-
PDF
- Split View
-
Views
-
Cite
Cite
Julius J Schmidt, Dan Nicolae Borchina, Mariet van't Klooster, Khalida Bulhan-Soki, Reuben Okioma, Larissa Herbst, Diego Sandoval Rodríguez, Vedran Premužić, Stefan Büttner, Birgit Bader, Wojciech Serednicki, Ewa Zasada, Michael Schmitz, Ralf A Quabach, Maria Hrincheva, Thomas Fühner, Jan T Kielstein, Interim analysis of the COSA (COVID-19 patients treated with the Seraph® 100 Microbind® Affinity filter) registry, Nephrology Dialysis Transplantation, Volume 37, Issue 4, April 2022, Pages 673–680, https://doi.org/10.1093/ndt/gfab347
Close - Share Icon Share
ABSTRACT
The Seraph® 100 Microbind® Affinity Blood Filter is a haemoperfusion device that is licensed for the reduction of pathogens, including several viruses, in the blood. It received Emergency Use Authorization for the treatment of severe coronavirus disease 2019 (COVID-19) by the Food and Drug Administration (FDA). Several studies have shown that the blood viral load of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) correlates with adverse outcomes and removal of the nucleocapsid of the SARS-CoV-2 virus by the Seraph® 100 has been recently demonstrated. The aim of this registry was to evaluate the safety and efficacy of Seraph® 100 treatment for COVID-19 patients.
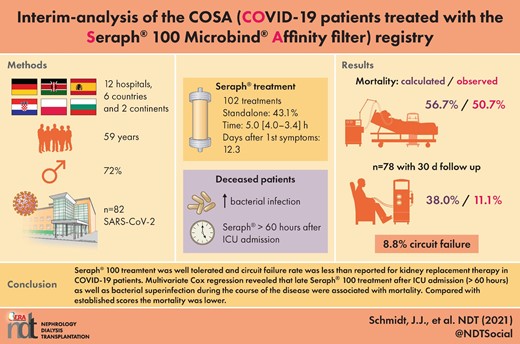
Twelve hospitals from six countries representing two continents documented patient and treatment characteristics as well as outcome parameters without reimbursement. Additionally, mortality and safety results of the device were reported. A total of 102 treatment sessions in 82 patients were documented in the registry. Four patients were excluded from mortality analysis due to incomplete outcome data, which were available in the other 78 patients.
Overall, a 30-day mortality rate of 46.2% in the 78 patients with complete follow-up was reported. The median treatment time was 5.00 h (4.00–13.42) and 43.1% of the treatments were performed as haemoperfusion only. Adverse events of the Seraph® 100 treatment were reported in 8.8% of the 102 treatments and represented the premature end of treatment due to circuit failure. Patients who died were treated later in their intensive care unit (ICU) stay and onset of COVID symptoms. They also had higher ferritin levels. Multivariate Cox regression revealed that delayed Seraph® 100 treatment after ICU admission (>60 h), as well as bacterial superinfection, were associated with mortality. While average predicted mortality rate according to Sequential Organ Failure Assessment (SOFA) score in ICU patients was 56.7%, the observed mortality was 50.7%. In non-ICU patients, Coronavirus Clinical Characterisation Consortium (4C) score average predicted a mortality rate of 38.0%, while the observed mortality rate was 11.1%.
The treatment of COVID-19 patients with Seraph® 100 is well tolerated and the circuit failure rate was lower than previously reported for kidney replacement therapy (KRT) in COVID-19 patients. Mortality correlated with late initiation of Seraph treatment after ICU admission and bacterial superinfection. Compared with predicted mortality according to 4C and SOFA scores, mortality of Seraph® 100-treated patients reported in the registry was lower.
What is already known about this subject?
The Seraph® 100, a biomimetic adsorber, has been shown to bind pathogens including SARS-CoV-2 from the blood.
The practicability, safety, and clinical impact of this intervention in critically ill COVID-19 patients is not known.
The aim of an online registry was to collect data on treatment coordinates, safety, and efficacy.
What this study adds?
The Seraph® 100 treatment was easy to operate either as a stand-alone hemoperfusion treatment or in combination with standardized kidney replacement procedures.
The clotting rate was remarkably low as compared to that reported for extracorporeal procedures, independently of type of anticoagulation chosen.
Observed mortality was lower than calculated mortality by established scores.
What impact this may have on practice or policy?
The data justify controlled prospective trials of the Seraph® 100 in critically ill COVID-19 patients.
The Seraph® 100 might be a treatment option for critically ill patients with COVID-19.
INTRODUCTION
For almost 2 years, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes the coronavirus disease 2019 (COVID-19), has had a serious impact on health and economics worldwide. Despite the recent advent of SARS-CoV-2 vaccines [1, 2], treatment options for those in critical condition are still needed, and yet pharmacologic interventions remain limited. Aside from dexamethasone [3] and encouraging preliminary data using recombinant interleukin-1 receptor antagonist [4], none of the many repurposed drugs that had been suggested for the treatment of COVID-19 has substantiated its therapeutic effectiveness in randomized prospective trials [5–8]. Both successful antibody-mediated strategies, the combination of the monoclonal antibodies casirivimab and imdevimab [9], as well as the pan-sarbecovirus monoclonal antibody sotrovimab [10], are aimed towards non-hospitalized patients in the early phase of the disease, i.e. ≤7 days or ≤5 days after onset of symptoms, respectively. On this background, several different extracorporeal treatments are currently being explored for their potential to improve the clinical course and outcomes of critically ill patients with COVID-19. Extracorporeal interventions of interest should eliminate the virus and blunt the immune response to avoid manifestations such as cytokine storm and multiorgan failure. Additionally, they could diminish the increased blood coagulation and clotting that result in venous, arterious and microvascular thrombosis. The evidence supporting the use of the various extracorporeal devices is neither substantial nor homogenous. Most interventions aim to reduce the cytokine storm [11]. The effect of therapeutic plasma exchange has been questioned [12], yet using fresh frozen plasma could optimize the von Willebrand factor/a disintegrin and metalloprotease with thrombospondin-1-like domains (vWF/ADAMTS-13) ratio [13]. The Seraph® 100 Microbind® Affinity filter has recently been introduced for the elimination of bacteria [14] and other pathogens from the blood [15]. Emergency Use Authorization (EUA) in patients with COVID-19 admitted to the ICU with confirmed or imminent respiratory failure was granted by the US FDA. The rationale for the approval was the fact that viral RNAemia is frequently (up to 78%) seen in critically ill patients, where it is related to the severity of the disease [16]. This has recently been confirmed in a meta-analysis of 21 studies including 2181 patients [17]. RNAemia was associated with severe cases of COVID-19 with an odds ratio (OR) of 5.43. In addition, SARS-CoV-2 RNAemia was a significant risk factor for unfavourable clinical outcomes including ICU admission and mortality. Furthermore, RNAemia was also a significant risk factor for invasive mechanical ventilation and multiple organ failure [17]. Recently, visualization of virus particles in plasma of COVID-19 patients indicated that SARS-CoV-2 RNAemia is at least in part due to viraemia [18]. As the SARS-CoV-2 spike glycoprotein binds tightly to immobilized heparin [19], the Seraph® 100 may decrease RNAemia/viraemia. Recently, it had been shown that the Seraph® 100 Microbind® Affinity filter removes the nucleocapsid protein of SARS-CoV-2 in critically ill COVID-19 patients [20]. As current evidence is based on individual cases or small case series [21–25], we aimed to collect treatment data prospectively in a standardized approach using a registry. Here, we report the interim analysis of the COVID-19 patients treated with the Seraph® 100 Microbind®Affinity filter (COSA) registry.
MATERIALS AND METHODS
This is a multicentre observational study (OBS) in 12 different hospitals (6 in Germany, 1 in Poland, 1 in Spain, 1 in Kenya, 2 in Croatia and 1 in Bulgaria) between March 2020 and October 2021. Clinical and biochemical data were gathered from every included patient. The registry for the evaluation of safety and effectiveness of the Seraph® 100 Microbind® Blood Filter in the therapy of COVID-19 Patients (COSA) has been registered at ClinicalTrials.gov (Identifier: NCT04361500). The registry was initially approved by the institutional review board (IRB) of the Hannover Medical School (8998_BO_K_2020). Data entry was not reimbursed.
