-
PDF
- Split View
-
Views
-
Cite
Cite
Nessn Azawi, Mia Jensen, Boye L Jensen, Jens P Gϕtze, Claus Bistrup, Lars Lund, Functional adaptation after kidney tissue removal in patients is associated with increased plasma atrial natriuretic peptide concentration, Nephrology Dialysis Transplantation, Volume 37, Issue 11, November 2022, Pages 2138–2149, https://doi.org/10.1093/ndt/gfab327
Close - Share Icon Share
ABSTRACT
Following nephrectomy, the remaining kidney tissue adapts by an increase in glomerular filtration rate (GFR). In rats, hyperfiltration can be transferred by plasma. We examined whether natriuretic peptides, atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) increase in plasma proportionally with kidney mass reduction and, if so, whether the increase relates to an increase in GFR.
Patients (n = 54) undergoing partial or total unilateral nephrectomy at two Danish centres were followed for 1 year in an observational study. Glomerular filtration rate was measured before, and 3 and 12 months after surgery. Natriuretic propeptides (proANP and proBNP) and aldosterone were measured in plasma before and at 24 h, 5 days, 21 days, 3 months and 12 months. Cyclic guanosine monophosphate (cGMP) was determined in urine.
There was no baseline difference in GFR between total and partial nephrectomy (90.1 mL/min/1.73 m2 ± 14.6 versus 82.9 ± 18; P = 0.16). Single-kidney GFR increased after 3 and 12 months (12.0 and 11.9 mL/min/1.73 m2, +23.3%). There was no change in measured GFR 3 and 12 months after partial nephrectomy. ProANP and proBNP increased 3-fold 24 h after surgery and returned to baseline after 5 days. The magnitude of acute proANP and proBNP increases did not relate to kidney mass removed. ProANP, not proBNP, increased 12 months after nephrectomy. Plasma aldosterone and urine cGMP did not change. Urine albumin/creatinine ratio increased transiently after partial nephrectomy. Blood pressure was similar between the groups.
ANP and BNP increase acutely in plasma with no relation to degree of kidney tissue ablation. After 1 year, only unilateral nephrectomy patients displayed increased plasma ANP, which could support adaptation.
What is already known about this subject?
Nephrectomy leads to rapid adaptation whereby the remaining kidney tissue increases its function. The mechanisms are not known but can be transferred by plasma in experimental animals.
Natriuretic peptide hormones atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) may increase glomerular filtration rate and are significantly extracted and metabolized by kidneys.
Atrial natriuretic factor infusion protects kidney function in patients at high risk of acute kidney injury.
What this study adds?
The present study was done to address whether natriuretic peptides increase proportionally to tissue removal and the increase associates with kidney functional adaptation in patients undergoing kidney surgery.
The study shows in patients with total unilateral nephrectomy and partial nephrectomy that both ANP and BNP increase in the first days after surgery independent of the amount of kidney tissue removed and the increase in natriuretic peptides is parallel to the 25% functional increase after unilateral nephrectomy.
Plasma aldosterone concentration does not change with time and type of kidney surgery and the renin–angiotensin system is therefore less likely to mediate adaptive changes.
After 1 year, plasma ANP is elevated only in total nephrectomy group compared with baseline and may contribute to the functional adaptation.
What impact this may have on practice or policy?
The study highlights a potential mediator role for natriuretic peptides in acute and long-term renal functional adaptation.
The study suggests that the pathway can be exploited pharmacologically by, for example, neprilysin inhibitors or guanylyl cyclase stimulators.
INTRODUCTION
The increase in the glomerular filtration rate (GFR) between resting state and maximum capacity is known as the renal functional reserve [1]. Living donor nephrectomy is associated with an increase in the single kidney and thus single nephron GFR (snGFR) after surgery reflecting the higher functional demand [2, 3]. The increase is acute and persistent [4]. While this was attributed previously to increases in renal blood flow, magnetic resonance imaging studies contest this view by showing that GFR increases in association with a relatively minor change or even a decrease in total kidney perfusion and cortical perfusion after nephrectomy [5]. Consistent data show that an increase in GFR is relatively more extensive than in renal plasma flow (RPF), suggesting differential and balanced effects on glomerular arterioles with preferential efferent resistance increase and/or afferent resistance decrease [5, 6]. Pioneering studies showed that infusion of plasma from rats nephrectomized before plasma harvest conferred an increase in snGFR, tubular fluid flow rate and sodium excretion upon transfusion into recipient rats [7], suggesting that circulating factors were responsible. Angiotensin II (ANGII) and aldosterone concentrations increased in plasma harvested from nephrectomized rats [7]. These sodium-retaining hormones are less likely as mediators of increased sodium excretion. The cardiac natriuretic peptides appear relevant. Natriuretic peptides, best documented for atrial natriuretic peptide (ANP), exert differential effects on glomerular arterioles, at least in rodents, with afferent relaxation and efferent constriction [8–12]. In patients with acute kidney failure, ANP infusion increases renal blood flow, filtration fraction and GFR [13, 14]. However, ANP concentration was not increased in plasma from uninephrectomized rats [7]. Brain natriuretic peptide (BNP) was not determined. BNP infusion to healthy humans at a slightly supraphysiological level increases urinary flow rate, sodium excretion and GFR, while RPF decreases [15]. Chronic kidney disease is associated with elevated plasma concentration of natriuretic peptides, best described for the more stable prohormone proBNP (often referred to as NT-proBNP) [13, 16]. Because of high renal extraction of natriuretic peptides and their molecular precursors [17], it seems likely that an abrupt decrease in GFR by removal of kidney tissue increases natriuretic peptide concentrations proportional to the loss of nephron mass and that such an acute increase could initiate and maintain the adaptive renal functional increase. Data show that natriuretic peptide receptors in podocytes confer protection of the filtration barrier [18]. There is now a possibility to test a relation between tissue removal and natriuretic peptides directly in human patients. Total unilateral nephrectomy is replaced progressively by ‘nephron-sparing’ surgical techniques, denoted partial nephrectomy, which has become the golden standard for treating small tumour masses. The present study was designed to characterize longitudinal changes in ANP and BNP plasma concentration in patients undergoing partial and total unilateral nephrectomy. The aim was to associate acute changes in natriuretic peptides to the degree of kidney mass reduction and to renal functional adaptation, blood pressure and kidney injury. Specifically, it was hypothesized that (i) magnitude of kidney mass reduction relates directly to plasma increase in natriuretic peptide concentrations, with larger sensitivity in proBNP than proANP, and (ii) such an increase relates directly to compensatory functional increase and inversely to kidney injury and blood pressure.
