-
PDF
- Split View
-
Views
-
Cite
Cite
Ana Melissa Rau Lertora, Adrián Santelli, Maria Fiol, Luis Riera, Begoña Etcheverry, Carmen Ardanuy Tisaire, Sara Marti, Josep Cruzado, Edoardo Melilli, MO1004: Non-Antibiotic Prophylaxis with D-MANOSE for Uti in DE-NOVO Kidney Transplant Recipients: The Manotras Study, Nephrology Dialysis Transplantation, Volume 37, Issue Supplement_3, May 2022, gfac088.006, https://doi.org/10.1093/ndt/gfac088.006
Close - Share Icon Share
Abstract
Urinary tract infections (UTIs) are one of the most frequent complications in the first 6 months after kidney transplantation. (1) D-Mannose, a simple sugar, could play a very important role in the prevention of urinary infections, inhibiting the attachment of bacterial type one fimbriae to the urothelium, reducing their ability to infect de host. Proanthocyanides (PAC) have also been shown in vitro activity to inhibit the adherence of Escherichia coli by a similar mechanism. Both have shown promising results in reducing the risk of UTI in healthy women, but its efficacy has not been evaluated in kidney transplant population yet. (2) In this single-cantre, double-blind, prospective, randomized clinical trial, we aimed to evaluate the impact and security of a 24-h prolonged release formulation of D-Mannose in de-novo kidney transplantation UTI's.
Between April 2019 and September 2020, 60 patients were recruited and randomized in 1:1 ratio according to treatment arm. Control group was treated with PAC, while the study group received PAC plus Mannose. Treatments were given as a prophylaxis strategy immediate after renal transplantation and during the first 3 months. All patients were followed for 6 months.
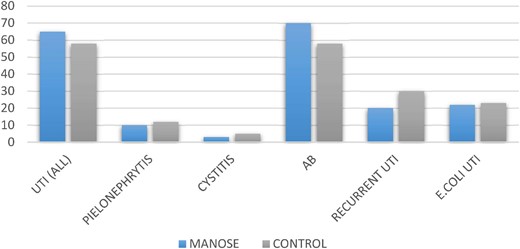
No statistically differences were observed in the baseline characteristics among study groups (Table 1). During the follow up; 61% of patients had at least one UTI episode (asymptomatic bacteriuria 56%, cystitis 6% and acute graft pyelonephritis 17%). Escherichia coli accounted for 28% of all UTI episodes, followed by Enterococcus faecalis (15%), Enterobacter cloacae (13%) and Klebsiella pneumoniae (13%). The main renal outcomes (eGFR at 6 months, acute rejection and DGF) did not differ among study groups.
We found no statistically differences recording in the incidence of UTI between groups (Fig. 1). At univariate analysis the surgical complications, and female gender were independently associated with UTI. {OR 5.92, [95% confidence interval (95% CI) 1.021–34.36]; P = .041 and OR 3.59 95% CI 1.051–12.27; P = .047} with no effect of treatment with D-mannose.
In this study, a prophylaxis strategy based on Mannose + PAC did not show a significant impact compared with PAC alone on UTI in the de-novo kidney transplant population.
Baseline demographics and clinical characteristics depending on treatment group
| . | Mannose + PAC . | PAC . | . |
|---|---|---|---|
| Characteristic . | n = 27 . | n = 27 . | P . |
| Sex (F/M), n (%) | 13/14 (48/52%) | 12/15 (44/55%) | 0.78 |
| Age (years), median (IQR) | 62 | 57 | 0.68 |
| Waiting time on dialysis (months), median (IQR) | 31 | 27 | 0.35 |
| Cause of ESKD | 0.83 | ||
| Undetermined, n (%) | 5 (18%) | 6 (22%) | |
| Diabetes, n (%) | 3 (11,1%) | 3 (11,1%) | |
| Vascular nephropathy, n (%) | 2 (7.4%) | 2(7,4%) | |
| Tubulo-interstitial nephritis, n (%) | 3 (11.1%) | 0 (0 %) | |
| ADPKD, n (%) | 2 (7,4%) | 7 (25,9%) | |
| Glomerulonephritis, n (%) | 8 (29,96%) | 7 (25,9%) | |
| Others, n (%) | 4 (14,1%) | 2 (7,4%) | |
| Type of donor, n (%) | 0.58 | ||
| Living | 13 (48%) | 12 (40%) | |
| Deceased | 14 (52%) | 16 (60%) | |
| Induction Therapy, n (%) | 0.26 | ||
| Basiliximab | 18 (66%) | 13 (52%) | |
| Thymoglobulin | 9 (33%) | 12 (48%) |
| . | Mannose + PAC . | PAC . | . |
|---|---|---|---|
| Characteristic . | n = 27 . | n = 27 . | P . |
| Sex (F/M), n (%) | 13/14 (48/52%) | 12/15 (44/55%) | 0.78 |
| Age (years), median (IQR) | 62 | 57 | 0.68 |
| Waiting time on dialysis (months), median (IQR) | 31 | 27 | 0.35 |
| Cause of ESKD | 0.83 | ||
| Undetermined, n (%) | 5 (18%) | 6 (22%) | |
| Diabetes, n (%) | 3 (11,1%) | 3 (11,1%) | |
| Vascular nephropathy, n (%) | 2 (7.4%) | 2(7,4%) | |
| Tubulo-interstitial nephritis, n (%) | 3 (11.1%) | 0 (0 %) | |
| ADPKD, n (%) | 2 (7,4%) | 7 (25,9%) | |
| Glomerulonephritis, n (%) | 8 (29,96%) | 7 (25,9%) | |
| Others, n (%) | 4 (14,1%) | 2 (7,4%) | |
| Type of donor, n (%) | 0.58 | ||
| Living | 13 (48%) | 12 (40%) | |
| Deceased | 14 (52%) | 16 (60%) | |
| Induction Therapy, n (%) | 0.26 | ||
| Basiliximab | 18 (66%) | 13 (52%) | |
| Thymoglobulin | 9 (33%) | 12 (48%) |
ESKD, end stage kidney decease; ADPKD, autosomic dominant kidney decease; CMV, citomegalovirius.