Study population and data collection
All polymerase chain reaction (PCR)-diagnosed COVID-19 pneumonia patients >18 years old treated with the Seraph® 100 were eligible for the study. Data collection included patient characteristics, such as age, sex, height, weight, comorbidities and dates of COVID-19 manifestation, hospital and ICU admission, Seraph® 100 treatment time, as well as death or ICU referral. Additionally, at three different time points (before Seraph® 100 treatment, 6 h after Seraph® 100 treatment and the day after Seraph® 100 treatment) information about SOFA and 4C scores and their defining variables, as well as different biochemical parameters, were collected. Comments and information about the Seraph® 100 procedure and its tolerability were gathered as well.
The primary endpoint was 30-day survival after Seraph® 100 treatment; secondary endpoints were reported adverse events, clotting rates, as well as time to ICU discharge.
Statistical analysis
Quantitative variables were expressed as median (IQR), while categorical variables were expressed as counts (proportions). Comparison between survivors and non-survivors were evaluated in univariate analysis (Chi-squared test for proportions and Mann–Whitney test for non-normally distributed variables). To identify predictors of mortality in the ICU subgroup, we applied Cox proportional hazards models. To obtain multivariable-adjusted estimates, variables with univariate P-values ≤0.1 were investigated simultaneously. Statistical significance was defined as P < 0.05.
Statistical analysis was performed using R Statistical Software (version 4.0.4; R Foundation for Statistical Computing, Vienna, Austria).
Ethical approval
This study was approved by the Ethics Committee of the Hannover Medical School as well as the respective Ethics Committees of the participating institutions.
RESULTS
Of the 102 entered datasheets in the registry, 20 represented repeated Seraph® 100 treatments. After exclusion of incompletely documented patients (n = 4), partly due to ongoing ICU treatment, 78 patients could be analysed (Figure 1). The median Seraph® 100 treatment time was 5.00 h (4.00–13.42) and the median treated blood volume was 71.3 L (43.7–109). The Seraph® 100 was used in 43.1% of the 102 treatments as a standalone treatment. While the majority of patients received one Seraph® 100 treatment, 1 patient received four treatments, 3 received three treatments and 11 patients received two treatments. Overall, 36 (46.2%) of the 78 patients died during the 30-day follow-up. Body weight, height, age and SOFA score were not different in the patients who succumbed from those in the patients who survived. However, 4C score was higher in non-survivors [11 (8–12) versus 12.5 (11.3–14); P = 0.015] and non-survivors had higher median ferritin levels [1980 (1040–8140) versus 1010 (777–2000) ng/mL; P = 0.0418]. Treatment with Seraph® 100 was initiated earlier after the onset of symptoms in the survivors than in those who succumbed [9.56 (6.6–14.6) versus 13.0 (11.0–19.5) days; P = 0.021]. Also, time from ICU submission to Seraph® 100 treatment was shorter in the survivors than in the non-survivors [1.73 (1.5–3.24) versus 4.58 (2.05–11.4) days; P = 0.0023] (Table 1). Mortality in ICU patients who started Seraph® 100 treatment within 60 h of ICU admission (n = 29) was 34.5%, while it was 62.5% (P = 0.04) in patients who started treatment after more than 60 h in the ICU (n = 40). Univariate and multivariate Cox regression modelling showed 30-day mortality was associated with the presence of a bacterial superinfection [hazard ratio (HR) 2.36 (95% confidence interval 1.18–4.7)] and delayed Seraph® 100 treatment [HR 1.1 (1.04–1.15)] after ICU admission (Table 2).
Patient characteristics of the entire cohort as well as for survivors and non-survivors, and significance level of the difference between survivors and non-survivors
| . | Overall (n = 78) . | Survivor (n = 42) . | Non-survivor (n = 36) . | P-value . |
|---|---|---|---|---|
| Sex | ||||
| Female (%) | 22 (28.2) | 12 (28.6) | 10 (27.8) | 1 |
| Male (%) | 56 (71.8) | 30 (71.4) | 26 (72.2) | |
| Age (years) | ||||
| n | 78 | 42 | 36 | 0.741 |
| Median (Q1, Q3) | 59.0 (50.3, 68.8) | 57.0 (50.3, 68.0) | 61.5 (49.8, 71.3) | |
| Ethnicity | ||||
| Black/African/Caribbean (%) | 13 (16.7) | 7 (16.7) | 6 (16.7) | 0.445 |
| Mixed/multiple ethnic groups (%) | 8 (10.3) | 4 (9.5) | 4 (11.1) | |
| White (%) | 51 (65.4) | 29 (69.0) | 22 (61.1) | |
| Asian (%) | 1 (1.3) | 0 (0) | 1 (2.8) | |
| Other ethnic group (%) | 2 (2.6) | 0 (0) | 2 (5.6) | |
| Missing (%) | 3 (3.8) | 2 (4.8) | 1 (2.8) | |
| Weight (kg) | ||||
| n | 75 | 42 | 33 | 0.847 |
| Median (Q1, Q3) | 90.0 (79.0, 100) | 90.0 (79.0, 109) | 90.0 (80.0, 96.0) | |
| Missing (%) | 3 (3.8) | 0 (0) | 3 (8.3) | |
| Height (cm) | ||||
| n | 72 | 39 | 33 | 0.995 |
| Median (Q1, Q3) | 173 (168, 178) | 174 (168, 178) | 173 (168, 177) | |