MATERIALS AND METHODS
Study population
In the period July 2016–August 2019, 74 adult patients were enrolled before their renal surgery from the Department of Urology at Odense University hospital and Zealand University hospital; Of them, only 54 patients were included in the study (Figure 1). All subjects underwent a standard staging procedure in patients with cancer and extended medical/nephrological evaluation before donor nephrectomies. Exclusion criteria for the study included severe hypertension, congestive heart failure New York Heart Association (NYHA) classification III–IV and renal insufficiency with estimated GFR (eGFR) <50% of normal value and for donors, diabetes and cancer. Twenty patients were excluded from the analysis due to withdrawing consent (n = 11), declining to participate (n = 6) and not meeting inclusion criteria (n = 3). Patients were divided into two groups: unilateral total nephrectomy (35 patients) and unilateral partial nephrectomy (19 patients). Total nephrectomy was subdivided into cancer nephrectomy (25 patients) and donor nephrectomy (10 patients). Patients underwent study evaluations at six time points: (i) pre-surgery, (ii) 24 h post-surgery, (iii) 5 days post-surgery, (iv) 21 post-surgery, (v) 3 months post-surgery and (vi) 12 months post-surgery. Informed consent was gathered from all patients before they participated in the study. The study has been approved by the Scientific Ethics Committee for the Region of Southern Denmark (S-20140044) and the transfer of biological material from Region Zealand to the University of Southern Denmark has been approved by The Data Inspectorate (2019-522-0171). The project is registered at ClinicalTrials.gov, identifier: NCT02646293. All patients underwent an extended nephrological evaluation before donor nephrectomy.
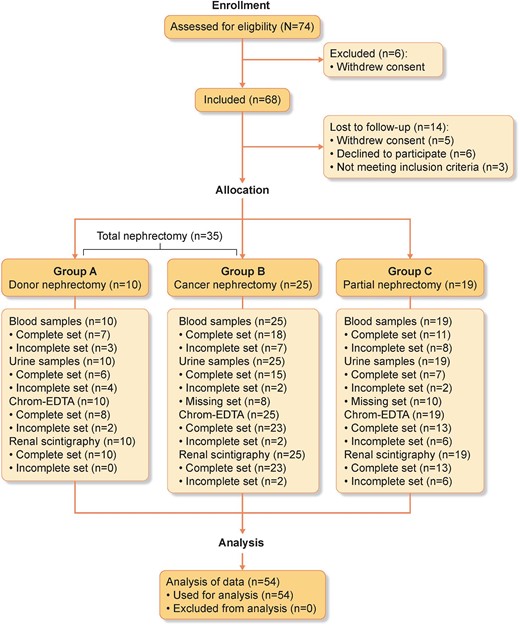
The consort flow diagram shows the number of subjects eligible and enrolled in the study and the subjects that completed the study with dropouts. Sixty-eight participants were included and divided into total (donor and cancer) and partial unilateral nephrectomy. Plasma and urine samples were collected at each visit. Kidney functional distribution was assessed by scintigraphy before surgery and at 3 and 12 months after surgery for partial nephrectomy. GFR was measured three times by indicator substance. Fourteen participants were lost to follow-up. Complete set: all samples or screenings were collected for all evaluation time points for the participant. Incomplete set: samples or screenings are missing for one or more time points for the participant. Missing set: participants who did not have their urine samples stored in a biobank. (Created with BioRender.com.)
Statistic power
The primary effect parameter was BNP in plasma. The minimal relevant difference in plasma concentration is estimated at 50 pg/mL change in plasma concentration of NT-proBNP was determined in a pilot study in 20 patients with a standard error of ∼10 pg/mL in 20 observations gives a standard deviation (SD) of ∼45 pg/mL. This provides a standardized difference of 50/45 = 1.1, and with a power of 90 and a significance level of 5%, the necessary sample size is ∼40 patients with 20 patients in each group (total nephrectomy versus partial nephrectomy).
Baseline data
Plasma samples, urine samples, pulse and blood pressure measurements were collected from all enrolled patients at the evaluation points. Biochemical parameters (plasma concentrations of creatinine, sodium, potassium, urine concentration of albumin and creatinine) were measured at the respective hospitals. The samples from each patient were stored at a research biobank at the respective hospital at −80°C for later measurements. All samples were measured in one setting. Patient characteristics, body compositions and medical history were stored together with the longitudinal data from the plasma and urine samples in a REDCap (Research Electronic Data Capture) database. REDCap is an electronic data capture tool hosted at Odense University hospital [19, 20]. REDCap is a secure, web-based software platform designed to support data capture for research studies.
Assessment of kidney function
Patients underwent a detailed assessment of kidney function before, after 3 months and 12 months post-nephrectomy mainly by 51Cr-EDTA (ethylenediaminetetraacetate) but in a few patients, 99mTc-DPTA (diethylenetriaminepentaacetic acid) was used. Injections were followed by timed collections of plasma referred to as measured GFR (mGFR). eGFR was calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Creatinine Equation for all evaluation points [21]. All patients underwent preoperative renal scintigraphy. Patients who underwent partial nephrectomy underwent two additional renal scintigraphy at 3 and 12 months post-nephrectomy (Supplementary data, Table S1 and Figure S1).
Estimation of compensatory function increase
Measurement of natriuretic peptides
The concentration of proBNP (NT-proBNP) was measured at the Department of Clinical Biochemistry and Pharmacology at Odense University hospital using electrochemiluminescence immunoassay (ECLIA) on Cobas 800 (Roche Diagnostic). ProANP (MR-proANP) was measured with a mid-regional assay on a Kryptor Plus platform (Thermo Fisher Scientific BRAHMS, Germany), with intra- and interassay coefficients of variation of <6.5%.
