successful AVF placement |
| Rus et al., 2003 [19] | Isometric hand grip exercise in non-AVF arm | Single-arm prospective study | 8 weeks | HD patients | 14 patients, 7 male and 7 female | 49 ± 11 | 50% | 35.7% | Change/increase in forearm circumference, venous diameter with and without tourniquet, arterial diameter and flow, HGS, EDV at 8 weeks |
| Leaf et al., 2003 [20] | IA: isometric hand grip exercise in one arm CA: opposite limb of the same patient | Split-body trial | 6 weeks | Nephrology outpatient, CKD with SCr >1.5 | 5 patients | 57 ± 9 | 100% | 60% | Change/increase in distal CV capacitance as measured by CSA with and without a tourniquet, increase in HGS |
| Wasse et al., 2011 [18] | IA: high-dose cholecalciferol 200 000 U once weekly for 3 weeks CA: placebo | RCT, double blind | 6 months after AVF creation | Adult ESRD patients receiving in-centre MHD and planned for AVF creation | 52 randomized, 25 to cholecalciferol, 27 to placebo. Final analysis: 44: 20 cholecalciferol and 24 placebo | IA: 49.9 ± 10.9 CA: 52.1 ± 14.9 | IA: 75% CA: 62.5% | IA: 45% CA: 50% | AVF maturation, defined as the ability to cannulate the AVF with two large-bore needles at ≥6 consecutive dialysis sessions and achievement of an AVF blood flow >300 ml/min assessed at 6 months after AVF creation |
| Hammes et al., 2021 [22] | Intermittent pneumatic compression of the arm using Fist Assist device | Single-arm prospective study | 84 hours/day for 3 months | CKD stage 4 and 5 patients anticipated to start HD | 37 patients (46 enrolled) | 62.1 ± 12.61 | 56.8% | 59.5% | Increase in upper arm and forearm cephalic vein diameter with and without blood pressure cuff, proportion of veins achieving threshold for suitability for AVF |
BFR: blood flow restriction; CA: control arm; CSA: cross-sectional area; CV: cephalic vein; HGS: hand grip strength; IA: intervention arm; MHD: maintenance haemodialysis; PSV: peak systolic velocity; RA: radial artery.
| Study and year . | Intervention . | Study design . | Mean follow-up . | Population source . | Study size . | Mean age . | Gender (% Males) . | Diabetics (%) . | Outcome measure . |
|---|---|---|---|---|---|---|---|---|---|
| Sauco et al., 2019 [15] | IA: isometric exercise, hand grip contractions; elastic band, arm flexion and extension CA: no exercise | RCT, open label | AVF surgery after 8 weeks of exercise and follow-up until 3 months after AVF creation | 4 centres in Madrid, Spain, CKD stage 4/5 | 167 screened,138 randomized, 68 in intervention and 70 in control | IA: 64.7 (21.1) CA: 67.9 (55.3) | IA: 79.2% CA:72.1% | IA: 52.8% CA: 55.7% | Primary: percentage PF by 12 weeks; secondary: mean increase in venous calibre, mean increase in PSV, % increase in distal AVF creation |
| Barbosa et al., 2017 [16] | IA: exercise (isometric and isotonic) BFR training CA: exercise without BFR | RCT, double blind | 8 weeks from recruitment | Nephrology outpatients, CKD 4/5 | 35 screened, 26 randomized, 12 in BFR and 14 in CA | IA: 61.33 ± 7.82 CA: 60.14 ± 10.67 | IA: 66.7% CA: 71.4% | IA: 75% CA: 21.4% | Primary outcome: increased CV diameter (mm) of at least 0.22 mm; secondary: increased CV distensibility (mm), increased RA diameter, increased PSV (cm/s) and mean velocity (cm/s) in the upper limbs, increased forearm circumference (cm) and increased HGS |
| Kumar et al., 2020 [17] | IA: isometric hand grip exercise CA: no exercise | RCT, open label | 8 weeks | Nephrology outpatients, CKD 4/5 | 36 patients, 18 intervention, 18 control. 2 in exercise arm lost to FU | IA: 48.6 ± 3.4 CA: 43.4 ± 3.7 | IA:75% CA: 77.8% | IA: 18.8% CA: 16.7% | Primary: mean change in the distal forearm CV diameter between the intervention and control group at weeks 4 and 8; secondary: HGS |
| Uy et al., 2012 [21] | IA: isometric hand grip exercise in one arm CA: opposite limb of same patient | Split-body trial | 8 weeks | Nephrology outpatients, CKD 4/5 | 15 patients (28 limbs examined, as no detectable distal veins in 2 non-exercise limbs) | 68.7 ± 4.2 | 46% | 20% | Primary: mean change in venous diameter between the control and study arm at weeks 4 and 8; secondary: proportion of subjects that achieved at least one CV diameter >2.5 mm, proportion of subjects who had a successful AVF placement |
| Rus et al., 2003 [19] | Isometric hand grip exercise in non-AVF arm | Single-arm prospective study | 8 weeks | HD patients | 14 patients, 7 male and 7 female | 49 ± 11 | 50% | 35.7% | Change/increase in forearm circumference, venous diameter with and without tourniquet, arterial diameter and flow, HGS, EDV at 8 weeks |
| Leaf et al., 2003 [20] | IA: isometric hand grip exercise in one arm CA: opposite limb of the same patient | Split-body trial | 6 weeks | Nephrology outpatient, CKD with SCr >1.5 | 5 patients | 57 ± 9 | 100% | 60% | Change/increase in distal CV capacitance as measured by CSA with and without a tourniquet, increase in HGS |