| Missing (%) | 6 (7.7) | 3 (7.1) | 3 (8.3) | |
| ICU admission | ||||
| No (%) | 9 (11.5) | 8 (19.0) | 1 (2.8) | 0.0592 |
| Yes (%) | 69 (88.5) | 34 (81.0) | 35 (97.2) | |
| SOFA score | ||||
| n | 71 | 39 | 32 | 0.194 |
| Median (Q1, Q3) | 9.00 (7.00, 12.5) | 9.00 (7.00, 12.0) | 11.0 (8.00, 13.3) | |
| Missing (%) | 7 (9.0) | 3 (7.1) | 4 (11.1) | |
| 4C score | ||||
| n | 47 | 29 | 18 | 0.0157 |
| Median (Q1, Q3) | 12.0 (9.50, 14.0) | 11.0 (8.00, 12.0) | 12.5 (11.3, 14.0) | |
| Missing (%) | 31 (39.7) | 13 (31.0) | 18 (50.0) | |
| Mechanical ventilation | ||||
| No (%) | 23 (29.5) | 15 (35.7) | 8 (22.2) | 0.262 |
| Yes (%) | 52 (66.7) | 25 (59.5) | 27 (75.0) | |
| Missing (%) | 3 (3.8) | 2 (4.8) | 1 (2.8) | |
| PaO2/FiO2 | ||||
| n | 67 | 36 | 31 | 0.692 |
| Median (Q1, Q3) | 119 (71.5, 167) | 120 (75.5, 170) | 111 (67.2, 152) | |
| Missing (%) | 11 (14.1) | 6 (14.3) | 5 (13.9) | |
| Vasopressors | ||||
| No (%) | 35 (44.9) | 20 (47.6) | 15 (41.7) | 0.734 |
| Yes (%) | 32 (41.0) | 16 (38.1) | 16 (44.4) | |
| Missing (%) | 11 (14.1) | 6 (14.3) | 5 (13.9) | |
| Bacterial superinfection | ||||
| No (%) | 47 (60.3) | 30 (71.4) | 17 (47.2) | 0.0517 |
| Yes (%) | 31 (39.7) | 12 (28.6) | 19 (52.8) | |
| KRT dependency | ||||
| No (%) | 54 (69.2) | 29 (69.0) | 25 (69.4) | 1 |
| Yes (%) | 22 (28.2) | 12 (28.6) | 10 (27.8) | |
| Missing (%) | 2 (2.6) | 1 (2.4) | 1 (2.8) | |
| ECMO | ||||
| No (%) | 74 (94.9) | 41 (97.6) | 33 (91.7) | 0.501 |
| Yes (%) | 4 (5.1) | 1 (2.4) | 3 (8.3) | |
| Intermittent haemodialysis | ||||
| No (%) | 54 (69.2) | 28 (66.7) | 26 (72.2) | 0.776 |
| Yes (%) | 24 (30.8) | 14 (33.3) | 10 (27.8) | |
| Seraph as standalone treatment | ||||
| No (%) | 39 (50.0) | 18 (42.9) | 21 (58.3) | 0.287 |
| Yes (%) | 36 (46.2) | 22 (52.4) | 14 (38.9) | |
| Missing (%) | 3 (3.8) | 2 (4.8) | 1 (2.8) | |
| CRP (mg/L) | ||||
| n | 73 | 40 | 33 | 0.907 |
| Median (Q1, Q3) | 155 (97.0, 279) | 149 (110, 24) | 162 (83.0, 386) | |
| Missing (%) | 5 (6.4) | 2 (4.8) | 3 (8.3) | |
| PCT (µg/L) | ||||
| n | 72 | 37 | 35 | 0.0884 |
| Median (Q1, Q3) | 1.50 (0.400, 10.9) | 0.600 (0.400, 6.00) | 4.40 (0.650, 13.8) | |
| Missing (%) | 6 (7.7) | 5 (11.9) | 1 (2.8) | |
| Leucocytes | ||||
| n | 76 | 41 | 35 | 0.26 |
| Median (Q1, Q3) | 11.0 (8.43, 14.8) | 10.3 (7.30, 14.8) | 11.9 (9.45, 14.9) | |
| Missing (%) | 2 (2.6) | 1 (2.4) | 1 (2.8) | |
| Ferritin (ng/mL) | ||||
| n | 48 | 30 | 18 | 0.0418 |
| Median (Q1, Q3) | 1260 (894, 2880) | 1010 (777, 2000) | 1980 (1040, 8140) | |
| Missing (%) | 30 (38.5) | 12 (28.6) | 18 (50.0) | |
| d-Dimer (mg/L) | ||||
| n | 66 | 38 | 28 | 0.364 |
| Median (Q1, Q3) | 3.53 (1.07, 25.1) | 2.41 (0.848, 24.7) | 4.18 (1.51, 23.3) | |
| Missing (%) | 12 (15.4) | 4 (9.5) | 8 (22.2) | |
| Length of ICU stay (days) | ||||
| n | 69 | 34 | 35 | 0.299 |
| Median (Q1, Q3) | 17.0 (10.0, 27.0) | 15.0 (7.50, 26.3) | 19.0 (14.0, 25.5) | |
| Missing (%) | 9 (11.5) | 8 (19.0) | 1 (2.8) | |
| Length of hospital stay (days) | ||||
| n | 78 | 42 | 36 | 0.173 |
| Median (Q1, Q3) | 22.0 (15.0, 33.3) | 23.5 (16.3, 37.0) | 20.0 (13.0, 30.3) | |
| Time from symptoms to Seraph treatment (days) | ||||
| n | 74 | 39 | 35 | 0.0211 |
| Median (Q1, Q3) | 12.3 (7.43, 15.5) | 9.56 (6.60, 14.6) | 13.0 (11.0, 19.5) | |
| Missing (%) | 4 (5.1) | 3 (7.1) | 1 (2.8) | |
| Time from ICU admission to Seraph treatment (days) | ||||
| n | 69 | 34 | 35 | 0.0023 |
| Median (Q1, Q3) | 2.55 (1.53, 6.00) | 1.73 (1.50, 3.24) | 4.58 (2.05, 11.4) | |
| Missing (%) | 9a (11.5) | 8 (19.0) | 1 (2.8) | |
| . | Overall (n = 78) . | Survivor (n = 42) . | Non-survivor (n = 36) . | P-value . |
|---|---|---|---|---|
| Sex | ||||
| Female (%) | 22 (28.2) | 12 (28.6) | 10 (27.8) | 1 |
| Male (%) | 56 (71.8) | 30 (71.4) | 26 (72.2) | |
| Age (years) | ||||
| n | 78 | 42 | 36 | 0.741 |
| Median (Q1, Q3) | 59.0 (50.3, 68.8) | 57.0 (50.3, 68.0) | 61.5 (49.8, 71.3) | |
| Ethnicity | ||||
| Black/African/Caribbean (%) | 13 (16.7) | 7 (16.7) | 6 (16.7) | 0.445 |
| Mixed/multiple ethnic groups (%) | 8 (10.3) | 4 (9.5) | 4 (11.1) | |
| White (%) | 51 (65.4) | 29 (69.0) | 22 (61.1) | |
| Asian (%) | 1 (1.3) | 0 (0) | 1 (2.8) | |
| Other ethnic group (%) | 2 (2.6) | 0 (0) | 2 (5.6) | |
| Missing (%) | 3 (3.8) | 2 (4.8) | 1 (2.8) | |
| Weight (kg) | ||||
| n | 75 | 42 | 33 | 0.847 |
| Median (Q1, Q3) | 90.0 (79.0, 100) | 90.0 (79.0, 109) | 90.0 (80.0, 96.0) | |
| Missing (%) | 3 (3.8) | 0 (0) | 3 (8.3) | |
| Height (cm) | ||||
| n | 72 | 39 | 33 | 0.995 |
| Median (Q1, Q3) | 173 (168, 178) | 174 (168, 178) | 173 (168, 177) | |
| Missing (%) | 6 (7.7) | 3 (7.1) | 3 (8.3) | |
| ICU admission | ||||
| No (%) | 9 (11.5) | 8 (19.0) | 1 (2.8) | 0.0592 |
| Yes (%) | 69 (88.5) | 34 (81.0) | 35 (97.2) | |
| SOFA score | ||||
| n | 71 | 39 | 32 | 0.194 |
| Median (Q1, Q3) | 9.00 (7.00, 12.5) | 9.00 (7.00, 12.0) | 11.0 (8.00, 13.3) | |