Measurements of cyclic guanosine monophosphate in urine
The cyclic guanosine monophosphate (cGMP) concentration was measured in urine by the cyclic GMP kit from Cayman Chemical (581021). The assay was based on competitive enzyme-linked immunoassay (ELISA), which detects signal interferences to measure the concentration of the antigen. Urine samples were diluted 1:500 with ELISA buffer.
Measurements of aldosterone in plasma
The concentration of aldosterone in EDTA-anticoagulated plasma was measured by aldosterone ELISA from Labor Diagnostika Nord GmbH & CoKG (MS E-5200). The assay was based on the competitive ELISA and the plasma samples were not diluted.
Statistics
All data sets were analysed by D'Agostino & Pearson omnibus normality test to determine Gaussian distribution, and non-normally distributed data were log-transformed to obtain a normal distribution. Depending on normal distribution, values are reported as mean ± standard error of mean (SEM) or median [21] and values in tables are reported as mean ± SD or median [interquartile range (IQR)]. Statistical analyses were performed by using the two-tailed t-test, one-way analysis of variance (ANOVA) or Kruskal–Wallis test and repeated measures ANOVA followed by Bonferroni's post-test. Pearson correlation coefficients were used to determine the correlation between two variables. All statistical analysis was performed by GraphPad Prism software version 9.
RESULTS
Fifty-four patients participated; 19 patients underwent partial nephrectomy and 35 underwent total nephrectomy, subdivided into 10 living donor nephrectomies and 25 cancer-related nephrectomies (consort diagram, Figure 1). The mean age was 69.1 years (SD, 1.7), and the male to female ratio was 32:22. The baseline and physiological characteristics of the study population are shown in Table 1. The nephrectomy group had equal sex distribution; the group subjected to partial nephrectomy had more males (Table 1). Age, body mass index (BMI) and weight were comparable between the three groups (Table 1). In the total nephrectomy patient group, hypertension was more prevalent in patients with cancer, and intake of antihypertensives was higher (+22%). Patients with cancer did not differ from healthy donors subjected to nephrectomy on other parameters (eGFR, urine albumin, urine protein excretion, plasma electrolytes and heart rate; Table 1). In subsequent analyses, the kidney donors were pooled with patients with total nephrectomy due to cancer.
Baseline characteristics of the nephrectomy patients from Odense and Roskilde
| . | Donor nephrectomy . | Cancer nephrectomy . | Partial nephrectomy . | P-value . |
|---|---|---|---|---|
| Participant characteristics | ||||
| Number of participants | 10 | 25 | 19 | |
| Median age, years | 56 ± 13.83 | 59.40 ± 14.84 | 60.47 ± 9.507 | 0.65 (ns) |
| Sex distribution (female/male) | 8 female/2 male | 10 female/15 male | 4 female/15 male | |
| Body composition | ||||
| Weight, kg | 76.88 ± 15.34 | 86.42 ± 20.83 | 87.70 ± 17.47 | 0.31 (ns) |
| Height, cm | 168.2 ± 4.392 | 173.1 ± 6.987 | 176.9 ± 7.534 | 0.0076** |
| BMI, kg/m2 | 26.99 ± 4.545 | 28.76 ± 6.627 | 27.94 ± 5.286 | 0.71 (ns) |
| Medical history | ||||
| Hypertension | 3 (30) | 13 (52) | 9 (47.36) | |
| Heart patient | 0 (0) | 2 (8) | 2 (10.52) | |
| Former known kidney patienta | 0 (0) | 4 (16) | 3 (15.79) | |
| DM type I | 0 (0) | 1 (4) | 0 (0) | |
| DM type II | 0 (0) | 3 (12) | 2 (10.52) | |
| Medication | ||||
| Antihypertensiveb | 3 (100) | 12 (92.3) | 7 (77.78) | |
| Diuretics | 1/3 | 7/12 | 0/7 | |
| β-blocker | 0/3 | 2/12 | 2/7 | |
| ACE inhibitor | 0/3 | 5/12 | 4/7 | |
| AT2 inhibitor | 1/3 | 5/12 | 2/7 | |
| Calcium antagonist | 1/3 | 4/12 | 2/7 | |
| α-blocker | 0/3 | 1/12 | 0/7 | |
| Combined α/β blocker | 0/3 | 1/12 | 0/7 | |
| Clinical and biochemical parameters | ||||
| Systolic BP, mmHg | 136 ± 20 | 165 ± 20 | 158 ± 23 | 0.0028** |
| Diastolic BP, mmHg | 82.1 ± 10.9 | 94.6 ± 14 | 88.9 ± 12.8 | 0.0403* |
| Heart rate, beats/min | 68.5 ± 14.4 | 81.5 ± 11.4 | 75.3 ± 18.3 | 0.09 (ns) |
| Urine albumin/creatinine, mg/mmol | 13.24 [28.03] | 21.57 [375.2] | 15.28 [90.64] | 0.38 (ns) |
| Urine total protein/creatinine, g/mmol | 0.021 ± 0.016 | 0.030 ± 0.031 | 0.027 ± 0.028 | 0.53 (ns) |
| Plasma creatinine, µmol/L | 69.50 [20.0] | 76.0 [76.0] | 81.0 [89.0] | 0.29 (ns) |
| Plasma sodium, mmol/L | 140.5 [5.0] | 139.0 [10.0] | 139.0 [11.0] | 0.08 (ns) |
| Plasma potassium, mmol/L | 4.1 ± 0.3 | 4.2 ± 0.3 | 4.3 ± 0.5 | 0.70 (ns) |
| eGFR, mL/min/1.73 m2 | 87.50 [16.00] | 87.00 [39.00] | 88.00 [49.00] | 0.83 (ns) |
| Other | ||||
| Smoker (y/n) | 2 yes/8 no | 15 yes/10 no | 12 yes/7 no |
| . | Donor nephrectomy . | Cancer nephrectomy . | Partial nephrectomy . | P-value . |