| Wasse et al., 2011 [18] | IA: high-dose cholecalciferol 200 000 U once weekly for 3 weeks CA: placebo | RCT, double blind | 6 months after AVF creation | Adult ESRD patients receiving in-centre MHD and planned for AVF creation | 52 randomized, 25 to cholecalciferol, 27 to placebo. Final analysis: 44: 20 cholecalciferol and 24 placebo | IA: 49.9 ± 10.9 CA: 52.1 ± 14.9 | IA: 75% CA: 62.5% | IA: 45% CA: 50% | AVF maturation, defined as the ability to cannulate the AVF with two large-bore needles at ≥6 consecutive dialysis sessions and achievement of an AVF blood flow >300 ml/min assessed at 6 months after AVF creation |
| Hammes et al., 2021 [22] | Intermittent pneumatic compression of the arm using Fist Assist device | Single-arm prospective study | 84 hours/day for 3 months | CKD stage 4 and 5 patients anticipated to start HD | 37 patients (46 enrolled) | 62.1 ± 12.61 | 56.8% | 59.5% | Increase in upper arm and forearm cephalic vein diameter with and without blood pressure cuff, proportion of veins achieving threshold for suitability for AVF |
| Study and year . | Intervention . | Study design . | Mean follow-up . | Population source . | Study size . | Mean age . | Gender (% Males) . | Diabetics (%) . | Outcome measure . |
|---|---|---|---|---|---|---|---|---|---|
| Sauco et al., 2019 [15] | IA: isometric exercise, hand grip contractions; elastic band, arm flexion and extension CA: no exercise | RCT, open label | AVF surgery after 8 weeks of exercise and follow-up until 3 months after AVF creation | 4 centres in Madrid, Spain, CKD stage 4/5 | 167 screened,138 randomized, 68 in intervention and 70 in control | IA: 64.7 (21.1) CA: 67.9 (55.3) | IA: 79.2% CA:72.1% | IA: 52.8% CA: 55.7% | Primary: percentage PF by 12 weeks; secondary: mean increase in venous calibre, mean increase in PSV, % increase in distal AVF creation |
| Barbosa et al., 2017 [16] | IA: exercise (isometric and isotonic) BFR training CA: exercise without BFR | RCT, double blind | 8 weeks from recruitment | Nephrology outpatients, CKD 4/5 | 35 screened, 26 randomized, 12 in BFR and 14 in CA | IA: 61.33 ± 7.82 CA: 60.14 ± 10.67 | IA: 66.7% CA: 71.4% | IA: 75% CA: 21.4% | Primary outcome: increased CV diameter (mm) of at least 0.22 mm; secondary: increased CV distensibility (mm), increased RA diameter, increased PSV (cm/s) and mean velocity (cm/s) in the upper limbs, increased forearm circumference (cm) and increased HGS |
| Kumar et al., 2020 [17] | IA: isometric hand grip exercise CA: no exercise | RCT, open label | 8 weeks | Nephrology outpatients, CKD 4/5 | 36 patients, 18 intervention, 18 control. 2 in exercise arm lost to FU | IA: 48.6 ± 3.4 CA: 43.4 ± 3.7 | IA:75% CA: 77.8% | IA: 18.8% CA: 16.7% | Primary: mean change in the distal forearm CV diameter between the intervention and control group at weeks 4 and 8; secondary: HGS |
| Uy et al., 2012 [21] | IA: isometric hand grip exercise in one arm CA: opposite limb of same patient | Split-body trial | 8 weeks | Nephrology outpatients, CKD 4/5 | 15 patients (28 limbs examined, as no detectable distal veins in 2 non-exercise limbs) | 68.7 ± 4.2 | 46% | 20% | Primary: mean change in venous diameter between the control and study arm at weeks 4 and 8; secondary: proportion of subjects that achieved at least one CV diameter >2.5 mm, proportion of subjects who had a successful AVF placement |
| Rus et al., 2003 [19] | Isometric hand grip exercise in non-AVF arm | Single-arm prospective study | 8 weeks | HD patients | 14 patients, 7 male and 7 female | 49 ± 11 | 50% | 35.7% | Change/increase in forearm circumference, venous diameter with and without tourniquet, arterial diameter and flow, HGS, EDV at 8 weeks |
| Leaf et al., 2003 [20] | IA: isometric hand grip exercise in one arm CA: opposite limb of the same patient | Split-body trial | 6 weeks | Nephrology outpatient, CKD with SCr >1.5 | 5 patients | 57 ± 9 | 100% | 60% | Change/increase in distal CV capacitance as measured by CSA with and without a tourniquet, increase in HGS |
| Wasse et al., 2011 [18] | IA: high-dose cholecalciferol 200 000 U once weekly for 3 weeks CA: placebo | RCT, double blind | 6 months after AVF creation | Adult ESRD patients receiving in-centre MHD and planned for AVF creation | 52 randomized, 25 to cholecalciferol, 27 to placebo. Final analysis: 44: 20 cholecalciferol and 24 placebo | IA: 49.9 ± 10.9 CA: 52.1 ± 14.9 | IA: 75% CA: 62.5% | IA: 45% CA: 50% | AVF maturation, defined as the ability to cannulate the AVF with two large-bore needles at ≥6 consecutive dialysis sessions and achievement of an AVF blood flow >300 ml/min assessed at 6 months after AVF creation |
| Hammes et al., 2021 [22] | Intermittent pneumatic compression of the arm using Fist Assist device | Single-arm prospective study | 84 hours/day for 3 months | CKD stage 4 and 5 patients anticipated to start HD | 37 patients (46 enrolled) | 62.1 ± 12.61 | 56.8% | 59.5% | Increase in upper arm and forearm cephalic vein diameter with and without blood pressure cuff, proportion of veins achieving threshold for suitability for AVF |
BFR: blood flow restriction; CA: control arm; CSA: cross-sectional area; CV: cephalic vein; HGS: hand grip strength; IA: intervention arm; MHD: maintenance haemodialysis; PSV: peak systolic velocity; RA: radial artery.
Of the eight included studies, four were RCTs, with three of them involving hand exercise [15–17] as the test intervention and one involved a drug, namely cholecalciferol, as the test intervention compared with placebo [18].