| Missing (%) | 7 (9.0) | 3 (7.1) | 4 (11.1) | |
| 4C score | ||||
| n | 47 | 29 | 18 | 0.0157 |
| Median (Q1, Q3) | 12.0 (9.50, 14.0) | 11.0 (8.00, 12.0) | 12.5 (11.3, 14.0) | |
| Missing (%) | 31 (39.7) | 13 (31.0) | 18 (50.0) | |
| Mechanical ventilation | ||||
| No (%) | 23 (29.5) | 15 (35.7) | 8 (22.2) | 0.262 |
| Yes (%) | 52 (66.7) | 25 (59.5) | 27 (75.0) | |
| Missing (%) | 3 (3.8) | 2 (4.8) | 1 (2.8) | |
| PaO2/FiO2 | ||||
| n | 67 | 36 | 31 | 0.692 |
| Median (Q1, Q3) | 119 (71.5, 167) | 120 (75.5, 170) | 111 (67.2, 152) | |
| Missing (%) | 11 (14.1) | 6 (14.3) | 5 (13.9) | |
| Vasopressors | ||||
| No (%) | 35 (44.9) | 20 (47.6) | 15 (41.7) | 0.734 |
| Yes (%) | 32 (41.0) | 16 (38.1) | 16 (44.4) | |
| Missing (%) | 11 (14.1) | 6 (14.3) | 5 (13.9) | |
| Bacterial superinfection | ||||
| No (%) | 47 (60.3) | 30 (71.4) | 17 (47.2) | 0.0517 |
| Yes (%) | 31 (39.7) | 12 (28.6) | 19 (52.8) | |
| KRT dependency | ||||
| No (%) | 54 (69.2) | 29 (69.0) | 25 (69.4) | 1 |
| Yes (%) | 22 (28.2) | 12 (28.6) | 10 (27.8) | |
| Missing (%) | 2 (2.6) | 1 (2.4) | 1 (2.8) | |
| ECMO | ||||
| No (%) | 74 (94.9) | 41 (97.6) | 33 (91.7) | 0.501 |
| Yes (%) | 4 (5.1) | 1 (2.4) | 3 (8.3) | |
| Intermittent haemodialysis | ||||
| No (%) | 54 (69.2) | 28 (66.7) | 26 (72.2) | 0.776 |
| Yes (%) | 24 (30.8) | 14 (33.3) | 10 (27.8) | |
| Seraph as standalone treatment | ||||
| No (%) | 39 (50.0) | 18 (42.9) | 21 (58.3) | 0.287 |
| Yes (%) | 36 (46.2) | 22 (52.4) | 14 (38.9) | |
| Missing (%) | 3 (3.8) | 2 (4.8) | 1 (2.8) | |
| CRP (mg/L) | ||||
| n | 73 | 40 | 33 | 0.907 |
| Median (Q1, Q3) | 155 (97.0, 279) | 149 (110, 24) | 162 (83.0, 386) | |
| Missing (%) | 5 (6.4) | 2 (4.8) | 3 (8.3) | |
| PCT (µg/L) | ||||
| n | 72 | 37 | 35 | 0.0884 |
| Median (Q1, Q3) | 1.50 (0.400, 10.9) | 0.600 (0.400, 6.00) | 4.40 (0.650, 13.8) | |
| Missing (%) | 6 (7.7) | 5 (11.9) | 1 (2.8) | |
| Leucocytes | ||||
| n | 76 | 41 | 35 | 0.26 |
| Median (Q1, Q3) | 11.0 (8.43, 14.8) | 10.3 (7.30, 14.8) | 11.9 (9.45, 14.9) | |
| Missing (%) | 2 (2.6) | 1 (2.4) | 1 (2.8) | |
| Ferritin (ng/mL) | ||||
| n | 48 | 30 | 18 | 0.0418 |
| Median (Q1, Q3) | 1260 (894, 2880) | 1010 (777, 2000) | 1980 (1040, 8140) | |
| Missing (%) | 30 (38.5) | 12 (28.6) | 18 (50.0) | |
| d-Dimer (mg/L) | ||||
| n | 66 | 38 | 28 | 0.364 |
| Median (Q1, Q3) | 3.53 (1.07, 25.1) | 2.41 (0.848, 24.7) | 4.18 (1.51, 23.3) | |
| Missing (%) | 12 (15.4) | 4 (9.5) | 8 (22.2) | |
| Length of ICU stay (days) | ||||
| n | 69 | 34 | 35 | 0.299 |
| Median (Q1, Q3) | 17.0 (10.0, 27.0) | 15.0 (7.50, 26.3) | 19.0 (14.0, 25.5) | |
| Missing (%) | 9 (11.5) | 8 (19.0) | 1 (2.8) | |
| Length of hospital stay (days) | ||||
| n | 78 | 42 | 36 | 0.173 |
| Median (Q1, Q3) | 22.0 (15.0, 33.3) | 23.5 (16.3, 37.0) | 20.0 (13.0, 30.3) | |
| Time from symptoms to Seraph treatment (days) | ||||
| n | 74 | 39 | 35 | 0.0211 |
| Median (Q1, Q3) | 12.3 (7.43, 15.5) | 9.56 (6.60, 14.6) | 13.0 (11.0, 19.5) | |
| Missing (%) | 4 (5.1) | 3 (7.1) | 1 (2.8) | |
| Time from ICU admission to Seraph treatment (days) | ||||
| n | 69 | 34 | 35 | 0.0023 |
| Median (Q1, Q3) | 2.55 (1.53, 6.00) | 1.73 (1.50, 3.24) | 4.58 (2.05, 11.4) | |
| Missing (%) | 9a (11.5) | 8 (19.0) | 1 (2.8) | |
ECMO, extracorporeal membrane oxygenation; CRP, C-reactive protein; PCT, procalcitonin.
Non-ICU patients.
Patient characteristics of the entire cohort as well as for survivors and non-survivors, and significance level of the difference between survivors and non-survivors
| . | Overall (n = 78) . | Survivor (n = 42) . | Non-survivor (n = 36) . | P-value . |
|---|---|---|---|---|
| Sex | ||||
| Female (%) | 22 (28.2) | 12 (28.6) | 10 (27.8) | 1 |
| Male (%) | 56 (71.8) | 30 (71.4) | 26 (72.2) | |
| Age (years) | ||||
| n | 78 | 42 | 36 | 0.741 |
| Median (Q1, Q3) | 59.0 (50.3, 68.8) | 57.0 (50.3, 68.0) | 61.5 (49.8, 71.3) | |
| Ethnicity | ||||
| Black/African/Caribbean (%) | 13 (16.7) | 7 (16.7) | 6 (16.7) | 0.445 |
| Mixed/multiple ethnic groups (%) | 8 (10.3) | 4 (9.5) | 4 (11.1) | |
| White (%) | 51 (65.4) | 29 (69.0) | 22 (61.1) | |
| Asian (%) | 1 (1.3) | 0 (0) | 1 (2.8) | |
| Other ethnic group (%) | 2 (2.6) | 0 (0) | 2 (5.6) | |
| Missing (%) | 3 (3.8) | 2 (4.8) | 1 (2.8) | |
| Weight (kg) | ||||
| n | 75 | 42 | 33 | 0.847 |
| Median (Q1, Q3) | 90.0 (79.0, 100) | 90.0 (79.0, 109) | 90.0 (80.0, 96.0) | |
| Missing (%) | 3 (3.8) | 0 (0) | 3 (8.3) | |
| Height (cm) | ||||
| n | 72 | 39 | 33 | 0.995 |
| Median (Q1, Q3) | 173 (168, 178) | 174 (168, 178) | 173 (168, 177) | |
| Missing (%) | 6 (7.7) | 3 (7.1) | 3 (8.3) | |
| ICU admission | ||||
| No (%) | 9 (11.5) | 8 (19.0) | 1 (2.8) | 0.0592 |
| Yes (%) | 69 (88.5) | 34 (81.0) | 35 (97.2) | |
| SOFA score | ||||
| n | 71 | 39 | 32 | 0.194 |
| Median (Q1, Q3) | 9.00 (7.00, 12.5) | 9.00 (7.00, 12.0) | 11.0 (8.00, 13.3) | |
| Missing (%) | 7 (9.0) | 3 (7.1) | 4 (11.1) | |
| 4C score | ||||
| n | 47 | 29 | 18 | 0.0157 |
