-
PDF
- Split View
-
Views
-
Cite
Cite
Paola Carioni, Francesco Bellocchio, Ana Bernardo, Stefano Stuard, Peter Kotanko, Len A Usvyat, Vratislava Kovarova, Otto Arkossy, Anke Winter, Jeroen Kooman, Federica Gervasoni, Antonio Tupputi, Yan Zhang, Hanjie Zhang, John Larkin, Luca Neri, FC087: Effectiveness of Covid-19 Vaccines in a Large European Haemodialysis Cohort, Nephrology Dialysis Transplantation, Volume 37, Issue Supplement_3, May 2022, gfac116.003, https://doi.org/10.1093/ndt/gfac116.003
Close - Share Icon Share
Abstract
To date, no large-scale study has evaluated the effectiveness of COVID-19 vaccines in hemodialysis patients. We sought to evaluate the effectiveness of vaccines against SARS-CoV-2 infections and death in haemodialysis patients registered in the Fresenius Medical Care (FMC) Nephrocare network.
In this historical, 1:1 matched cohort study, we analysed electronic health records (EHR) of individuals receiving in-center haemodialysis therapy in FMC European dialysis clinics from 1 December 2020, to 31 May 2021 (study period). For each vaccinated patient, an unvaccinated patient was selected among patients registered in the same country and attending a dialysis session within +/–3 days from the vaccination date. Matching without replacement was based on demographics, clinical characteristics, past COVID-19 infections and a risk score representing the local (dialysis centre) background risk of infection at each vaccination date. The infection risk score was calculated from an artificial Intelligence model predicting the risk of COVID-19 outbreak in each clinic over a 2-week prediction horizon. The infection risk score was based on trends in regional COVID-19 epidemic metrics, FMC COVID-19 reporting system and clinical practice patterns. The index date was the date of the first vaccination for the vaccinated and the matching treatment date for the unvaccinated controls. To overcome violation of the proportional hazard assumption, we estimated the effectiveness of the COVID-19 vaccines in preventing infection and mortality rates as 1—hazard ratio estimated from a time-dependent extended Cox regression stratified by country and vaccine type.
We included 44 458 patients, 22 229 vaccinated and matched 22 229 unvaccinated. Distribution of covariates was balanced across study arms after matching (Figure 1A). In the effectiveness analysis on mRNA vaccines, we observed 850 SARS-CoV-2 infections and 201 COVID19-related deaths among the 28 110 patients (14 055 vaccinated and 14 055 unvaccinated) during a mean follow up time of 44 ± 40 days. In the effectiveness analysis of viral-vector vaccines, we observed 297 SARS-CoV-2 infections and 64 COVID19-related deaths among 12 888 patients (6444 vaccinated and 6444 unvaccinated) during a mean a follow-up time of 48 ± 32 days (Figure 1B). We observed 18.5/100 patient-year and 8.5/100 patient-year fewer infections and 5.4/100 patient-year and 5.2/100 patient-year fewer COVID-19-related deaths among patients vaccinated with mRNA and viral-vector vaccines respectively, as compared to matched unvaccinated controls. The effectiveness of COVID-19 vaccines concerning both symptomatic infections and COVID-related death along the follow up period is shown in Figure 2.
In this matched, historical cohort study, we observed a strong reduction in both SARS-CoV-2 symptomatic infection and COVID-19-related death among dialysis patients receiving an mRNA vaccine. Despite seemingly less protective against symptomatic infections, we observed similar reduction in COVID-19 mortality rate among patients receiving a viral-carrier vaccine.
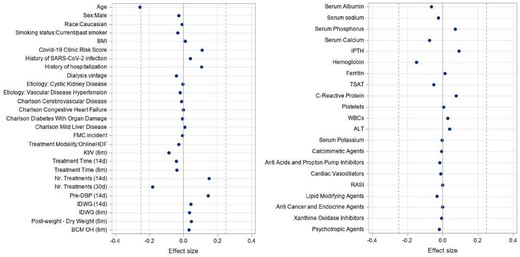
Forest Plot demonstrating covariate distribution balance between exposure groups. Effect Sizes calculated as Cohen's d or Cromer's Negative coefficient indicates that mean or relative frequency was greater among vaccinated patients. Effect Size 0.12 negligible Effect Size-0.1-0.2: small.

Absolute frequency and incidence density (95% confidence intervall of events across exposure groups.
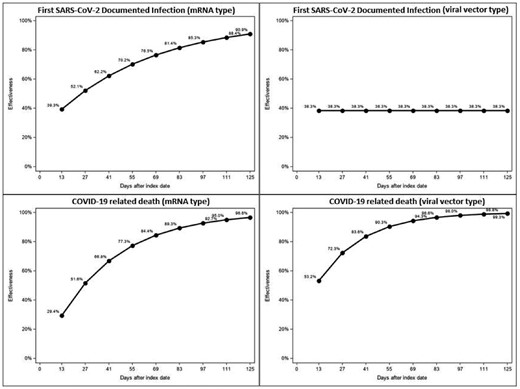
Effectiveness (1-HR) estimates by vaccine type concerning symptomatic, documented infection and COVID-19 related death. Estimates were obtained from extended, cox regression with time-varying covariate.
- hemodialysis
- artificial intelligence
- viral vector
- demography
- disease outbreaks
- follow-up
- rna, messenger
- vaccination
- vaccines
- infections
- mortality
- epidemics
- electronic medical records
- cox proportional hazards models
- clinical practice patterns
- infection risk
- mrna vaccines
- sars-cov-2
- covid-19
- covid-19 vaccines





Comments