Three eligible non-randomized studies tested the efficacy of different regimens of hand exercises on vascular structural and functional parameters. These three studies were single-arm prospective studies in which the intervention was performed on a cohort of patients with advanced CKD or ESKD [19–21]. However, in the studies by Leaf et al. [20] and Uy et al. [21], the pre- and post-intervention variables were compared between the exercise limb and the non-exercise limb (split-body trial design). This was not possible in the study conducted by Rus et al. [19], as the patients included were HD patients with an AVF in one arm. Yet another non-randomized study involved testing the efficacy of intermittent pneumatic compression of the arm with the Fist Assist device [22].
As hand exercise was the most common type of intervention studied in the preoperative period, these studies are summarized in Table 2.
Summary of exercise regimens and effect on various vessel structure and function parameters across selected studies.
| Author and year . | Intervention, co-interventions and controls . | Grip strength . | Effect size on vein calibre in intervention arm . | Effect size on vein calibre in control arm . | Artery parameters . |
|---|---|---|---|---|---|
| Sauco et al., 2021 [15] | Two sets of 30 ISM hand grip contractions twice a day. At noon: slow contraction session using elastic band with arm in flexion and extension. Comparator: no exercise. Duration 8 weeks | Significant increase of 4.33 kg (SD 4.5) in IA (P < .001); non-significant increase of 3.4 kg (SD 2.5) in the CA (P = .46) | Mean increase of 0.71 mm (SD 0.49; P < .001) at 8 weeks | Mean decrease of 0.112 mm (SD 0.48; P = .121) at 8 weeks | Increase in calibre by 0.12 mm (SD 0.31; P = .008) in IA Decrease in calibre by 0.02 mm (SD 0.32; P = .55) in CA |
| Barbosa et al., 2018 [16] | ISM + IST with tensiometer for BFR in intervention arm. Exercise only without BFR in control. Duration 8 weeks | Significant increase in dynamometry: in CA, 2.36 kg increase (P = .003); in IA, 2.25 kg increase (P = .06). No significant difference in increase in strength between arms, inter P = .302 | Mean increase of 0.20, 0.16 and 0.15 mm for the segments 2, 10 and 20 cm in CV (P = .16, .20 and .33 at these segments) | Mean increase in CV diameter of 0.24, 0.39 and 0.17 mm for the segments 2, 10 and 20 cm (P = .008, .001 and .237at these segments) | No significant changes in mean velocity at all segments across both groups (P = .279, .150 and .341 at 2, 10 and 20 cm) |
| Kumar et al., 2020 [17] | ISM handgrip exercise: 20 repetitions/min, 30 min/day. Comparator: no exercise. Duration 8 weeks | Increased by median of 4 kg on exercise arm; no significant increase in control arm | Mean change 0.31 ± 0.04 at 4 weeks (P < .05) without tourniquet and 0.40 ± 0.06 with tourniquet (P < .05) | No significant change with or without tourniquet | Not measured |
| Uy et al., 2012 [21] | ISM forearm strengthening exercises in preferred access arm: 10 sets of 20 repetitions/min. Comparator: non-exercise arm. Duration 8 weeks | Increase in the exercised arm from 0 to 8 weeks: 24.50 ± 2.05 kg to 27.04 ± 2.20 kg (P = .025). No change in control arm: 26.68 ± 2.60 kg to 26.82 ± 2.40 kg P = .929 | Mean changes in distal sites: 0.48 ± 0.16 at 4 weeks and 0.32 ± 0.20 at 8 weeks; in proximal sites: 0.59 ± 0.25 mm at 4 weeks and 0.52 ± 0.32 at 8 weeks | Mean changes in distal sites: 0.81 ± 0.20 at 4 weeks and 0.65 ± 0.15 at 8 weeks; in proximal sites: 0.71 ± 0.20 mm at 4 weeks and 0.43 ± 0.20 at 8 weeks. No significant difference in proximal or distal sites, as diameter increased in both arms (inter P = .209, .217, .726 and .826) | Not assessed |
| Rus et al., 2003 [19] | ISM hand grip exercise using a rubber ring: 4.5 cm inner and 7.5 cm outer diameter, maximal compression force 50 N. Frequency and intensity: trained and supervised by dialysis staff on HD days | Maximal hand grip strength measured with dynamometer increased significantly from 24.1 ± 2.95 mm to 26.2 ± 3.06 mm after 4 weeks and to 28.5 ± 3.17 mm after 8 weeks (P < .001) | Average vein diameter without tourniquet remained unchanged for 4 weeks but significantly increased after 8 weeks (P = .015). Average vein diameter after tourniquet significantly greater after 4 weeks (P = .007) and even greater at 8 weeks (P < .001). However, distensibility remained unchanged | No control arm | No significant change in endothelium-dependent vasodilation |