| Median (Q1, Q3) | 12.0 (9.50, 14.0) | 11.0 (8.00, 12.0) | 12.5 (11.3, 14.0) | |
| Missing (%) | 31 (39.7) | 13 (31.0) | 18 (50.0) | |
| Mechanical ventilation | ||||
| No (%) | 23 (29.5) | 15 (35.7) | 8 (22.2) | 0.262 |
| Yes (%) | 52 (66.7) | 25 (59.5) | 27 (75.0) | |
| Missing (%) | 3 (3.8) | 2 (4.8) | 1 (2.8) | |
| PaO2/FiO2 | ||||
| n | 67 | 36 | 31 | 0.692 |
| Median (Q1, Q3) | 119 (71.5, 167) | 120 (75.5, 170) | 111 (67.2, 152) | |
| Missing (%) | 11 (14.1) | 6 (14.3) | 5 (13.9) | |
| Vasopressors | ||||
| No (%) | 35 (44.9) | 20 (47.6) | 15 (41.7) | 0.734 |
| Yes (%) | 32 (41.0) | 16 (38.1) | 16 (44.4) | |
| Missing (%) | 11 (14.1) | 6 (14.3) | 5 (13.9) | |
| Bacterial superinfection | ||||
| No (%) | 47 (60.3) | 30 (71.4) | 17 (47.2) | 0.0517 |
| Yes (%) | 31 (39.7) | 12 (28.6) | 19 (52.8) | |
| KRT dependency | ||||
| No (%) | 54 (69.2) | 29 (69.0) | 25 (69.4) | 1 |
| Yes (%) | 22 (28.2) | 12 (28.6) | 10 (27.8) | |
| Missing (%) | 2 (2.6) | 1 (2.4) | 1 (2.8) | |
| ECMO | ||||
| No (%) | 74 (94.9) | 41 (97.6) | 33 (91.7) | 0.501 |
| Yes (%) | 4 (5.1) | 1 (2.4) | 3 (8.3) | |
| Intermittent haemodialysis | ||||
| No (%) | 54 (69.2) | 28 (66.7) | 26 (72.2) | 0.776 |
| Yes (%) | 24 (30.8) | 14 (33.3) | 10 (27.8) | |
| Seraph as standalone treatment | ||||
| No (%) | 39 (50.0) | 18 (42.9) | 21 (58.3) | 0.287 |
| Yes (%) | 36 (46.2) | 22 (52.4) | 14 (38.9) | |
| Missing (%) | 3 (3.8) | 2 (4.8) | 1 (2.8) | |
| CRP (mg/L) | ||||
| n | 73 | 40 | 33 | 0.907 |
| Median (Q1, Q3) | 155 (97.0, 279) | 149 (110, 24) | 162 (83.0, 386) | |
| Missing (%) | 5 (6.4) | 2 (4.8) | 3 (8.3) | |
| PCT (µg/L) | ||||
| n | 72 | 37 | 35 | 0.0884 |
| Median (Q1, Q3) | 1.50 (0.400, 10.9) | 0.600 (0.400, 6.00) | 4.40 (0.650, 13.8) | |
| Missing (%) | 6 (7.7) | 5 (11.9) | 1 (2.8) | |
| Leucocytes | ||||
| n | 76 | 41 | 35 | 0.26 |
| Median (Q1, Q3) | 11.0 (8.43, 14.8) | 10.3 (7.30, 14.8) | 11.9 (9.45, 14.9) | |
| Missing (%) | 2 (2.6) | 1 (2.4) | 1 (2.8) | |
| Ferritin (ng/mL) | ||||
| n | 48 | 30 | 18 | 0.0418 |
| Median (Q1, Q3) | 1260 (894, 2880) | 1010 (777, 2000) | 1980 (1040, 8140) | |
| Missing (%) | 30 (38.5) | 12 (28.6) | 18 (50.0) | |
| d-Dimer (mg/L) | ||||
| n | 66 | 38 | 28 | 0.364 |
| Median (Q1, Q3) | 3.53 (1.07, 25.1) | 2.41 (0.848, 24.7) | 4.18 (1.51, 23.3) | |
| Missing (%) | 12 (15.4) | 4 (9.5) | 8 (22.2) | |
| Length of ICU stay (days) | ||||
| n | 69 | 34 | 35 | 0.299 |
| Median (Q1, Q3) | 17.0 (10.0, 27.0) | 15.0 (7.50, 26.3) | 19.0 (14.0, 25.5) | |
| Missing (%) | 9 (11.5) | 8 (19.0) | 1 (2.8) | |
| Length of hospital stay (days) | ||||
| n | 78 | 42 | 36 | 0.173 |
| Median (Q1, Q3) | 22.0 (15.0, 33.3) | 23.5 (16.3, 37.0) | 20.0 (13.0, 30.3) | |
| Time from symptoms to Seraph treatment (days) | ||||
| n | 74 | 39 | 35 | 0.0211 |
| Median (Q1, Q3) | 12.3 (7.43, 15.5) | 9.56 (6.60, 14.6) | 13.0 (11.0, 19.5) | |
| Missing (%) | 4 (5.1) | 3 (7.1) | 1 (2.8) | |
| Time from ICU admission to Seraph treatment (days) | ||||
| n | 69 | 34 | 35 | 0.0023 |
| Median (Q1, Q3) | 2.55 (1.53, 6.00) | 1.73 (1.50, 3.24) | 4.58 (2.05, 11.4) | |
| Missing (%) | 9a (11.5) | 8 (19.0) | 1 (2.8) | |
| . | Overall (n = 78) . | Survivor (n = 42) . | Non-survivor (n = 36) . | P-value . |
|---|---|---|---|---|
| Sex | ||||
| Female (%) | 22 (28.2) | 12 (28.6) | 10 (27.8) | 1 |
| Male (%) | 56 (71.8) | 30 (71.4) | 26 (72.2) | |
| Age (years) | ||||
| n | 78 | 42 | 36 | 0.741 |
| Median (Q1, Q3) | 59.0 (50.3, 68.8) | 57.0 (50.3, 68.0) | 61.5 (49.8, 71.3) | |
| Ethnicity | ||||
| Black/African/Caribbean (%) | 13 (16.7) | 7 (16.7) | 6 (16.7) | 0.445 |
| Mixed/multiple ethnic groups (%) | 8 (10.3) | 4 (9.5) | 4 (11.1) | |
| White (%) | 51 (65.4) | 29 (69.0) | 22 (61.1) | |
| Asian (%) | 1 (1.3) | 0 (0) | 1 (2.8) | |
| Other ethnic group (%) | 2 (2.6) | 0 (0) | 2 (5.6) | |
| Missing (%) | 3 (3.8) | 2 (4.8) | 1 (2.8) | |
| Weight (kg) | ||||
| n | 75 | 42 | 33 | 0.847 |
| Median (Q1, Q3) | 90.0 (79.0, 100) | 90.0 (79.0, 109) | 90.0 (80.0, 96.0) | |
| Missing (%) | 3 (3.8) | 0 (0) | 3 (8.3) | |
| Height (cm) | ||||
| n | 72 | 39 | 33 | 0.995 |
| Median (Q1, Q3) | 173 (168, 178) | 174 (168, 178) | 173 (168, 177) | |
| Missing (%) | 6 (7.7) | 3 (7.1) | 3 (8.3) | |
| ICU admission | ||||
| No (%) | 9 (11.5) | 8 (19.0) | 1 (2.8) | 0.0592 |
| Yes (%) | 69 (88.5) | 34 (81.0) | 35 (97.2) | |
| SOFA score | ||||
| n | 71 | 39 | 32 | 0.194 |
| Median (Q1, Q3) | 9.00 (7.00, 12.5) | 9.00 (7.00, 12.0) | 11.0 (8.00, 13.3) | |
| Missing (%) | 7 (9.0) | 3 (7.1) | 4 (11.1) | |
| 4C score | ||||
| n | 47 | 29 | 18 | 0.0157 |
| Median (Q1, Q3) | 12.0 (9.50, 14.0) | 11.0 (8.00, 12.0) | 12.5 (11.3, 14.0) | |
| Missing (%) | 31 (39.7) | 13 (31.0) | 18 (50.0) | |
| Mechanical ventilation | ||||
| No (%) | 23 (29.5) | 15 (35.7) | 8 (22.2) | 0.262 |
| Yes (%) | 52 (66.7) | 25 (59.5) | 27 (75.0) | |
| Missing (%) | 3 (3.8) | 2 (4.8) | 1 (2.8) | |
| PaO2/FiO2 | ||||
| n | 67 | 36 | 31 | 0.692 |
