-
PDF
- Split View
-
Views
-
Cite
Cite
Ian A Strohbehn, Sarah Street, Donald Chute, Harish Seethapathy, Meghan Lee, Rituvanthikaa Seethapathy, Zsofia D Drobni, Osama Rahma, Tomas G Neilan, Leyre Zubiri, Kerry Reynolds, Meghan E Sise, Immune checkpoint inhibitor–induced thyroiditis is a risk factor for acute and chronic kidney dysfunction, Nephrology Dialysis Transplantation, Volume 37, Issue 1, January 2022, Pages 187–189, https://doi.org/10.1093/ndt/gfab240
Close - Share Icon Share
Acute and chronic kidney function decline have been associated with the use of immune checkpoint inhibitors (ICIs) for cancer [1–5]. ICIs are monoclonal antibodies targeting cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed death protein 1 (PD-1) and programmed death ligand 1 (PD-L1) that unleash an adaptive immune response against cancer, but they can also lead to autoimmune side effects called immune-related adverse events (irAEs) that affect up to 60–80% of patients. The most common kidney irAE is tubulointerstitial nephritis; however, other glomerular diseases and accelerated chronic kidney disease (CKD) have also been reported after ICIs [2, 3, 6, 7]. Patients who develop kidney irAEs often concurrently develop extrarenal irAEs [3]. Given the risks of immune activation in preexisting kidney disease, we hypothesized that extrarenal irAEs would be a surrogate for an inflammatory state and therefore be a risk factor for kidney function decline. ICI-induced thyroiditis is a common and easily diagnosed irAE that results from lymphocytic infiltration of the thyroid gland and is typically present within the first few months after starting ICIs [8, 9]. We aimed to determine if ICI-induced thyroiditis was a risk factor for acute and chronic kidney function decline after ICIs.
We performed a retrospective cohort study including all adults who received ICIs for cancer as part of monotherapy or combined with other antineoplastic therapies at Mass General Brigham between 2010 and 2019. Patients were excluded if they had a previous history of thyroid disease defined by International Classification of Diseases (ICD), Revision 9 or 10 codes or a prescription for levothyroxine at baseline. The follow-up period began on the date of each patient’s first ICI exposure and patients were followed up until death or 31 December 2020. This protocol was approved by the Mass General Brigham Institutional Review Board.
Detailed clinical data on all patients were reviewed using the Research Patient Data Registry, the electronic health record database for Mass General Brigham Healthcare. Information collected included demographics, comorbidities and baseline medications. Longitudinally, we evaluated laboratory tests, including creatinine, thyroid-stimulating hormone (TSH), free thyroxine 4 (fT4) and creatinine, as well as new medications. ICI-induced thyroiditis was defined by the first thyroid dysfunction after ICI initiation; thyrotoxicosis was defined by concurrent TSH values <0.5 μg/dL and fT4 >1.9 ng/dL and hypothyroidism was defined by the initiation of thyroid replacement therapy [Grade 2 per the Common Terminology Criteria for Adverse Events (CTCAE) guidelines] anytime after Day 14 of ICI therapy [10]. Acute kidney injury (AKI) was defined by a ≥1.5-fold increase in serum creatinine from baseline (creatinine value just prior to starting ICIs) within 12 months of starting ICIs. A sustained estimated glomerular filtration rate (eGFR) decline was defined by a >20% decline from baseline sustained for ≥90 days with no intervening values within 20% of baseline. New-onset CKD was determined among patients without baseline CKD who newly developed an eGFR <60 mL/min/1.73 m2 sustained for ≥90 days.
To determine the association between thyroiditis and adverse kidney outcomes, we performed Cox proportional hazard models to estimate unadjusted and adjusted hazard ratios (aHRs). The selection of covariates in multivariable models was based on clinical and biological plausibility and by Akaike and Bayesian Information Criteria. All comparisons were two-sided, with P-values <0.05 considered significant, and we present the 95% confidence intervals. We performed the statistical analyses with Stata version 16 (StataCorp, College Station, TX, USA).
We identified 9419 adult patients treated with ICIs between 2010 and 2019 (Figure 1). After exclusions, 3109 patients who survived ≥1 year were included. The mean age for the total cohort was 62.8 years [standard deviation (SD) 13.1], 43% were male and 91% were White. The most frequent type of malignancy was melanoma (27%), followed by thoracic malignancies (26%). Most of the patients received PD-1/PD-L1 inhibitors (86%); CTLA-4 inhibitor monotherapy (5%) and PD-1/CTLA-4 combination therapy (9%) were less common. Hypertension (51%), diabetes mellitus (19%), coronary artery disease (25%), congestive heart failure (8%) and CKD (17%) were frequent comorbidities.