Baseline demographics and clinical characteristics depending on treatment group
| . | Mannose + PAC . | PAC . | . |
|---|---|---|---|
| Characteristic . | n = 27 . | n = 27 . | P . |
| Sex (F/M), n (%) | 13/14 (48/52%) | 12/15 (44/55%) | 0.78 |
| Age (years), median (IQR) | 62 | 57 | 0.68 |
| Waiting time on dialysis (months), median (IQR) | 31 | 27 | 0.35 |
| Cause of ESKD | 0.83 | ||
| Undetermined, n (%) | 5 (18%) | 6 (22%) | |
| Diabetes, n (%) | 3 (11,1%) | 3 (11,1%) | |
| Vascular nephropathy, n (%) | 2 (7.4%) | 2(7,4%) | |
| Tubulo-interstitial nephritis, n (%) | 3 (11.1%) | 0 (0 %) | |
| ADPKD, n (%) | 2 (7,4%) | 7 (25,9%) | |
| Glomerulonephritis, n (%) | 8 (29,96%) | 7 (25,9%) | |
| Others, n (%) | 4 (14,1%) | 2 (7,4%) | |
| Type of donor, n (%) | 0.58 | ||
| Living | 13 (48%) | 12 (40%) | |
| Deceased | 14 (52%) | 16 (60%) | |
| Induction Therapy, n (%) | 0.26 | ||
| Basiliximab | 18 (66%) | 13 (52%) | |
| Thymoglobulin | 9 (33%) | 12 (48%) |
| . | Mannose + PAC . | PAC . | . |
|---|---|---|---|
| Characteristic . | n = 27 . | n = 27 . | P . |
| Sex (F/M), n (%) | 13/14 (48/52%) | 12/15 (44/55%) | 0.78 |
| Age (years), median (IQR) | 62 | 57 | 0.68 |
| Waiting time on dialysis (months), median (IQR) | 31 | 27 | 0.35 |
| Cause of ESKD | 0.83 | ||
| Undetermined, n (%) | 5 (18%) | 6 (22%) | |
| Diabetes, n (%) | 3 (11,1%) | 3 (11,1%) | |
| Vascular nephropathy, n (%) | 2 (7.4%) | 2(7,4%) | |
| Tubulo-interstitial nephritis, n (%) | 3 (11.1%) | 0 (0 %) | |
| ADPKD, n (%) | 2 (7,4%) | 7 (25,9%) | |
| Glomerulonephritis, n (%) | 8 (29,96%) | 7 (25,9%) | |
| Others, n (%) | 4 (14,1%) | 2 (7,4%) | |
| Type of donor, n (%) | 0.58 | ||
| Living | 13 (48%) | 12 (40%) | |
| Deceased | 14 (52%) | 16 (60%) | |
| Induction Therapy, n (%) | 0.26 | ||
| Basiliximab | 18 (66%) | 13 (52%) | |
| Thymoglobulin | 9 (33%) | 12 (48%) |
ESKD, end stage kidney decease; ADPKD, autosomic dominant kidney decease; CMV, citomegalovirius.
- antibiotics
- hemodialysis
- kidney diseases
- diabetes mellitus
- renal transplantation
- glomerulonephritis
- basiliximab
- diabetes mellitus, type 2
- surgical complications
- urinary tract infections
- cystitis
- demography
- enterobacter cloacae
- enterococcus faecalis
- bacterial fimbria
- follow-up
- klebsiella pneumoniae
- mannose
- monosaccharides
- neoadjuvant therapy
- nephritis
- polycystic kidney, autosomal dominant
- pyelonephritis
- rejection (psychology)
- tissue transplants
- urothelium
- infections
- arm
- cytomegalovirus
- dialysis procedure
- kidney
- gender
- escherichia coli
- bacteriuria, asymptomatic
- risk reduction
- waiting time
- prevention
- univariate analysis
- thymoglobulin
- donors





Comments