| Median (Q1, Q3) | 119 (71.5, 167) | 120 (75.5, 170) | 111 (67.2, 152) | |
| Missing (%) | 11 (14.1) | 6 (14.3) | 5 (13.9) | |
| Vasopressors | ||||
| No (%) | 35 (44.9) | 20 (47.6) | 15 (41.7) | 0.734 |
| Yes (%) | 32 (41.0) | 16 (38.1) | 16 (44.4) | |
| Missing (%) | 11 (14.1) | 6 (14.3) | 5 (13.9) | |
| Bacterial superinfection | ||||
| No (%) | 47 (60.3) | 30 (71.4) | 17 (47.2) | 0.0517 |
| Yes (%) | 31 (39.7) | 12 (28.6) | 19 (52.8) | |
| KRT dependency | ||||
| No (%) | 54 (69.2) | 29 (69.0) | 25 (69.4) | 1 |
| Yes (%) | 22 (28.2) | 12 (28.6) | 10 (27.8) | |
| Missing (%) | 2 (2.6) | 1 (2.4) | 1 (2.8) | |
| ECMO | ||||
| No (%) | 74 (94.9) | 41 (97.6) | 33 (91.7) | 0.501 |
| Yes (%) | 4 (5.1) | 1 (2.4) | 3 (8.3) | |
| Intermittent haemodialysis | ||||
| No (%) | 54 (69.2) | 28 (66.7) | 26 (72.2) | 0.776 |
| Yes (%) | 24 (30.8) | 14 (33.3) | 10 (27.8) | |
| Seraph as standalone treatment | ||||
| No (%) | 39 (50.0) | 18 (42.9) | 21 (58.3) | 0.287 |
| Yes (%) | 36 (46.2) | 22 (52.4) | 14 (38.9) | |
| Missing (%) | 3 (3.8) | 2 (4.8) | 1 (2.8) | |
| CRP (mg/L) | ||||
| n | 73 | 40 | 33 | 0.907 |
| Median (Q1, Q3) | 155 (97.0, 279) | 149 (110, 24) | 162 (83.0, 386) | |
| Missing (%) | 5 (6.4) | 2 (4.8) | 3 (8.3) | |
| PCT (µg/L) | ||||
| n | 72 | 37 | 35 | 0.0884 |
| Median (Q1, Q3) | 1.50 (0.400, 10.9) | 0.600 (0.400, 6.00) | 4.40 (0.650, 13.8) | |
| Missing (%) | 6 (7.7) | 5 (11.9) | 1 (2.8) | |
| Leucocytes | ||||
| n | 76 | 41 | 35 | 0.26 |
| Median (Q1, Q3) | 11.0 (8.43, 14.8) | 10.3 (7.30, 14.8) | 11.9 (9.45, 14.9) | |
| Missing (%) | 2 (2.6) | 1 (2.4) | 1 (2.8) | |
| Ferritin (ng/mL) | ||||
| n | 48 | 30 | 18 | 0.0418 |
| Median (Q1, Q3) | 1260 (894, 2880) | 1010 (777, 2000) | 1980 (1040, 8140) | |
| Missing (%) | 30 (38.5) | 12 (28.6) | 18 (50.0) | |
| d-Dimer (mg/L) | ||||
| n | 66 | 38 | 28 | 0.364 |
| Median (Q1, Q3) | 3.53 (1.07, 25.1) | 2.41 (0.848, 24.7) | 4.18 (1.51, 23.3) | |
| Missing (%) | 12 (15.4) | 4 (9.5) | 8 (22.2) | |
| Length of ICU stay (days) | ||||
| n | 69 | 34 | 35 | 0.299 |
| Median (Q1, Q3) | 17.0 (10.0, 27.0) | 15.0 (7.50, 26.3) | 19.0 (14.0, 25.5) | |
| Missing (%) | 9 (11.5) | 8 (19.0) | 1 (2.8) | |
| Length of hospital stay (days) | ||||
| n | 78 | 42 | 36 | 0.173 |
| Median (Q1, Q3) | 22.0 (15.0, 33.3) | 23.5 (16.3, 37.0) | 20.0 (13.0, 30.3) | |
| Time from symptoms to Seraph treatment (days) | ||||
| n | 74 | 39 | 35 | 0.0211 |
| Median (Q1, Q3) | 12.3 (7.43, 15.5) | 9.56 (6.60, 14.6) | 13.0 (11.0, 19.5) | |
| Missing (%) | 4 (5.1) | 3 (7.1) | 1 (2.8) | |
| Time from ICU admission to Seraph treatment (days) | ||||
| n | 69 | 34 | 35 | 0.0023 |
| Median (Q1, Q3) | 2.55 (1.53, 6.00) | 1.73 (1.50, 3.24) | 4.58 (2.05, 11.4) | |
| Missing (%) | 9a (11.5) | 8 (19.0) | 1 (2.8) | |
ECMO, extracorporeal membrane oxygenation; CRP, C-reactive protein; PCT, procalcitonin.
Non-ICU patients.
Adverse events were reported in 9 (8.8%) of the 102 treatments. Circuit failure was the most commonly reported adverse event, occurring in nine of the treatments. Other side effects also occurred within the group of nine patients, including hypotension in two patients and shivering and fever in one patient. No adverse event was declared as serious (Table 3).
| . | Univariate . | Multivariatea . | ||||
|---|---|---|---|---|---|---|
| . | HR . | 95% CI . | P-value . | HR . | 95% CI . | P-value . |
| Bacterial infection (yes) | 2 | 1.1–3.9 | 0.032 | 2.36 | 1.18–4.7 | 0.014 |
| ECMO (yes) | 2.7 | 0.83–8.9 | 0.1 | 2.04 | 0.57–7.35 | 0.275 |
| Time from ICU admission to Seraph treatment (days) | 1.1 | 1–1.1 | <0.001 | 1.10 | 1.04–1.15 | <0.001 |
| . | Univariate . | Multivariatea . | ||||
|---|---|---|---|---|---|---|
| . | HR . | 95% CI . | P-value . | HR . | 95% CI . | P-value . |
| Bacterial infection (yes) | 2 | 1.1–3.9 | 0.032 | 2.36 | 1.18–4.7 | 0.014 |
| ECMO (yes) | 2.7 | 0.83–8.9 | 0.1 | 2.04 | 0.57–7.35 | 0.275 |
| Time from ICU admission to Seraph treatment (days) | 1.1 | 1–1.1 | <0.001 | 1.10 | 1.04–1.15 | <0.001 |
ECMO, extracorporeal membrane oxygenation.
n = 69, events = 35.
| . | Univariate . | Multivariatea . | ||||
|---|---|---|---|---|---|---|
| . | HR . | 95% CI . | P-value . | HR . | 95% CI . | P-value . |
| Bacterial infection (yes) | 2 | 1.1–3.9 | 0.032 | 2.36 | 1.18–4.7 | 0.014 |
| ECMO (yes) | 2.7 | 0.83–8.9 | 0.1 | 2.04 | 0.57–7.35 | 0.275 |
| Time from ICU admission to Seraph treatment (days) | 1.1 | 1–1.1 | <0.001 | 1.10 | 1.04–1.15 | <0.001 |
| . | Univariate . | Multivariatea . | ||||
|---|---|---|---|---|---|---|
| . | HR . | 95% CI . | P-value . | HR . | 95% CI . | P-value . |
| Bacterial infection (yes) | 2 | 1.1–3.9 | 0.032 | 2.36 | 1.18–4.7 | 0.014 |
| ECMO (yes) | 2.7 | 0.83–8.9 | 0.1 | 2.04 | 0.57–7.35 | 0.275 |
| Time from ICU admission to Seraph treatment (days) | 1.1 | 1–1.1 | <0.001 | 1.10 | 1.04–1.15 | <0.001 |
ECMO, extracorporeal membrane oxygenation.
n = 69, events = 35.