| Leaf et al., 2003 [20] | 6 weeks of ISM exercise at 30–40% MVC for 80–360 sec with increased frequency over time and repetitive isotonic squeezing of a squash ball or racquet ball. Non-exercise arm was comparator. Duration and frequency: 4 times per week for 6 weeks. Controlled heating employed | Paradoxically, the increase in venous size was unaccompanied by an increase in hand grip strength | 2-fold increase in CV diameter noted in most patients (P < .05) | Cephalic vein size increase was ≈0.4 mm in non-exercise arm (P = non-significant) | Not assessed |
| Author and year . | Intervention, co-interventions and controls . | Grip strength . | Effect size on vein calibre in intervention arm . | Effect size on vein calibre in control arm . | Artery parameters . |
|---|---|---|---|---|---|
| Sauco et al., 2021 [15] | Two sets of 30 ISM hand grip contractions twice a day. At noon: slow contraction session using elastic band with arm in flexion and extension. Comparator: no exercise. Duration 8 weeks | Significant increase of 4.33 kg (SD 4.5) in IA (P < .001); non-significant increase of 3.4 kg (SD 2.5) in the CA (P = .46) | Mean increase of 0.71 mm (SD 0.49; P < .001) at 8 weeks | Mean decrease of 0.112 mm (SD 0.48; P = .121) at 8 weeks | Increase in calibre by 0.12 mm (SD 0.31; P = .008) in IA Decrease in calibre by 0.02 mm (SD 0.32; P = .55) in CA |
| Barbosa et al., 2018 [16] | ISM + IST with tensiometer for BFR in intervention arm. Exercise only without BFR in control. Duration 8 weeks | Significant increase in dynamometry: in CA, 2.36 kg increase (P = .003); in IA, 2.25 kg increase (P = .06). No significant difference in increase in strength between arms, inter P = .302 | Mean increase of 0.20, 0.16 and 0.15 mm for the segments 2, 10 and 20 cm in CV (P = .16, .20 and .33 at these segments) | Mean increase in CV diameter of 0.24, 0.39 and 0.17 mm for the segments 2, 10 and 20 cm (P = .008, .001 and .237at these segments) | No significant changes in mean velocity at all segments across both groups (P = .279, .150 and .341 at 2, 10 and 20 cm) |
| Kumar et al., 2020 [17] | ISM handgrip exercise: 20 repetitions/min, 30 min/day. Comparator: no exercise. Duration 8 weeks | Increased by median of 4 kg on exercise arm; no significant increase in control arm | Mean change 0.31 ± 0.04 at 4 weeks (P < .05) without tourniquet and 0.40 ± 0.06 with tourniquet (P < .05) | No significant change with or without tourniquet | Not measured |
| Uy et al., 2012 [21] | ISM forearm strengthening exercises in preferred access arm: 10 sets of 20 repetitions/min. Comparator: non-exercise arm. Duration 8 weeks | Increase in the exercised arm from 0 to 8 weeks: 24.50 ± 2.05 kg to 27.04 ± 2.20 kg (P = .025). No change in control arm: 26.68 ± 2.60 kg to 26.82 ± 2.40 kg P = .929 | Mean changes in distal sites: 0.48 ± 0.16 at 4 weeks and 0.32 ± 0.20 at 8 weeks; in proximal sites: 0.59 ± 0.25 mm at 4 weeks and 0.52 ± 0.32 at 8 weeks | Mean changes in distal sites: 0.81 ± 0.20 at 4 weeks and 0.65 ± 0.15 at 8 weeks; in proximal sites: 0.71 ± 0.20 mm at 4 weeks and 0.43 ± 0.20 at 8 weeks. No significant difference in proximal or distal sites, as diameter increased in both arms (inter P = .209, .217, .726 and .826) | Not assessed |
| Rus et al., 2003 [19] | ISM hand grip exercise using a rubber ring: 4.5 cm inner and 7.5 cm outer diameter, maximal compression force 50 N. Frequency and intensity: trained and supervised by dialysis staff on HD days | Maximal hand grip strength measured with dynamometer increased significantly from 24.1 ± 2.95 mm to 26.2 ± 3.06 mm after 4 weeks and to 28.5 ± 3.17 mm after 8 weeks (P < .001) | Average vein diameter without tourniquet remained unchanged for 4 weeks but significantly increased after 8 weeks (P = .015). Average vein diameter after tourniquet significantly greater after 4 weeks (P = .007) and even greater at 8 weeks (P < .001). However, distensibility remained unchanged | No control arm | No significant change in endothelium-dependent vasodilation |
| Leaf et al., 2003 [20] | 6 weeks of ISM exercise at 30–40% MVC for 80–360 sec with increased frequency over time and repetitive isotonic squeezing of a squash ball or racquet ball. Non-exercise arm was comparator. Duration and frequency: 4 times per week for 6 weeks. Controlled heating employed | Paradoxically, the increase in venous size was unaccompanied by an increase in hand grip strength | 2-fold increase in CV diameter noted in most patients (P < .05) | Cephalic vein size increase was ≈0.4 mm in non-exercise arm (P = non-significant) | Not assessed |
CA: control arm; CV: cephalic vein; IA: intervention arm; ISM: isometric; IST: isotonic; MVC: maximal voluntary contraction; RA: radial artery.
Summary of exercise regimens and effect on various vessel structure and function parameters across selected studies.