|---|---|---|---|---|
| Participant characteristics | ||||
| Number of participants | 10 | 25 | 19 | |
| Median age, years | 56 ± 13.83 | 59.40 ± 14.84 | 60.47 ± 9.507 | 0.65 (ns) |
| Sex distribution (female/male) | 8 female/2 male | 10 female/15 male | 4 female/15 male | |
| Body composition | ||||
| Weight, kg | 76.88 ± 15.34 | 86.42 ± 20.83 | 87.70 ± 17.47 | 0.31 (ns) |
| Height, cm | 168.2 ± 4.392 | 173.1 ± 6.987 | 176.9 ± 7.534 | 0.0076** |
| BMI, kg/m2 | 26.99 ± 4.545 | 28.76 ± 6.627 | 27.94 ± 5.286 | 0.71 (ns) |
| Medical history | ||||
| Hypertension | 3 (30) | 13 (52) | 9 (47.36) | |
| Heart patient | 0 (0) | 2 (8) | 2 (10.52) | |
| Former known kidney patienta | 0 (0) | 4 (16) | 3 (15.79) | |
| DM type I | 0 (0) | 1 (4) | 0 (0) | |
| DM type II | 0 (0) | 3 (12) | 2 (10.52) | |
| Medication | ||||
| Antihypertensiveb | 3 (100) | 12 (92.3) | 7 (77.78) | |
| Diuretics | 1/3 | 7/12 | 0/7 | |
| β-blocker | 0/3 | 2/12 | 2/7 | |
| ACE inhibitor | 0/3 | 5/12 | 4/7 | |
| AT2 inhibitor | 1/3 | 5/12 | 2/7 | |
| Calcium antagonist | 1/3 | 4/12 | 2/7 | |
| α-blocker | 0/3 | 1/12 | 0/7 | |
| Combined α/β blocker | 0/3 | 1/12 | 0/7 | |
| Clinical and biochemical parameters | ||||
| Systolic BP, mmHg | 136 ± 20 | 165 ± 20 | 158 ± 23 | 0.0028** |
| Diastolic BP, mmHg | 82.1 ± 10.9 | 94.6 ± 14 | 88.9 ± 12.8 | 0.0403* |
| Heart rate, beats/min | 68.5 ± 14.4 | 81.5 ± 11.4 | 75.3 ± 18.3 | 0.09 (ns) |
| Urine albumin/creatinine, mg/mmol | 13.24 [28.03] | 21.57 [375.2] | 15.28 [90.64] | 0.38 (ns) |
| Urine total protein/creatinine, g/mmol | 0.021 ± 0.016 | 0.030 ± 0.031 | 0.027 ± 0.028 | 0.53 (ns) |
| Plasma creatinine, µmol/L | 69.50 [20.0] | 76.0 [76.0] | 81.0 [89.0] | 0.29 (ns) |
| Plasma sodium, mmol/L | 140.5 [5.0] | 139.0 [10.0] | 139.0 [11.0] | 0.08 (ns) |
| Plasma potassium, mmol/L | 4.1 ± 0.3 | 4.2 ± 0.3 | 4.3 ± 0.5 | 0.70 (ns) |
| eGFR, mL/min/1.73 m2 | 87.50 [16.00] | 87.00 [39.00] | 88.00 [49.00] | 0.83 (ns) |
| Other | ||||
| Smoker (y/n) | 2 yes/8 no | 15 yes/10 no | 12 yes/7 no |
Data are presented as mean ± SD, number (percentage) or median [IQR].
*P < 0.05.
**P < 0.01.
Former known kidney patients cover those participants who previously had or have renal conditions as cysts or fibrosis, renal surgeries, or an autoimmune disease affecting the kidneys.
Antihypertensive covers either one or in a combination of the presented drugs. DM, diabetes mellitus; AT2, angiotensin II receptor; ACE, angiotensin-converting enzyme; ns, non-significant; BP, blood pressure.
Baseline characteristics of the nephrectomy patients from Odense and Roskilde
| . | Donor nephrectomy . | Cancer nephrectomy . | Partial nephrectomy . | P-value . |
|---|---|---|---|---|
| Participant characteristics | ||||
| Number of participants | 10 | 25 | 19 | |
| Median age, years | 56 ± 13.83 | 59.40 ± 14.84 | 60.47 ± 9.507 | 0.65 (ns) |
| Sex distribution (female/male) | 8 female/2 male | 10 female/15 male | 4 female/15 male | |
| Body composition | ||||
| Weight, kg | 76.88 ± 15.34 | 86.42 ± 20.83 | 87.70 ± 17.47 | 0.31 (ns) |
| Height, cm | 168.2 ± 4.392 | 173.1 ± 6.987 | 176.9 ± 7.534 | 0.0076** |
| BMI, kg/m2 | 26.99 ± 4.545 | 28.76 ± 6.627 | 27.94 ± 5.286 | 0.71 (ns) |
| Medical history | ||||
| Hypertension | 3 (30) | 13 (52) | 9 (47.36) | |
| Heart patient | 0 (0) | 2 (8) | 2 (10.52) | |
| Former known kidney patienta | 0 (0) | 4 (16) | 3 (15.79) | |
| DM type I | 0 (0) | 1 (4) | 0 (0) | |
| DM type II | 0 (0) | 3 (12) | 2 (10.52) | |
| Medication | ||||
| Antihypertensiveb | 3 (100) | 12 (92.3) | 7 (77.78) | |
| Diuretics | 1/3 | 7/12 | 0/7 | |
| β-blocker | 0/3 | 2/12 | 2/7 | |
| ACE inhibitor | 0/3 | 5/12 | 4/7 | |
| AT2 inhibitor | 1/3 | 5/12 | 2/7 | |
| Calcium antagonist | 1/3 | 4/12 | 2/7 | |
| α-blocker | 0/3 | 1/12 | 0/7 | |
| Combined α/β blocker | 0/3 | 1/12 | 0/7 | |
| Clinical and biochemical parameters | ||||
| Systolic BP, mmHg | 136 ± 20 | 165 ± 20 | 158 ± 23 | 0.0028** |
| Diastolic BP, mmHg | 82.1 ± 10.9 | 94.6 ± 14 | 88.9 ± 12.8 | 0.0403* |
| Heart rate, beats/min | 68.5 ± 14.4 | 81.5 ± 11.4 | 75.3 ± 18.3 | 0.09 (ns) |
| Urine albumin/creatinine, mg/mmol | 13.24 [28.03] | 21.57 [375.2] | 15.28 [90.64] | 0.38 (ns) |
| Urine total protein/creatinine, g/mmol | 0.021 ± 0.016 | 0.030 ± 0.031 | 0.027 ± 0.028 | 0.53 (ns) |
| Plasma creatinine, µmol/L | 69.50 [20.0] | 76.0 [76.0] | 81.0 [89.0] | 0.29 (ns) |
| Plasma sodium, mmol/L | 140.5 [5.0] | 139.0 [10.0] | 139.0 [11.0] | 0.08 (ns) |
| Plasma potassium, mmol/L | 4.1 ± 0.3 | 4.2 ± 0.3 | 4.3 ± 0.5 | 0.70 (ns) |
| eGFR, mL/min/1.73 m2 | 87.50 [16.00] | 87.00 [39.00] | 88.00 [49.00] | 0.83 (ns) |
| Other | ||||
| Smoker (y/n) | 2 yes/8 no | 15 yes/10 no | 12 yes/7 no |