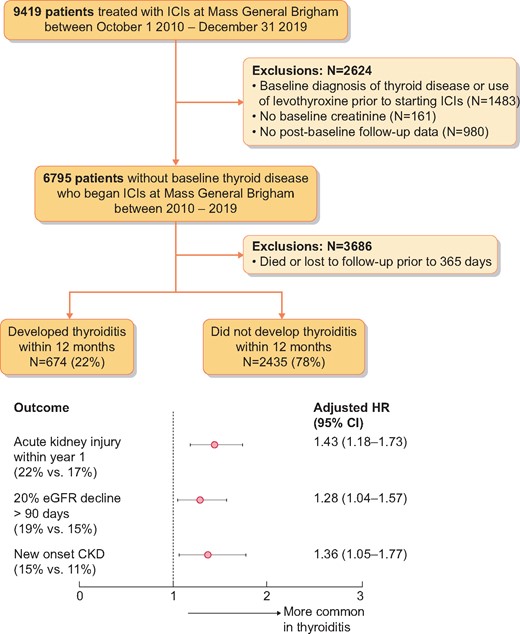
Patient flow and outcomes. Among patients surviving 1 year, Cox proportional hazards models were performed to predict each of the three renal outcomes among patients experiencing thyroiditis within 12 months compared with those who did not develop thyroiditis within 12 months.
A total of 674 patients (22%) developed thyroiditis. The median time to thyroiditis after receipt of the first dose of ICI was 2.9 months [interquartile range (IQR) 1.5–5.5]. Creatinine was measured a median of 23 times (IQR 17–30) during the first year of follow-up. The multivariable models were adjusted for age, comorbidities (diabetes, coronary artery disease, hypertension and congestive heart failure), baseline creatinine, use of proton pump inhibitors, cancer type and ICI class. ICI-induced thyroiditis was independently associated with AKI [aHR 1.43 (95% CI 1.18–1.73); P < 0.001], sustained 20% eGFR decline [aHR 1.28 (95% CI 1.04–1.57); P = 0.018] and new-onset CKD [aHR 1.36 (95% CI 1.06–1.77); P = 0.018] (Figure 1; full models are shown in Supplementary data, Table S1A–C).
Acute and chronic kidney function decline have been reported after ICIs [1–5]. We chose to evaluate thyroiditis as a novel risk factor because it is a common, early-onset irAE that is well-defined by laboratory studies and medication records, thereby making the diagnosis easy and accurate and allowing us to utilize our large database of patients receiving ICIs to test the association of this irAE with kidney function decline. To our knowledge, this is the first study to determine the association between a nonrenal irAE and kidney function decline. Possible mechanisms include increased risk of acute or chronic interstitial nephritis among patients experiencing irAEs such as thyroiditis, or an overly robust immune response induced by ICIs accelerating the progression of preexisting kidney disease. Outside the realm of ICI use, autoimmune thyroiditis is associated with autoantibodies to thyroglobulin and thyroid peroxidase. Autoantibodies have been less commonly detected in patients with ICI-induced thyroid disease, and antibody-mediated kidney disease has only rarely been reported after ICIs [7, 8, 11]. Prospective studies are needed to better phenotype AKI and CKD occurring after ICIs, as important data (such as proteinuria and serologic studies) are commonly missing in retrospective databases, limiting our understanding of mechanisms.
Our study has several limitations. First, we used an indirect method to identify patients with thyroiditis and could have missed patients with subclinical thyroid dysfunction. We evaluated only patients surviving ≥1 year to decrease the risk of survival bias leading to higher rates of AKI and CKD. However, given that ICI-induced thyroiditis is associated with increased survival, immortal time bias may still be a confounder [12–14]. Given the size of our cohort, we were unable to determine the etiology of all cases of AKI; nonimmune mechanisms likely caused many cases of AKI, particularly in patients with lung cancer and other malignancies where platinum-based chemotherapies and pemetrexed was commonly used. Finally, although our cohort is large, it lacks racial and ethnic diversity. Future studies will be needed to determine the association of other irAEs with kidney function decline.
In summary, we have shown that ICI-induced thyroiditis is a novel risk factor for AKI, CKD and sustained eGFR decline in patients surviving at least 1 year after ICI. Thus kidney function should be closely monitored in ICI-treated patients who develop extrarenal irAEs.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
ACKNOWLEDGEMENTS
The patients described in this cohort are a subset of those reported in a publication in Annals of Oncology (n = 6596) [14] of the effect of thyroiditis on survival.
FUNDING
M.E.S. is supported by the National Institutes of Health (NIH; K23 DK 117014) and the Claflin Distinguished Scholars Award. T.G.N. is supported by the NIH (R01HL137562, R01HL130539 and K24HL150238) and in part through a kind gift from A. Curtis Greer and Pamela Kohlberg. The NIH had no role in the study design; collection, analysis and interpretation of data; writing the report; or the decision to submit the report for publication.
CONFLICT OF INTEREST STATEMENT
L.Z. serves as a consultant for Merck and reports a grant from Sociedad Española de Oncología Médica (SEOM) to study immune-related toxicities. T.G.N. has been a consultant to and received fees from Intrinsic Imaging, H3-Biomedicine, Amgen and AbbVie, outside of the current work. T.G.N. also reports consultant fees from Bristol Myers Squibb for a scientific advisory board focused on myocarditis related to ICIs and an unrestricted grant from AstraZeneca to study atherosclerosis related to ICIs. O.R. received research support from Merck. Speaker for activities is supported by educational grants from Bristol Myers Squibb and Merck. Consultant for Merck, Celgene, Five Prime, GlaxoSmithKline, Bayer, Roche/Genentech, Puretech, Imvax and Sobi. In addition, O.R. has a patent (Methods of using pembrolizumab and trebananib) pending. The remaining authors declared no conflicts of interest.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.





Comments