| Author and year . | Intervention, co-interventions and controls . | Grip strength . | Effect size on vein calibre in intervention arm . | Effect size on vein calibre in control arm . | Artery parameters . |
|---|---|---|---|---|---|
| Sauco et al., 2021 [15] | Two sets of 30 ISM hand grip contractions twice a day. At noon: slow contraction session using elastic band with arm in flexion and extension. Comparator: no exercise. Duration 8 weeks | Significant increase of 4.33 kg (SD 4.5) in IA (P < .001); non-significant increase of 3.4 kg (SD 2.5) in the CA (P = .46) | Mean increase of 0.71 mm (SD 0.49; P < .001) at 8 weeks | Mean decrease of 0.112 mm (SD 0.48; P = .121) at 8 weeks | Increase in calibre by 0.12 mm (SD 0.31; P = .008) in IA Decrease in calibre by 0.02 mm (SD 0.32; P = .55) in CA |
| Barbosa et al., 2018 [16] | ISM + IST with tensiometer for BFR in intervention arm. Exercise only without BFR in control. Duration 8 weeks | Significant increase in dynamometry: in CA, 2.36 kg increase (P = .003); in IA, 2.25 kg increase (P = .06). No significant difference in increase in strength between arms, inter P = .302 | Mean increase of 0.20, 0.16 and 0.15 mm for the segments 2, 10 and 20 cm in CV (P = .16, .20 and .33 at these segments) | Mean increase in CV diameter of 0.24, 0.39 and 0.17 mm for the segments 2, 10 and 20 cm (P = .008, .001 and .237at these segments) | No significant changes in mean velocity at all segments across both groups (P = .279, .150 and .341 at 2, 10 and 20 cm) |
| Kumar et al., 2020 [17] | ISM handgrip exercise: 20 repetitions/min, 30 min/day. Comparator: no exercise. Duration 8 weeks | Increased by median of 4 kg on exercise arm; no significant increase in control arm | Mean change 0.31 ± 0.04 at 4 weeks (P < .05) without tourniquet and 0.40 ± 0.06 with tourniquet (P < .05) | No significant change with or without tourniquet | Not measured |
| Uy et al., 2012 [21] | ISM forearm strengthening exercises in preferred access arm: 10 sets of 20 repetitions/min. Comparator: non-exercise arm. Duration 8 weeks | Increase in the exercised arm from 0 to 8 weeks: 24.50 ± 2.05 kg to 27.04 ± 2.20 kg (P = .025). No change in control arm: 26.68 ± 2.60 kg to 26.82 ± 2.40 kg P = .929 | Mean changes in distal sites: 0.48 ± 0.16 at 4 weeks and 0.32 ± 0.20 at 8 weeks; in proximal sites: 0.59 ± 0.25 mm at 4 weeks and 0.52 ± 0.32 at 8 weeks | Mean changes in distal sites: 0.81 ± 0.20 at 4 weeks and 0.65 ± 0.15 at 8 weeks; in proximal sites: 0.71 ± 0.20 mm at 4 weeks and 0.43 ± 0.20 at 8 weeks. No significant difference in proximal or distal sites, as diameter increased in both arms (inter P = .209, .217, .726 and .826) | Not assessed |
| Rus et al., 2003 [19] | ISM hand grip exercise using a rubber ring: 4.5 cm inner and 7.5 cm outer diameter, maximal compression force 50 N. Frequency and intensity: trained and supervised by dialysis staff on HD days | Maximal hand grip strength measured with dynamometer increased significantly from 24.1 ± 2.95 mm to 26.2 ± 3.06 mm after 4 weeks and to 28.5 ± 3.17 mm after 8 weeks (P < .001) | Average vein diameter without tourniquet remained unchanged for 4 weeks but significantly increased after 8 weeks (P = .015). Average vein diameter after tourniquet significantly greater after 4 weeks (P = .007) and even greater at 8 weeks (P < .001). However, distensibility remained unchanged | No control arm | No significant change in endothelium-dependent vasodilation |
| Leaf et al., 2003 [20] | 6 weeks of ISM exercise at 30–40% MVC for 80–360 sec with increased frequency over time and repetitive isotonic squeezing of a squash ball or racquet ball. Non-exercise arm was comparator. Duration and frequency: 4 times per week for 6 weeks. Controlled heating employed | Paradoxically, the increase in venous size was unaccompanied by an increase in hand grip strength | 2-fold increase in CV diameter noted in most patients (P < .05) | Cephalic vein size increase was ≈0.4 mm in non-exercise arm (P = non-significant) | Not assessed |
| Author and year . | Intervention, co-interventions and controls . | Grip strength . | Effect size on vein calibre in intervention arm . | Effect size on vein calibre in control arm . | Artery parameters . |
|---|---|---|---|---|---|
| Sauco et al., 2021 [15] | Two sets of 30 ISM hand grip contractions twice a day. At noon: slow contraction session using elastic band with arm in flexion and extension. Comparator: no exercise. Duration 8 weeks | Significant increase of 4.33 kg (SD 4.5) in IA (P < .001); non-significant increase of 3.4 kg (SD 2.5) in the CA (P = .46) | Mean increase of 0.71 mm (SD 0.49; P < .001) at 8 weeks | Mean decrease of 0.112 mm (SD 0.48; P = .121) at 8 weeks | Increase in calibre by 0.12 mm (SD 0.31; P = .008) in IA Decrease in calibre by 0.02 mm (SD 0.32; P = .55) in CA |
| Barbosa et al., 2018 [16] | ISM + IST with tensiometer for BFR in intervention arm. Exercise only without BFR in control. Duration 8 weeks | Significant increase in dynamometry: in CA, 2.36 kg increase (P = .003); in IA, 2.25 kg increase (P = .06). No significant difference in increase in strength between arms, inter P = .302 | Mean increase of 0.20, 0.16 and 0.15 mm for the segments 2, 10 and 20 cm in CV (P = .16, .20 and .33 at these segments) | Mean increase in CV diameter of 0.24, 0.39 and 0.17 mm for the segments 2, 10 and 20 cm (P = .008, .001 and .237at these segments) | No significant changes in mean velocity at all segments across both groups (P = .279, .150 and .341 at 2, 10 and 20 cm) |
| Kumar et al., 2020 [17] | ISM handgrip exercise: 20 repetitions/min, 30 min/day. Comparator: no exercise. Duration 8 weeks | Increased by median of 4 kg on exercise arm; no significant increase in control arm | Mean change 0.31 ± 0.04 at 4 weeks (P < .05) without tourniquet and 0.40 ± 0.06 with tourniquet (P < .05) | No significant change with or without tourniquet | Not measured |
| Uy et al., 2012 [21] | ISM forearm strengthening exercises in preferred access arm: 10 sets of 20 repetitions/min. Comparator: non-exercise arm. Duration 8 weeks | Increase in the exercised arm from 0 to 8 weeks: 24.50 ± 2.05 kg to 27.04 ± 2.20 kg (P = .025). No change in control arm: 26.68 ± 2.60 kg to 26.82 ± 2.40 kg P = .929 | Mean changes in distal sites: 0.48 ± 0.16 at 4 weeks and 0.32 ± 0.20 at 8 weeks; in proximal sites: 0.59 ± 0.25 mm at 4 weeks and 0.52 ± 0.32 at 8 weeks | Mean changes in distal sites: 0.81 ± 0.20 at 4 weeks and 0.65 ± 0.15 at 8 weeks; in proximal sites: 0.71 ± 0.20 mm at 4 weeks and 0.43 ± 0.20 at 8 weeks. No significant difference in proximal or distal sites, as diameter increased in both arms (inter P = .209, .217, .726 and .826) | Not assessed |
| Rus et al., 2003 [19] | ISM hand grip exercise using a rubber ring: 4.5 cm inner and 7.5 cm outer diameter, maximal compression force 50 N. Frequency and intensity: trained and supervised by dialysis staff on HD days | Maximal hand grip strength measured with dynamometer increased significantly from 24.1 ± 2.95 mm to 26.2 ± 3.06 mm after 4 weeks and to 28.5 ± 3.17 mm after 8 weeks (P < .001) | Average vein diameter without tourniquet remained unchanged for 4 weeks but significantly increased after 8 weeks (P = .015). Average vein diameter after tourniquet significantly greater after 4 weeks (P = .007) and even greater at 8 weeks (P < .001). However, distensibility remained unchanged | No control arm | No significant change in endothelium-dependent vasodilation |
| Leaf et al., 2003 [20] | 6 weeks of ISM exercise at 30–40% MVC for 80–360 sec with increased frequency over time and repetitive isotonic squeezing of a squash ball or racquet ball. Non-exercise arm was comparator. Duration and frequency: 4 times per week for 6 weeks. Controlled heating employed | Paradoxically, the increase in venous size was unaccompanied by an increase in hand grip strength | 2-fold increase in CV diameter noted in most patients (P < .05) | Cephalic vein size increase was ≈0.4 mm in non-exercise arm (P = non-significant) | Not assessed |
CA: control arm; CV: cephalic vein; IA: intervention arm; ISM: isometric; IST: isotonic; MVC: maximal voluntary contraction; RA: radial artery.