Reported treatment characteristics, adverse events and system failures per reported treatments
| . | Overall (n = 102) . | Survivor (n = 58) . | Non-survivor (n = 44) . |
|---|---|---|---|
| Seraph treatment time (h) | |||
| n | 102 | 58 | 44 |
| Median (Q1, Q3) | 5.00 (4.00, 13.4) | 4.17 (4.00, 5.23) | 6.00 (4.21, 23.5) |
| Treated blood volume (L) | |||
| n | 64 | 32 | 32 |
| Median (Q1, Q3) | 71.3 (43.7, 109) | 66.8 (48.0, 84.0) | 76.9 (8.53, 158) |
| Missing (%) | 38 (37.3) | 26 (44.8) | 12 (27.3) |
| Seraph as standalone treatment | |||
| No (%) | 55 (53.9) | 30 (51.7) | 25 (56.8) |
| Yes (%) | 44 (43.1) | 26 (44.8) | 18 (40.9) |
| Missing (%) | 3 (2.9) | 2 (3.4) | 1 (2.3) |
| Adverse event | |||
| No (%) | 83 (81.4) | 49 (84.5) | 34 (77.3) |
| Yes (%) | 9 (8.8) | 6 (10.3) | 3 (6.8) |
| Missing (%) | 10 (9.8) | 3 (5.2) | 7 (15.9) |
| Vascular access | |||
| Double lumen catheter (%) | 62 (60.8) | 30 (51.7) | 32 (72.7) |
| Shunt/AV fistula (%) | 7 (6.9) | 5 (8.6) | 2 (4.5) |
| ECMO circuit (%) | 1 (1.0) | 0 (0) | 1 (2.3) |
| Missing (%) | 32 (31.4) | 23 (39.7) | 9 (20.5) |
| Hours | |||
| n | 64 | 32 | 32 |
| Median (Q1, Q3) | 5.50 (4.00, 24.0) | 4.00 (4.00, 10.0) | 16.8 (4.00, 24.0) |
| Missing (%) | 38 (37.3) | 26 (44.8) | 12 (27.3) |
| Anticoagulation | |||
| Citrate (%) | 15 (14.7) | 3 (5.2) | 12 (27.3) |
| Heparin (%) | 66 (64.7) | 41 (70.7) | 25 (56.8) |
| Other (%) | 8 (7.8) | 6 (10.3) | 2 (4.5) |
| Missing (%) | 13 (12.7) | 8 (13.8) | 5 (11.4) |
| Clotting event | |||
| No (%) | 92 (90.2) | 52 (89.7) | 40 (90.9) |
| Yes (%) | 9 (8.8) | 6 (10.3) | 3 (6.8) |
| Missing (%) | 1 (1.0) | 0 (0) | 1 (2.3) |
| . | Overall (n = 102) . | Survivor (n = 58) . | Non-survivor (n = 44) . |
|---|---|---|---|
| Seraph treatment time (h) | |||
| n | 102 | 58 | 44 |
| Median (Q1, Q3) | 5.00 (4.00, 13.4) | 4.17 (4.00, 5.23) | 6.00 (4.21, 23.5) |
| Treated blood volume (L) | |||
| n | 64 | 32 | 32 |
| Median (Q1, Q3) | 71.3 (43.7, 109) | 66.8 (48.0, 84.0) | 76.9 (8.53, 158) |
| Missing (%) | 38 (37.3) | 26 (44.8) | 12 (27.3) |
| Seraph as standalone treatment | |||
| No (%) | 55 (53.9) | 30 (51.7) | 25 (56.8) |
| Yes (%) | 44 (43.1) | 26 (44.8) | 18 (40.9) |
| Missing (%) | 3 (2.9) | 2 (3.4) | 1 (2.3) |
| Adverse event | |||
| No (%) | 83 (81.4) | 49 (84.5) | 34 (77.3) |
| Yes (%) | 9 (8.8) | 6 (10.3) | 3 (6.8) |
| Missing (%) | 10 (9.8) | 3 (5.2) | 7 (15.9) |
| Vascular access | |||
| Double lumen catheter (%) | 62 (60.8) | 30 (51.7) | 32 (72.7) |
| Shunt/AV fistula (%) | 7 (6.9) | 5 (8.6) | 2 (4.5) |
| ECMO circuit (%) | 1 (1.0) | 0 (0) | 1 (2.3) |
| Missing (%) | 32 (31.4) | 23 (39.7) | 9 (20.5) |
| Hours | |||
| n | 64 | 32 | 32 |
| Median (Q1, Q3) | 5.50 (4.00, 24.0) | 4.00 (4.00, 10.0) | 16.8 (4.00, 24.0) |
| Missing (%) | 38 (37.3) | 26 (44.8) | 12 (27.3) |
| Anticoagulation | |||
| Citrate (%) | 15 (14.7) | 3 (5.2) | 12 (27.3) |
| Heparin (%) | 66 (64.7) | 41 (70.7) | 25 (56.8) |
| Other (%) | 8 (7.8) | 6 (10.3) | 2 (4.5) |
| Missing (%) | 13 (12.7) | 8 (13.8) | 5 (11.4) |
| Clotting event | |||
| No (%) | 92 (90.2) | 52 (89.7) | 40 (90.9) |
| Yes (%) | 9 (8.8) | 6 (10.3) | 3 (6.8) |
| Missing (%) | 1 (1.0) | 0 (0) | 1 (2.3) |
AV, vascular access; ECMO, extracorporeal membrane oxygenation.
Reported treatment characteristics, adverse events and system failures per reported treatments
| . | Overall (n = 102) . | Survivor (n = 58) . | Non-survivor (n = 44) . |
|---|---|---|---|
| Seraph treatment time (h) | |||
| n | 102 | 58 | 44 |
| Median (Q1, Q3) | 5.00 (4.00, 13.4) | 4.17 (4.00, 5.23) | 6.00 (4.21, 23.5) |
| Treated blood volume (L) | |||
| n | 64 | 32 | 32 |
| Median (Q1, Q3) | 71.3 (43.7, 109) | 66.8 (48.0, 84.0) | 76.9 (8.53, 158) |
| Missing (%) | 38 (37.3) | 26 (44.8) | 12 (27.3) |
| Seraph as standalone treatment | |||
| No (%) | 55 (53.9) | 30 (51.7) | 25 (56.8) |
| Yes (%) | 44 (43.1) | 26 (44.8) | 18 (40.9) |
| Missing (%) | 3 (2.9) | 2 (3.4) | 1 (2.3) |
| Adverse event | |||
| No (%) | 83 (81.4) | 49 (84.5) | 34 (77.3) |
| Yes (%) | 9 (8.8) | 6 (10.3) | 3 (6.8) |
| Missing (%) | 10 (9.8) | 3 (5.2) | 7 (15.9) |
| Vascular access | |||
| Double lumen catheter (%) | 62 (60.8) | 30 (51.7) | 32 (72.7) |
| Shunt/AV fistula (%) | 7 (6.9) | 5 (8.6) | 2 (4.5) |
| ECMO circuit (%) | 1 (1.0) | 0 (0) | 1 (2.3) |
| Missing (%) | 32 (31.4) | 23 (39.7) | 9 (20.5) |
| Hours | |||
| n | 64 | 32 | 32 |
| Median (Q1, Q3) | 5.50 (4.00, 24.0) | 4.00 (4.00, 10.0) | 16.8 (4.00, 24.0) |
| Missing (%) | 38 (37.3) | 26 (44.8) | 12 (27.3) |
| Anticoagulation | |||
| Citrate (%) | 15 (14.7) | 3 (5.2) | 12 (27.3) |
| Heparin (%) | 66 (64.7) | 41 (70.7) | 25 (56.8) |
| Other (%) | 8 (7.8) | 6 (10.3) | 2 (4.5) |
| Missing (%) | 13 (12.7) | 8 (13.8) | 5 (11.4) |
| Clotting event | |||
| No (%) | 92 (90.2) | 52 (89.7) | 40 (90.9) |
| Yes (%) | 9 (8.8) | 6 (10.3) | 3 (6.8) |
| Missing (%) | 1 (1.0) | 0 (0) | 1 (2.3) |
| . | Overall (n = 102) . | Survivor (n = 58) . | Non-survivor (n = 44) . |
|---|---|---|---|
| Seraph treatment time (h) | |||
| n | 102 | 58 | 44 |
| Median (Q1, Q3) | 5.00 (4.00, 13.4) | 4.17 (4.00, 5.23) | 6.00 (4.21, 23.5) |
| Treated blood volume (L) | |||
| n | 64 | 32 | 32 |
| Median (Q1, Q3) | 71.3 (43.7, 109) | 66.8 (48.0, 84.0) | 76.9 (8.53, 158) |
| Missing (%) | 38 (37.3) | 26 (44.8) | 12 (27.3) |
| Seraph as standalone treatment | |||
| No (%) | 55 (53.9) | 30 (51.7) | 25 (56.8) |
| Yes (%) | 44 (43.1) | 26 (44.8) | 18 (40.9) |
| Missing (%) | 3 (2.9) | 2 (3.4) | 1 (2.3) |
| Adverse event | |||
| No (%) | 83 (81.4) | 49 (84.5) | 34 (77.3) |
| Yes (%) | 9 (8.8) | 6 (10.3) | 3 (6.8) |
| Missing (%) | 10 (9.8) | 3 (5.2) | 7 (15.9) |
| Vascular access | |||
| Double lumen catheter (%) | 62 (60.8) | 30 (51.7) | 32 (72.7) |
| Shunt/AV fistula (%) | 7 (6.9) | 5 (8.6) | 2 (4.5) |
| ECMO circuit (%) | 1 (1.0) | 0 (0) | 1 (2.3) |
| Missing (%) | 32 (31.4) | 23 (39.7) | 9 (20.5) |
| Hours | |||
| n | 64 | 32 | 32 |
| Median (Q1, Q3) | 5.50 (4.00, 24.0) | 4.00 (4.00, 10.0) | 16.8 (4.00, 24.0) |
| Missing (%) | 38 (37.3) | 26 (44.8) | 12 (27.3) |
| Anticoagulation | |||
| Citrate (%) | 15 (14.7) | 3 (5.2) | 12 (27.3) |
| Heparin (%) | 66 (64.7) | 41 (70.7) | 25 (56.8) |
| Other (%) | 8 (7.8) | 6 (10.3) | 2 (4.5) |
| Missing (%) | 13 (12.7) | 8 (13.8) | 5 (11.4) |
| Clotting event | |||
| No (%) | 92 (90.2) | 52 (89.7) | 40 (90.9) |
| Yes (%) | 9 (8.8) | 6 (10.3) | 3 (6.8) |
| Missing (%) | 1 (1.0) | 0 (0) | 1 (2.3) |
AV, vascular access; ECMO, extracorporeal membrane oxygenation.