| . | Donor nephrectomy . | Cancer nephrectomy . | Partial nephrectomy . | P-value . |
|---|---|---|---|---|
| Participant characteristics | ||||
| Number of participants | 10 | 25 | 19 | |
| Median age, years | 56 ± 13.83 | 59.40 ± 14.84 | 60.47 ± 9.507 | 0.65 (ns) |
| Sex distribution (female/male) | 8 female/2 male | 10 female/15 male | 4 female/15 male | |
| Body composition | ||||
| Weight, kg | 76.88 ± 15.34 | 86.42 ± 20.83 | 87.70 ± 17.47 | 0.31 (ns) |
| Height, cm | 168.2 ± 4.392 | 173.1 ± 6.987 | 176.9 ± 7.534 | 0.0076** |
| BMI, kg/m2 | 26.99 ± 4.545 | 28.76 ± 6.627 | 27.94 ± 5.286 | 0.71 (ns) |
| Medical history | ||||
| Hypertension | 3 (30) | 13 (52) | 9 (47.36) | |
| Heart patient | 0 (0) | 2 (8) | 2 (10.52) | |
| Former known kidney patienta | 0 (0) | 4 (16) | 3 (15.79) | |
| DM type I | 0 (0) | 1 (4) | 0 (0) | |
| DM type II | 0 (0) | 3 (12) | 2 (10.52) | |
| Medication | ||||
| Antihypertensiveb | 3 (100) | 12 (92.3) | 7 (77.78) | |
| Diuretics | 1/3 | 7/12 | 0/7 | |
| β-blocker | 0/3 | 2/12 | 2/7 | |
| ACE inhibitor | 0/3 | 5/12 | 4/7 | |
| AT2 inhibitor | 1/3 | 5/12 | 2/7 | |
| Calcium antagonist | 1/3 | 4/12 | 2/7 | |
| α-blocker | 0/3 | 1/12 | 0/7 | |
| Combined α/β blocker | 0/3 | 1/12 | 0/7 | |
| Clinical and biochemical parameters | ||||
| Systolic BP, mmHg | 136 ± 20 | 165 ± 20 | 158 ± 23 | 0.0028** |
| Diastolic BP, mmHg | 82.1 ± 10.9 | 94.6 ± 14 | 88.9 ± 12.8 | 0.0403* |
| Heart rate, beats/min | 68.5 ± 14.4 | 81.5 ± 11.4 | 75.3 ± 18.3 | 0.09 (ns) |
| Urine albumin/creatinine, mg/mmol | 13.24 [28.03] | 21.57 [375.2] | 15.28 [90.64] | 0.38 (ns) |
| Urine total protein/creatinine, g/mmol | 0.021 ± 0.016 | 0.030 ± 0.031 | 0.027 ± 0.028 | 0.53 (ns) |
| Plasma creatinine, µmol/L | 69.50 [20.0] | 76.0 [76.0] | 81.0 [89.0] | 0.29 (ns) |
| Plasma sodium, mmol/L | 140.5 [5.0] | 139.0 [10.0] | 139.0 [11.0] | 0.08 (ns) |
| Plasma potassium, mmol/L | 4.1 ± 0.3 | 4.2 ± 0.3 | 4.3 ± 0.5 | 0.70 (ns) |
| eGFR, mL/min/1.73 m2 | 87.50 [16.00] | 87.00 [39.00] | 88.00 [49.00] | 0.83 (ns) |
| Other | ||||
| Smoker (y/n) | 2 yes/8 no | 15 yes/10 no | 12 yes/7 no |
Data are presented as mean ± SD, number (percentage) or median [IQR].
*P < 0.05.
**P < 0.01.
Former known kidney patients cover those participants who previously had or have renal conditions as cysts or fibrosis, renal surgeries, or an autoimmune disease affecting the kidneys.
Antihypertensive covers either one or in a combination of the presented drugs. DM, diabetes mellitus; AT2, angiotensin II receptor; ACE, angiotensin-converting enzyme; ns, non-significant; BP, blood pressure.
Kidney function and blood pressure changes in response to renal surgery
The measured (m)GFR and estimated (e)GFR were not different before surgery and between the total nephrectomy and partial nephrectomy groups (Figure 2A and B). Following total nephrectomy, mGFR was significantly lower after 3 months and remained at the same, lower, level after 12 months at 62 mL/min/1.73 m2 (P < 0.0001) (Figure 2A). For partial nephrectomy, there was no significant reduction in mGFR at 3 and 12 months post-surgery (Figure 2B). GFR measured by Cr-EDTA and mTc-EDTA was higher in patients who underwent partial nephrectomy than total nephrectomy at 3 and 12 months (Figure 2A and B). Estimated GFR showed similar changes with higher time resolution (Figure 2C). Of note, after total nephrectomy, there was a significant progressive increase in eGFR from 24 h post-surgery to 1 year post-surgery (Figure 2C; P < 0.0001). The eGFR for patients with partial nephrectomy showed a significant decrease (P < 0.05) at 24 h, while there was no difference between the following time points, as observed for mGFR (P > 0.05, Figure 2D). The skGFR increased significantly after total nephrectomy from renal scintigraphy-estimated distribution 50.3 to 62.2 mL/min/1.73 m2 (P < 0.0001, Figure 2E). The mean skGFR compensatory increase was 11.9 mL/min/1.73 m2, corresponding to a 23.3% increase. The estimated GFR for partial nephrectomy (pnGFR) was calculated by estimating a maximal 40% nephron reduction at the relevant side. The pnGFR post-surgery did not increase significantly (P > 0.05) (Figure 2F). In the partial nephrectomy group, follow-up scintigraphy demonstrated significant changes in functional distribution between kidneys such that after 12 months, the healthy kidney increased function while the kidney with mass removal lowered its contribution (Supplementary data, Table S1 and Figure S1). Office systolic and diastolic blood pressures were not different between the two surgery groups at baseline and while an acute and similar decline in blood pressure was observed 24 h after surgery, there were no changes from baseline at any other point of recording (Figure 2G and H).
