-
PDF
- Split View
-
Views
-
Cite
Cite
Daniela Picciotto, Marco Frascio, Alessandro Laudon, Lucia Macciò, Federica Bui, Valentina Zanetti, Francesca Viazzi, Giacomo Garibotto, Giuliano Brunori, Daniela Verzola, Pasquale Esposito, MO582: Impaired HIF-1 Regulation in Skeletal Muscle of Patients With Advanced-Stage Chronic Kidney Disease, Nephrology Dialysis Transplantation, Volume 37, Issue Supplement_3, May 2022, gfac074.027, https://doi.org/10.1093/ndt/gfac074.027
Close - Share Icon Share
Abstract
Tiredness and fatigue are common symptoms in patients with chronic kidney disease (CKD), but their underlying mechanisms are unknown and treatments unavailable. Patients with CKD display abnormalities along the entire oxygen cascade, with impaired diffusive and convective oxygen transport, thus contributing to a reduced tissue oxygen supply. Hypoxic adaptation is largely regulated by hypoxia-inducible factor 1 (HIF-1α), encoded by the HIF-1Α gene [1], and peroxisome proliferator-activated receptor-gamma coactivator-1 alpha (PGC-1α). In response to hypoxia or anemia, the muscle HIF-1α target genes increase oxygen transport through angiogenesis, responsiveness to insulin, cell proliferation and apoptosis/survival [2]. PGC-1α controls the expression of genes involved in mitochondrial biogenesis, energy homeostasis and glucose metabolism. PGC-1α is correlated with a total-body aerobic capacity [3], and its decrease has been detected in muscles of elderly persons and type 2 diabetes patients [3,4]. We hypothesized that desensitization of the HIF-1α driven oxygen-sensing mechanisms occurs in CKD patients.
HIF-1α, PGC1α gene and protein expression, studied by Rt–PCR and immunohistochemistry, were assessed in the rectus abdominis muscle biopsies from 31 CKD patients with non-dialysis CKD 5 (18 M/13 F, eGFR 8 ± 1 mL/min) and were compared with those obtained in 10 subjects with normal renal function (7M/3F).
HIF-1α, PGC1α expression was studied also in C2C12 myotubes exposed to 10% normal serum or uremic serum (US) for 48 h. In addition, mitofusin-2 (MFN2), nuclear factor erythroid 2-related factor 2 (NRF2), and oxidative phosphorylation (OXPHOS) related to mitochondria integrity were monitored by Rt–PCR and/or western blot. Changes in the membrane potential were quantified by JC1 staining and fluorimeter analysis.
Despite anemia (Hb 9.5 ± 1 g/dL), HIF-1α mRNA was severely blunted in the muscle of CKD patients, as well as PGC1α that resulted down-regulated in 62.5% of them (P < 0.05) (Fig. 1). Log HIF-1α mRNA was directly related to log eGFR (r = 0.632, P < 0.02), suggesting that the hypoxic response in muscle progressively down-regulates as the renal function declines. In cultured myotubes, US decreases PGC1α, HIF1α, MFN2, NRF2 and OXPHOS and membrane potential (P < 0.05–0.01).
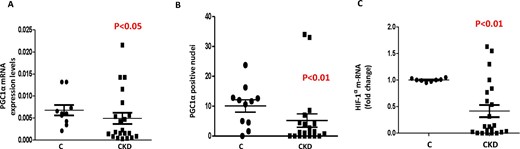
Gene and protein expression of hypoxia-inducible factor 1 (HIF-1α) and 1184 peroxisome proliferator-activated receptor-gamma coactivator-1 alpha (PGC-1α) in 1185 muscle biopsy of non-dialysis CKD patients and healthy controls (C).
In patients with non-dialysis CKD, PGC1α and HIF1α are down-regulated, as well as in an in vitro model that resembles the uremic milieu. On the one hand, these findings are in keeping with impaired oxidative metabolism in the advanced stage of CKD, while on the other hand, they may account for the fatigue often referred by these patients. Moreover, our study suggests that the HIF prolyl hydroxylase inhibitor (HIF-PHIs), currently in clinical development, might be targeted on muscle metabolism and function and tested in the treatment of myopathy and fatigue in CKD.
REFERENCES
- anemia
- angiogenesis
- apoptosis
- oxygen
- hemodialysis
- glucose metabolism
- western blotting
- immunohistochemistry
- mitochondria
- renal function
- kidney failure, chronic
- hypoxia
- diabetes mellitus, type 2
- biopsy
- pancreatic beta cell
- fatigue
- homeostasis
- origin of life
- genes
- membrane potentials
- skeletal muscles
- myopathy
- oxidative phosphorylation
- peroxisome
- procollagen-proline dioxygenase
- rectus abdominis
- reverse transcriptase polymerase chain reaction
- rna, messenger
- dialysis procedure
- cell respiration
- oxygen delivery
- oxygen transport
- desensitization
- biopsy of muscle
- older adult
- hypoxia-inducible factor 1
- candidate disease gene
- skeletal myocytes
- aerobic capacity
- mfn2 gene
- receptor desensitization
- personal integrity
- muscle metabolism





Comments