In summary, isometric hand grip exercise was the most common type of exercise advocated and was part of an exercise regime in all the included studies. Additional isotonic exercises were included in studies by Leaf et al. [20] and Barbosa et al. [16]. Important additional co-interventions included blood flow restriction using a tensiometer applied to the exercise arm, where the tensiometer was inflated to 50 mmHg of systolic blood pressure in the study by Barbosa et al. [16], and temperature control during exercise in the study by Leaf et al. [20] to standardize the effect of temperature on venous and arterial calibre.
Risk of bias
The risk of bias was assessed by the above-mentioned tools and the results are summarized in Table 3. Of the four RCTs, one had a high risk of bias [15], two had some concerns [16, 17] and only one had a low risk of bias [18]. All four non-RCTs had serious risk of bias due to the small number of participants [20, 22] or the split-body trial design [19, 21], as the systemic effect of exercise is likely to affect vessel parameters in both upper limbs.
Comparison of effect size of various preoperative interventions on AVF outcomes and some salient outcome measures tested by these interventions.
| Intervention type . | Source of data . | Effect size on forearm cephalic vein diameter . | Effect on AVF maturation . | Effect on primary failure/thrombosis/re-interventions/re-anastomosis/plasties . | Proportion of veins achieving the threshold for AVF creation . |
|---|---|---|---|---|---|
| Cholecalciferol supplementation | 1 RCT [17] | Not tested | AVF maturation at 6 months: 45% in cholecalciferol group and 54% in the placebo group (P = .8) | Not tested | Not tested |
| Hand exercise | Meta-analysis of 4 trials conducted by us (2 RCT and 2 non-RCT) [15, 17, 20, 21] | Mean increase in vein diameter of 0.24 mm (CI 0.03–0.45). If only RCTs are included, the mean increase is 0.29 mm (CI 0.11–0.47) | Not tested | Only one RCT [15] tested this outcome and found no difference between the control and intervention arms (50% lower primary failure in exercise arm, although not statistically significant) | One RCT [15] demonstrated significantly high rate of distal AVF creation in the exercise arm versus the control arm: 75% versus 51% (P = .008). In another study [21], potential access sites increased from 12 at baseline to 33 (P < .001) and 23 (P = .047), after 4 and 8 weeks of exercise, respectively |
| Intermittent pneumatic compression (Fist Assist device) | Single-arm prospective study [22] | Mean increase in forearm cephalic vein diameter by 0.41 mm (CI 0.12–0.7; P = .006) | Not tested | Not tested | 30% of forearm and 43% of upper arm veins crossed the threshold needed for AVF creation from being under the threshold |
| Intervention type . | Source of data . | Effect size on forearm cephalic vein diameter . | Effect on AVF maturation . | Effect on primary failure/thrombosis/re-interventions/re-anastomosis/plasties . | Proportion of veins achieving the threshold for AVF creation . |
|---|---|---|---|---|---|
| Cholecalciferol supplementation | 1 RCT [17] | Not tested | AVF maturation at 6 months: 45% in cholecalciferol group and 54% in the placebo group (P = .8) | Not tested | Not tested |
| Hand exercise | Meta-analysis of 4 trials conducted by us (2 RCT and 2 non-RCT) [15, 17, 20, 21] | Mean increase in vein diameter of 0.24 mm (CI 0.03–0.45). If only RCTs are included, the mean increase is 0.29 mm (CI 0.11–0.47) | Not tested | Only one RCT [15] tested this outcome and found no difference between the control and intervention arms (50% lower primary failure in exercise arm, although not statistically significant) | One RCT [15] demonstrated significantly high rate of distal AVF creation in the exercise arm versus the control arm: 75% versus 51% (P = .008). In another study [21], potential access sites increased from 12 at baseline to 33 (P < .001) and 23 (P = .047), after 4 and 8 weeks of exercise, respectively |
| Intermittent pneumatic compression (Fist Assist device) | Single-arm prospective study [22] | Mean increase in forearm cephalic vein diameter by 0.41 mm (CI 0.12–0.7; P = .006) | Not tested | Not tested | 30% of forearm and 43% of upper arm veins crossed the threshold needed for AVF creation from being under the threshold |
Comparison of effect size of various preoperative interventions on AVF outcomes and some salient outcome measures tested by these interventions.