![Assessment of kidney function by 51Cr-EDTA/technetium-99mTc-DPTA clearance (mGFR) and estimated by plasma creatinine in patients before and after kidney surgery (eGFR). (A) mGFR for patients subjected to total nephrectomy (n = 31). (B) Partial nephrectomy (n = 14) showed a significant decrease following surgery. The eGFR in patients subjected to (C) total nephrectomy (n = 35) and (D) partial nephrectomy (n = 18) was determined by the CKD-EPI creatinine equation and showed a significant decrease that was transient for total nephrectomy. (E) Single kidney GFR (skGFR) (n = 29) at baseline calculated by Equation (1) and single kidney GFR measured at 3 months after unilateral nephrectomy. (F) GFR measured in patients 3 months after nephron-sparing partial nephrectomy (pnGFR) (n = 14) as compared with baseline GFR based on removal of 40% of one kidney calculated by Equation (2). (G, H) Systolic and diastolic blood pressures in patients subjected to (G) total and (H) partial nephrectomy. Data (A, B and G, H) are shown as mean ± SEM, (C, D) as median [interquartile range (IQR)] and (E, F) in scatter plots, mean values are indicated by the line. Results were interpreted as significant if P < 0.05.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ndt/37/11/10.1093_ndt_gfab327/2/m_gfab327fig2.jpeg?Expires=1748021282&Signature=MHyR1zy08ASTsyx6dgNoteiCy54p6cMpNGvzml838B2ONaraEpxiEwrpvYscNEdzaSfJ6R8FYoz5m47xixb-6LK0fUuKShWiJee4~jN6G0p5Y9JQDXdoRzXXF2bjfP8qqOAHeWPee3Qi57RSogr6Fvljo10Hez6gfbBzJmRt9tRofx9Be8IjlCtKLTQHdbtlZ0ePCItG~bK8Dd3uqmwsmtNg6VAyh0goywMoID8RKT23m7S6KWs79MXaBnKbXWqcTNKoXDDYcqLRpRs3OqLUcCrw6Aoho4y--2yMupK4P812cInIWw1gnnyK5-RAWmqsCMDWh0Ymge7zIe6VqYGGYg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Assessment of kidney function by 51Cr-EDTA/technetium-99mTc-DPTA clearance (mGFR) and estimated by plasma creatinine in patients before and after kidney surgery (eGFR). (A) mGFR for patients subjected to total nephrectomy (n = 31). (B) Partial nephrectomy (n = 14) showed a significant decrease following surgery. The eGFR in patients subjected to (C) total nephrectomy (n = 35) and (D) partial nephrectomy (n = 18) was determined by the CKD-EPI creatinine equation and showed a significant decrease that was transient for total nephrectomy. (E) Single kidney GFR (skGFR) (n = 29) at baseline calculated by Equation (1) and single kidney GFR measured at 3 months after unilateral nephrectomy. (F) GFR measured in patients 3 months after nephron-sparing partial nephrectomy (pnGFR) (n = 14) as compared with baseline GFR based on removal of 40% of one kidney calculated by Equation (2). (G, H) Systolic and diastolic blood pressures in patients subjected to (G) total and (H) partial nephrectomy. Data (A, B and G, H) are shown as mean ± SEM, (C, D) as median [interquartile range (IQR)] and (E, F) in scatter plots, mean values are indicated by the line. Results were interpreted as significant if P < 0.05.
Change in plasma concentration of natriuretic peptides after renal surgery
Plasma proBNP concentration increased significantly and transiently 24 h after total nephrectomy (P < 0.0001) and remained stable at baseline level after that (P > 0.05) (Figure 3A). Plasma proBNP increased significantly 24 h after partial nephrectomy (P < 0.0001) (Figure 3B). There were no significant differences in proBNP plasma concentration profiles with time between patients who underwent total and partial nephrectomy at all points of evaluation (P > 0.05) (Figure 3E). Plasma concentration of proANP increased significantly and transiently 24 h after total nephrectomy. One year after nephrectomy, there was a significantly higher plasma level of proANP (Figure 3C, Supplementary data, Table S2). After partial nephrectomy, proANP increased significantly and was not different from baseline at other measuring points during follow-up (Figure 3D). There were no significant differences in proANP between total and partial nephrectomy at any measuring points following surgery (Figure 3F).
![Plasma concentrations of NT-proBNP and MR-proANP. NT-proBNP for patients subjected to (A) total nephrectomy (n = 33) and (B) partial nephrectomy (n = 19). There was a significant transient increase following surgery. MR-proANP increased significantly following surgery in patients subjected to (C) total nephrectomy (n = 32) and (D) partial nephrectomy (n = 18). (E, F) Comparison of NT-proBNP and MR-ProANP for both surgical interventions at all time points. There was no significant difference between types of surgery. Data are shown as median [interquartile range (IQR)] and results were interpreted as significant if P < 0.05.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ndt/37/11/10.1093_ndt_gfab327/2/m_gfab327fig3.jpeg?Expires=1748021282&Signature=RQj6Xk9Qd9YIyjLBD4tpZBJzqqpDX2CUeNGKd6h7DAumiyphV9W87OjD8INPl~V62VLve4z-0kVf9yy1kG8DHRNlZWwcu47YP-q6w~z8qtU4BE5iEyDA1RcKzLovjuOaO3UgB1B853dIugBWtEF1EOcVDZb7HXVq6ujYYgnLOJppu~lm~htt~7klnVmlWXWlHm~ntjV0mSUyFRpgEPAAnDXIw2RnkwfxJSDDsWV7EMe9wOjlHYN0XsJ3baUvc00ArRah~M9RmZk63piPudX9TtCFh1pYFp~S~DFDSiwqMEXeZtCpXH6kgCgXceNE07W9JVJSfR40tCLVKFDyucONwA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Plasma concentrations of NT-proBNP and MR-proANP. NT-proBNP for patients subjected to (A) total nephrectomy (n = 33) and (B) partial nephrectomy (n = 19). There was a significant transient increase following surgery. MR-proANP increased significantly following surgery in patients subjected to (C) total nephrectomy (n = 32) and (D) partial nephrectomy (n = 18). (E, F) Comparison of NT-proBNP and MR-ProANP for both surgical interventions at all time points. There was no significant difference between types of surgery. Data are shown as median [interquartile range (IQR)] and results were interpreted as significant if P < 0.05.