DISCUSSION
To our knowledge, this report is the largest cohort of patients with COVID-19 who had been treated with the Seraph® 100 Microbind® Affinity Blood Filter.
The Seraph® 100 has been licensed in the European Union in 2019 for the removal of pathogens from the blood. The functional basis of the device is an adsorption substrate of ultra-high molecular weight polyethylene beads coated with endpoint-attached heparin. Bacteria, viruses, fungi and toxins have been shown to bind to the immobilized heparin in a similar way to the interaction with heparan sulphate on the cell surface [15]. Due to this biomimetic feature, pathogens bind irreversibly to the heparin on the polyethylene beads and are thereby removed from the bloodstream. Indeed, recent data have shown that the Seraph® 100 Microbind® Affinity filter removes the nucleocapsid protein of SARS-CoV-2 [20] and might therefore improve the clinical course of critically ill COVID-19 patients in whom viraemia correlates and predicts adverse outcomes. This comes as no surprise as heparin binding is a frequent feature in viruses.
This ability allows viruses to bind to heparan sulphate proteoglycans on the surface of host cells—a common precondition to entering the cells through internalization. For SARS-CoV-2, it has been shown that it not only binds to heparin, but also that ACE2-mediated coronavirus entry can be mitigated by heparin, a heparan sulphate-related glycan, or by genetic ablation of biosynthetic enzymes for the cell surface heparan sulphate proteoglycans [26].
Mortality in the COSA registry
The mortality of 46.2% in our cohort is comparable to the 37.3% mortality in the Purify OBS study [27], although we describe a higher percentage of patients on mechanical ventilation (66.7% versus 56.6%) and generally fewer Seraph® 100 treatments per patient.
While the average predicted mortality rate according to SOFA score in ICU patients was 56.7%, the observed mortality was 50.7%. The SOFA scoring system, which has been used since 1996 to evaluate organ failure, can predict the severity and outcome of diseases such as sepsis [28]. It has also been of predictive value in COVID-19 with an OR of 5.56 in an early report from China [29]. A late report in patients with COVID-19 pneumonia receiving oxygen therapy for 4 h or longer, before undergoing endotracheal intubation, showed poor performance of the SOFA score to discriminate death from survival, and SOFA score was found to be inferior even to using age alone [30].
In non-ICU patients, 4C score average predicted a mortality rate of 38.0%, while the observed mortality rate Seraph® 100-treated patients was 11.1%. However, the 4C score was calculated on the day of Seraph® 100 treatment and not during hospital admission, for which the score was originally validated. Therefore, our observed differences in mortality between calculated and observed mortality must be interpreted with caution.
Timing of Seraph® 100 treatment and mortality
Treatment with Seraph® 100 was initiated earlier after the onset of symptoms in the survivors than in those who succumbed. Also, time from ICU admission to Seraph® 100 treatment was shorter in the survivors than in the non-survivors. Mortality in ICU patients who started Seraph® 100 treatment within 60 h of ICU admission was 34.5% (predicted 51.7%) and almost 50% lower than in those patients in which Seraph® 100 treatment was initiated after >60 h in the ICU (Figure 2). This is reminiscent of data in a patient with AKI in the ICU undergoing KRT. The late start of KRT (relative to ICU admission) was associated with greater mortality, irrespective of urea levels [31].
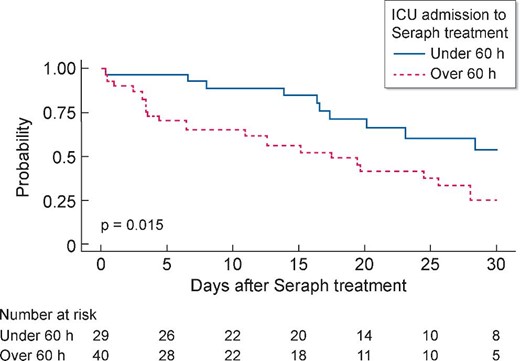
Kaplan–Meier analysis of survival in patients stratified for ICU admission <60 and >60 h during 30 days (P log-rank test <0.015).
Mortality and inflammation
Early reports from China showed that a fulminant inflammatory response to the viral infection correlates with disease severity [32]. In our cohort, ferritin was the only inflammatory marker that was significantly different between survivors and non-survivors, which is in line with the importance of ferritin as the only inflammatory marker in a score predicting mortality within 14 days after intubation [33]. However, in multivariate Cox regression analysis, ferritin levels were not significantly associated with mortality in our study.
Low rate of circuit failure
A single centre analysis showed that >30% of haemofilters failed in under 9 h due to clotting and the median circuit life was 21 h [34]. In our analysis, filter clotting was as low as 8.8%, although more than half of the treatments were performed in conjunction with renal replacement therapy.
Study limitations
The inherent limitation of a registry in comparison with a prospective randomized trial is the lack of a control group. Nonetheless, the collection of data will in our view help to focus future prospective randomized trials in terms of inclusion/exclusion criteria, as well as which variables to investigate. Ongoing prospective clinical trials in Europe (NCT04547257) and the USA (NCT04606498) will further clarify the role of the Seraph® 100 in the treatment of COVID-19 patients.
In conclusion, treatment of critically ill patients with the Seraph® 100 was well tolerated with a low rate of reported clotting events. Early initiation of treatment after ICU admission was associated with increased survival. Reported mortality was lower than expected according to SOFA and 4C scores. However, due to the inherent limitations of registry data, prospective studies are needed to evaluate the possible benefits of the Seraph® 100 in COVID-19 pneumonia.
DATA AVAILABILITY STATEMENT
The datasets during and/or analysed during the current study are available from the corresponding author on reasonable request.
ACKNOWLEDGEMENTS
We thank Ms Alina Siegert for her logistical support.
FUNDING
Infrastructural and IT support was provided by GORTA, Ireland's longest-running international development organization. GORTA had an influence neither on the design of the study nor on data collection, analysis and interpretation of data, nor the writing and content of the manuscript.
AUTHORS’ CONTRIBUTIONS
J.J.S. and J.T.K. designed the study and applied for the initial IRB approval. J.J.S., J.T.K., D.N.B. and T.F. analysed the data, designed the figures and wrote the initial draft of the manuscript. Sequence of authorship was related to the number of recruited patients per centre. All centres recruiting more than five patients have two authors on the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
S.B., T.F., J.T.K. and J.J.S. received research support from ExThera Medical. J.T.K. received speaker fees from Fresenius Medical Care. S.B. received speaker fees from CytoSorbents.
REFERENCES
- coronavirus
- hemoperfusion
- follow-up
- intensive care unit
- nucleocapsid
- reimbursement mechanisms
- renal replacement therapy
- safety
- superinfection
- united states food and drug administration
- viral load result
- viremia
- ferritin measurement
- mortality
- viruses
- organ failure
- pathogenic organism
- severe acute respiratory syndrome
- cox proportional hazards models
- medical devices
- interim analysis
- affinity
- adverse event
- emergency use authorization
- filters
- child occupational self assessment
- sequential organ failure assessment scores
- sars-cov-2
- covid-19





Comments