| Intervention type . | Source of data . | Effect size on forearm cephalic vein diameter . | Effect on AVF maturation . | Effect on primary failure/thrombosis/re-interventions/re-anastomosis/plasties . | Proportion of veins achieving the threshold for AVF creation . |
|---|---|---|---|---|---|
| Cholecalciferol supplementation | 1 RCT [17] | Not tested | AVF maturation at 6 months: 45% in cholecalciferol group and 54% in the placebo group (P = .8) | Not tested | Not tested |
| Hand exercise | Meta-analysis of 4 trials conducted by us (2 RCT and 2 non-RCT) [15, 17, 20, 21] | Mean increase in vein diameter of 0.24 mm (CI 0.03–0.45). If only RCTs are included, the mean increase is 0.29 mm (CI 0.11–0.47) | Not tested | Only one RCT [15] tested this outcome and found no difference between the control and intervention arms (50% lower primary failure in exercise arm, although not statistically significant) | One RCT [15] demonstrated significantly high rate of distal AVF creation in the exercise arm versus the control arm: 75% versus 51% (P = .008). In another study [21], potential access sites increased from 12 at baseline to 33 (P < .001) and 23 (P = .047), after 4 and 8 weeks of exercise, respectively |
| Intermittent pneumatic compression (Fist Assist device) | Single-arm prospective study [22] | Mean increase in forearm cephalic vein diameter by 0.41 mm (CI 0.12–0.7; P = .006) | Not tested | Not tested | 30% of forearm and 43% of upper arm veins crossed the threshold needed for AVF creation from being under the threshold |
| Intervention type . | Source of data . | Effect size on forearm cephalic vein diameter . | Effect on AVF maturation . | Effect on primary failure/thrombosis/re-interventions/re-anastomosis/plasties . | Proportion of veins achieving the threshold for AVF creation . |
|---|---|---|---|---|---|
| Cholecalciferol supplementation | 1 RCT [17] | Not tested | AVF maturation at 6 months: 45% in cholecalciferol group and 54% in the placebo group (P = .8) | Not tested | Not tested |
| Hand exercise | Meta-analysis of 4 trials conducted by us (2 RCT and 2 non-RCT) [15, 17, 20, 21] | Mean increase in vein diameter of 0.24 mm (CI 0.03–0.45). If only RCTs are included, the mean increase is 0.29 mm (CI 0.11–0.47) | Not tested | Only one RCT [15] tested this outcome and found no difference between the control and intervention arms (50% lower primary failure in exercise arm, although not statistically significant) | One RCT [15] demonstrated significantly high rate of distal AVF creation in the exercise arm versus the control arm: 75% versus 51% (P = .008). In another study [21], potential access sites increased from 12 at baseline to 33 (P < .001) and 23 (P = .047), after 4 and 8 weeks of exercise, respectively |
| Intermittent pneumatic compression (Fist Assist device) | Single-arm prospective study [22] | Mean increase in forearm cephalic vein diameter by 0.41 mm (CI 0.12–0.7; P = .006) | Not tested | Not tested | 30% of forearm and 43% of upper arm veins crossed the threshold needed for AVF creation from being under the threshold |
Primary and secondary outcomes
The outcome measures used for all the exercise studies were predominantly related to a change in vessel calibre or flow at the end of the exercise period. Only the RCT by Aragoncillo Sauco et al. [15] followed patients beyond AVF creation until 12 weeks post-surgery to look at more hard outcomes such as percentage primary failure at 12 weeks as the primary outcome. The cholecalciferol study by Wasse et al. [18] was another RCT that looked at the percentage or proportion of AVF maturation at 6-months after AVF creation using a standard definition of AVF maturation. Neither hand exercise nor cholecaliferol was found to improve the rate of AVF maturation or primary failure.
As summarized in Table 2, hand exercise was generally found to have a positive effect on vein calibre. with most of the studies demonstrating a significant increase as compared with no intervention. Effect size was lower in those studies that used the opposite limb of the same patients as a control, possibly because of the systemic effect of exercise affecting the non-exercise arm as well.
Meta-analysis of effect in four hand exercise studies was undertaken [15, 17, 20, 21] and the results are summarized in Fig. 2. Two studies were not included due to the lack of a comparative arm or addition of a co-intervention, namely blood flow restriction, to hand exercise. The overall effect size of hand exercise on distal cephalic vein calibre without a tourniquet is ≈0.24 mm (95% CI 0.03–0.45). The heterogeneity is minimal at ≈16%. As can be seen in the forest plot in Fig. 3, the effect size from the RCTs was significantly higher with narrow CIs as compared with non-RCTs, possibly because the non-RCTs used the non-exercise arm of the same patients as a control and hence the systemic influence of exercise on the non-exercise arm underestimated the effect size of hand exercise on the cephalic vein in the exercise arm.
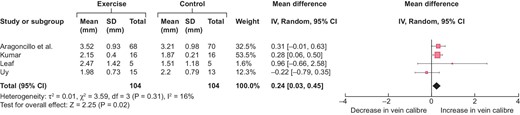
Forest plot of the effect of hand exercise on end-of-follow-up distal cephalic vein calibre (in mm) without a torniquet.
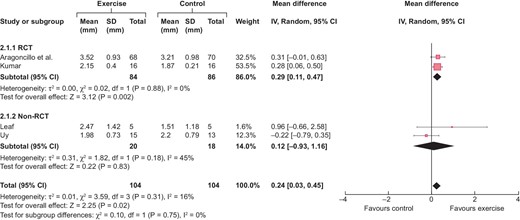
Forest plot of the effect of hand exercise on end-of-follow-up distal cephalic vein calibre (in mm) without a tourniquet, categorised according to study design.
The study by Rus et al. [19] also looked at the effect of hand exercise on endothelium-dependent vasodilation, but no significant effect was found.