Urine cGMP and albumin excretion and plasma aldosterone after renal surgery
Urine cGMP/creatinine concentration ratio did not change after total nephrectomy (P > 0.05) (Figure 4A). There was a tendency that cGMP/creatinine ratio increased after partial nephrectomy, but not significantly (P > 0.05) (Figure 4B). Urine albumin/creatinine ratio (ACR) after total nephrectomy did not change throughout the follow-up period (P > 0.05), but patients had borderline increased ACR at baseline (ACR >30 mg/g) (Figure 4C). ACR increased significantly 24 h after partial nephrectomy (P > 0.0001), and it was normalized after 21 days (Figure 4D). Eight out of 19 (42%) patients who received partial nephrectomy underwent surgery with an off-clamp technique, where the renal artery is not occluded and the rest experienced warm ischaemia with clamp technique for less than 25 min. There were no significant differences between the two groups pre- and 24 h post-surgery in ACR (P > 0.05) (Figure 4E). Plasma aldosterone concentration was not different at baseline between the two surgery groups and did not change with time at any observation point within or between groups (Figure 4F).
![Urine excretion of cyclic GMP and albumin before and after surgery. Data show the ratio between urine concentration of cGMP and creatinine for (A) total nephrectomy (n = 24) and (B) partial nephrectomy (n = 8). There was no significant difference following surgery. The urine ratio of albumin over creatinine concentrations before and after total nephrectomy (C, n = 35) showed no significant change while patients (D) subjected to partial nephrectomy (n = 18) showed a transient increase (P < 0.05). Panel (E) shows urine albumin to creatinine ratio before and after partial nephrectomy surgery was performed with and without arterial clamping. (F) Plasma aldosterone concentration before and after surgery for patients from both surgical intervention groups, median [interquartile range (IQR)]. Data (C–F) are shown as median IQR and (A, B) as mean ± SEM.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ndt/37/11/10.1093_ndt_gfab327/2/m_gfab327fig4.jpeg?Expires=1748021282&Signature=c3W8-vHizXacj2nyuITUgXM0YBr8cirfmtSY0HfOkCmk6-TEyKlc9qLT1WyuBSxgketR9a-gr34tMiJZAoECVSbQPJluTvibmOupVOuDb-6nW5YAQblbOm2ew3GPkRSjidVRyXHmg-6CTp3kUE4g4c75RjU45ato7yepiONQjXknGP3ucd2A4fUuy4w9m2ilPu~2~OL~PyDdzOXBxGjq2VzK7izbk7N25GLXBNVejmy0sjJeKY~rsJMiHnZK4lp-wyoY~24cJoeIbNujwM1VmlenfzHg8m7Injpt5PRjBQncG1WWeFUg9us4VH7FIu3kIT~w-s6u3thkzzwTpG5HMA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Urine excretion of cyclic GMP and albumin before and after surgery. Data show the ratio between urine concentration of cGMP and creatinine for (A) total nephrectomy (n = 24) and (B) partial nephrectomy (n = 8). There was no significant difference following surgery. The urine ratio of albumin over creatinine concentrations before and after total nephrectomy (C, n = 35) showed no significant change while patients (D) subjected to partial nephrectomy (n = 18) showed a transient increase (P < 0.05). Panel (E) shows urine albumin to creatinine ratio before and after partial nephrectomy surgery was performed with and without arterial clamping. (F) Plasma aldosterone concentration before and after surgery for patients from both surgical intervention groups, median [interquartile range (IQR)]. Data (C–F) are shown as median IQR and (A, B) as mean ± SEM.
DISCUSSION
We show in the present study that surgical removal of variable amounts of kidney tissue is associated with similar, acute and transient increases in plasma concentration of natriuretic peptide prohormones proBNP and proANP, refuting a proportional relation between the amount of acute nephron loss and plasma increase in natriuretic peptides. In contrast, at 1-year follow-up, a differential change in natriuretic peptides was evident with elevated plasma proANP but not proBNP. The study corroborates functional adaptive increase in skGFR following renal surgery with no long-term change in blood pressure, plasma aldosterone or filtration barrier injury; it shows that the adaptation occurs between 1 and 20 days post-nephrectomy. It shows that partial nephrectomy, despite warm ischaemia with transient filtration barrier injury, is associated with a significantly minor loss in GFR and significant adaption by changes in functional distribution after 12 months. In perspective, ANP signalling could contribute significantly to the maintenance of adaptive increase in kidney function and the new steady state that is achieved. The data point to general anesthesia, supine position, fluid infusion and surgical stress as potential contributors to acute natriuretic peptide responses in addition to kidney tissue removal, while blood pressure increase could be ruled out as a stimulus. The peptide pro-forms are more stable in plasma and better reflect secretion. ANP and BNP depend differentially on degradative enzyme pathways, with neprilysin being relatively more important for ANP [23] and dipeptidyl peptidase-4 (DPP-IV) being more relevant for BNP [24]. With this in mind, the similar acute plasma concentration changes upon surgery most likely reflect increased secretion. The adaptive increase in GFR after nephrectomy occurs due to structural and haemodynamic changes of the remaining glomeruli [25]. The present study showed a 23% compensatory increase in skGFR. In similarly aged, uni-nephrectomized probands, an skGFR increase of 38% was reported after 6 months [26]. This was not done prospectively in the same individuals but estimated from a control group. The mechanisms for adaptation have been extensively reviewed [27–31] and likely comprise combined changes in glomerular capillary pressure and ultrafiltration coefficient, Kf [26]. Adaptive hyperfiltration is preserved in healthy individuals over the age of 55 years and the hyperfiltration is only slightly smaller compared with younger subjects. The loss of ultrafiltration capacity within aging kidneys results from a reduction in the total number of glomeruli [26]. The average age of the included subjects was 59 years; thus, a reduced functional reserve could contribute to explain why a modest 23% increase was observed after nephrectomy [32, 33]. Natriuretic peptides increase in plasma with age. Although we did not include a time-control group, there was no difference in plasma concentrations of BNP and ANP within 12 months in the partial nephrectomy group, which indicates that age-related changes are less likely as confounders within the 12 months duration of the study [34, 35]. On the other hand, the age-related increase across an 80-year lifespan would fit with a relation between physiological loss of nephron mass and plasma natriuretic peptide concentration. After partial nephrectomy, mGFR was not significantly lower at 3 and 12 months after tissue removal, while eGFR was significantly lower 24 h after surgery. This transient acute decline in eGFR within the first 24 h in the patients with partial nephrectomy could contribute to the concomitant acute increase in natriuretic peptides, although a much smaller amount of tissue was removed and function rapidly recovered. We conclude that the approach was ‘nephron-sparing’ despite a transient filtration barrier injury and drop in eGFR. This is in agreement with long-term follow-up studies from large centres that document improved kidney functional outcomes and lower mortality of partial nephrectomy than total nephrectomy [36, 37]. The capacity to increase GFR above baseline can be induced by dopamine infusion or increased protein intake/amino acid loading. This approach can assist in determining how well donors adapt to kidney donation [38]. As to the mediator of functional adaptation, a humoral factor is likely: a ‘renotropic’ factor is present in rat plasma 20 h after total kidney removal [39], and the putative factor conveys to normal recipient rats an increased salt and water excretion and increase in snGFR [7]. This stimulating factor in rat plasma was present after 24 h and then waned [40]. We speculate that observed early changes in natriuretic peptide plasma concentration in patients would be compatible with such an acute and transient effect in human plasma. In support of such a role for natriuretic peptides, it has been shown that excessive plasma expansion by fluid loading kidney donors and recipients immediately preceding and during kidney transfer is associated with very rapid and large glomerular filtration and perfusion rate increases, in both donor and recipient [41]. The observations also fit the paradoxical protection and increased perfusion in the remaining kidney afforded by unilateral nephrectomy 24 h before an acute experimental ischaemic insult to the remaining kidney [42]. Infusion of ANP or its analogues protects kidney function in patients prone to develop acute kidney injury (AKI) [13]. Thus, in follow-up association studies in humans, unilateral donor nephrectomy was not associated with an increased risk of major adverse cardiovascular events [43–45]. This is a remarkable difference compared with a highly significant increase in cardiovascular risk if nephron loss is due to chronic progressive kidney disease [44–46]. The patients included in the present study suffered from various comorbidities, including heart disease (n = 4), diabetes (n = 6) and hypertension (n = 25), and there were many smokers (n = 29). These factors could alter the baseline level and responsiveness of natriuretic peptides, but comorbidities were equally distributed across groups [47, 48]. The present data show that the use of proBNP as a biomarker for heart disease would not be compromised by surgical nephron removal, which did not alter proBNP plasma concentrations at 1-year follow-up. A significant suppression of the renin–angiotensin–aldosterone system would be a relevant parameter to contribute to functional adaptation, but aldosterone plasma concentrations did not change acutely or after 1 year, which makes this a less like factor to contribute to the dynamic changes. As a surrogate for kidney natriuretic peptide receptor activation, urine cGMP excretion increased numerically but not statistically in the early post-nephrectomy phase. The lack of significant signal despite plasma natriuretic peptide increases could be explained by the observation that the ANP/BNP receptor NPR-A is associated predominantly with the arterial vasculature in the human kidney and not found in tubules [49]. The potential spill-over of cGMP from the comparatively minor arterial versus tubular cell mass was not detected in urine. Moreover, the transient response in plasma natriuretic peptides creates a narrow window where cGMP would change. Nitric oxide (NO) has been implicated in the compensatory kidney adaptation in mice [50, 51], but an upregulation in NO formation and signalling in kidneys would be expected to increase cGMP. The ACR showed a rapid transient increase after partial nephrectomy. This was likely due to parenchymal injury associated with tissue removal and resolved 21 days after surgery. The procedure saves nephrons and glomerular filtration, and does not cause permanent injury to the filtration barrier.
CONCLUSION
It is concluded that natriuretic peptide secretion increases acutely and transiently concomitantly with renal functional adaptation following partial and total unilateral nephrectomy. At 1-year follow-up, plasma proANP but not proBNP is elevated, following total nephrectomy. Thus, after 1 year, the amount of kidney tissue removal relates to plasma proANP increase. In perspective, natriuretic peptides may contribute to functional adaptation and kidney barrier protection. Only intervention studies can reveal causality and mechanistic involvement of natriuretic peptides.
ACKNOWLEDGEMENTS
The authors thank Jesper K. Andreasen for technical assistance. The study was supported by research infrastructure of The Open Patient Data Exploratory Network (OPEN) at Odense University Hospital.
FUNDING
Funding for the study was provided by grants from The Danish Kidney Research Fund—Nyreforeningen and The Regions of Zeeland and Southern Denmark.
AUTHORS’ CONTRIBUTIONS
N.A. and L.L. applied for the ethical approval, recruited patients, organized follow-up visits, collected and analysed clinical data, and drafted the manuscript; C.B. helped design the study, analysed and interpreted the data, and drafted the manuscript; J.P.G. did plasma proANP analyses, helped analyse data, and drafted figures and manuscript; M.J. analysed urine for cGMP, analysed plasma samples for hormones, analysed data, prepared figures, did statistical analyses, organized REDcap database and drafted manuscript; B.L.J. helped design the study, organized the analyses; helped with data analysis and figures, and drafted the manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.





Comments