The only single-arm prospective cohort study on intermittent pneumatic compression using a Fist Assist device found a significant improvement in baseline cephalic vein diameter on fixed landmarks of the forearm and upper arm with or without blood pressure cuff application and also increased the proportion of cephalic veins that is suitable for AVF creation [22].
Adverse events of the intervention
A common finding seen with hand exercise regimens in all the studies is that, according to logbooks, the adherence rate decreases after 4 weeks, as well as grip strength measurements. In the study by Aragoncillo Sauco et al. [15], 5 of 68 patients dropped out of the elastic band exercise due to shoulder pain and only 60% followed the suggested exercise regimen. Otherwise, overall engagement with exercise was good in all studies, especially in the first 4 weeks. Adherence to the Fist Assist device was also reasonable, with at least 300 recorded sessions in a 3-month period and no adverse events.
Publication bias and moderator analyses
The funnel plot of the four studies (see Fig. 4) did not indicate any asymmetry; however, the number of studies is small. Similarly, owing to the small number of studies, moderator analysis were not possible.
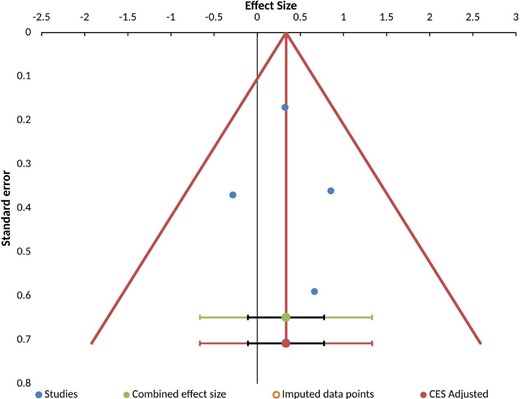
Funnel plot of eligible studies indicating symmetry and hence absence of publication bias.
DISCUSSION
This systematic review and meta-analysis assessed the efficacy of various forms of preoperative interventions aimed at improving AVF outcomes. The striking finding was the paucity of intervention studies applied to this cohort of patients. Hand exercise was the predominant form of intervention and was used with varying combinations of isometric or isotonic exercise with or without the addition of blood flow restriction. Although the exercise regimens were variable, they were usually applied for ≈6–8 weeks, but peak engagement occurred in the first 4 weeks, after which it appeared to decrease. Most of the exercise regimen seemed to be well tolerated, apart from slow arm contractions using the elastic band, which is associated with shoulder pain, leading to discontinuation of exercise.
Although the effect size of hand exercise on cephalic vein size seems to be quite small in terms of an increase in diameter, this might translate to a better outcome, as flow in a vessel is inversely related to the fourth power of its radius. Unfortunately, only one exercise study, by Aragoncillo Sauco et al. [15], followed patients until AVF maturation to assess the effect on primary failure and AVF maturation, which is the hard and desirable outcome measure. Although the study demonstrated a decrease in primary failure in the exercise arm compared with the non-exercise arm, it did not reach statistical significance, due to the overall low rate of primary failure.
Two studies employed a split-body trial design, where the non-exercise arm was used as the control. There have been studies [23] that have shown that exercise with one limb leads to systemic vasodilation and changes in vessel calibre in the opposite limb. As typically the dominant arm was the non-exercise arm in these studies (as AVFs are usually created in the dominant arm), exercise with the non-dominant arm may have more influence on the dominant arm. This can lead to underestimation of the effect size of hand exercise.
The latest Kidney Disease Outcomes Quality Initiative guidelines [24] suggest not relying on vessel diameter but to test vessel function, as recent studies on AVF maturation have revealed that vessel function parameters are good predictors of AVF maturation [25]. Only one study in this analysis tried to study the effect of hand exercise on endothelial-dependent vasodilation [19], but it was undertaken among patients who were already on HD and hence known to have severely impaired endothelial function.
There were not enough studies to do a moderator analysis to see which subgroup of patients might benefit more from exercise.
The other preoperative intervention that was tested was cholecaciferol supplementation, which was tested in a well-conducted double-blind RCT and was not found to be effective in improving the rate of AVF maturation.
Finally, the Fist Assist device [22] demonstrated reasonable safety and efficacy in improving upper arm and forearm cephalic vein calibre and also in increasing the proportion of veins suitable for AVF creation. This is one example where a proven intervention after AVF creation [27] has been tested and proven to be effective in the preoperative period [22].
Several interventions, including infrared radiation [26], that have been employed after AVF creation to assist with the maturation could also be potentially tried in the preoperative period, which is the window of opportunity available to improve AVF outcomes.
The limitations of our review are restriction of studies to those in English, inability to do moderator analysis due to the paucity of studies, the paucity of hard outcome data and an inability to account for error in diameter measurements by ultrasound, which is influenced by operator experience, body temperature, environmental temperature, medications etc. However, the strength of the review is identification of gaps in knowledge in the preoperative interventions and the positive effect of exercise on vein calibre that might be enhanced with other co-interventions, thereby improving AVF outcome.
CONCLUSION
There is a pressing need to do large-scale RCTs with hard endpoints of preoperative interventions that can improve the structure and function of upper arm vessels prior to AVF creation in order to facilitate favourable remodelling after AVF creation. Multiple co-interventions employing innovative trial designs will help in identifying the synergistic effects of such interventions and filling the current gaps in knowledge.
FUNDING
None.
AUTHORS’ CONTRIBUTIONS
S.R. was responsible for the design, analysis and interpretation of data and drafting of the manuscript. O.S. was responsible for analysis of data and study selection. S.S. was responsible for the design, analysis and interpretation of data and critical review of the manuscript. K.L. was responsible for statistical analysis, interpretation of data and critical review of the manuscript. H.U. was responsible for analysis and interpretation of data and the risk of bias analysis. K.F. was responsible for the concept, design, analysis and interpretation of data, critical review of the manuscript and final approval of the manuscript submitted.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared upon reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this article have not been published previously in whole or part.





Comments