-
PDF
- Split View
-
Views
-
Cite
Cite
Yong Zhong, Qiaoling Zhou, MO124
THE SINGLE-CELL TRANSCRIPTOMIC OF HUMAN IDIOPATHIC MEMBRANOUS NEPHROPATHY*, Nephrology Dialysis Transplantation, Volume 36, Issue Supplement_1, May 2021, gfab092.002, https://doi.org/10.1093/ndt/gfab092.002Close - Share Icon Share
Abstract
Idiopathic membranous nephropathy (IMN) is an organ-specific autoimmune disease of the kidney glomerulus. Although substantial advances have been made in the understanding of the molecular bases of IMN in the last 10 years, there remain largely unanswered questions.
To define the transcriptomic landscape at the single-cell resolution, we analyzed kidney samples from 6 patients with idiopathic membranous nephropathy (IMN) and 2 healthy control subjects using single-cell RNA sequencing.
Based on transcriptional expression patterns, we identified all previously described cell types in the kidney. Also, we identified a novel population of the epithelial cell which is mainly from IMN patients. GO enrichment analysis showed that DEGs were enriched in the regulation of apoptosis and type I interferon signaling pathway in mesangial cells, while DEGs were enriched in the regulation of programmed cell death, and various cytokine-mediated signaling pathway in endothelial cells and the regulation of protein modification in pericytes. KEGG enrichment analysis revealed that DEGs were mainly associated with the IL-17 signaling pathway, TNF signaling pathway, NOD-like receptor signaling pathway as well as MAPK signaling pathway in endothelial cells as well as pericytes DEGs of proximal tubules cells (PT) and PCs between IMN and control subjects were both enriched in IL-17 signaling, TNF signaling, NOD-like receptor signaling. Moreover, the cell-cell crosstalk highlighted the extensive communication of mesangial cells, which infers great importance in IMN. IMN with massive proteinuria displayed elevated genes participating in the inflammatory signaling pathways that may be involved in the pathogenesis of the progression of IMN.
Overall, we applied single-cell RNA sequencing to IMN to uncover intercellular interactions, elucidate key pathways underlying the pathogenesis, and identify novel therapeutic targets of IMN.
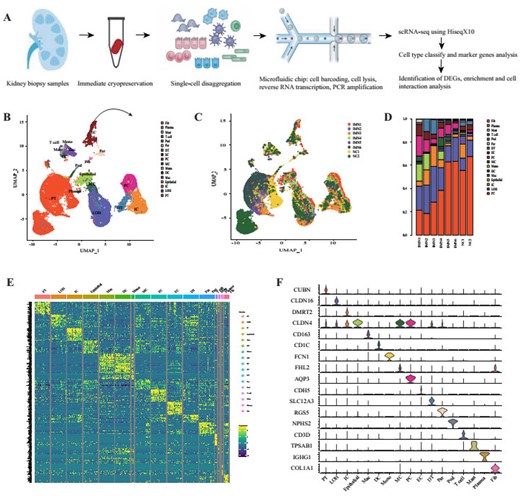
Cell lineage analysis by comprehensive single-cell RNA-sequencing in IMN and control subjects
(A) Schematic of the scRNA-seq pipeline. kidney (n=6) samples from patients with IMN or healthy control subjects (n=2) were collected at the time of clinically indicated renal biopsy or live kidney donation, respectively. Kidney biopsies were enzymatically disaggregated into single-cell suspensions and loaded onto a microfluidic device for cell barcoding, cell lysis, reverse RNA transcription, and then scRNA-seq as well as various analysis. (B)Seventeen distinct cell clusters were visualized by UMAP plotting, with each cell color-coded for its associated subtypes. The color of the cells represented group origin. (C) UMAP plot of cell clusters from different subjects of IMN patients and control. The color of cells reflected the individual origin. (D) Bar plots of the percent contribution of cell clusters in kidneys from different subjects. Blocks represented different subjects, and block height was in proportion to the number of cells. (E) Heatmap of the top 20 most differentially expressed genes in each cluster to identify mutually exclusive gene sets, which were then used to determine the cell lineage of each cluster. Each column represented a cell cluster, and each row corresponded to a marker gene for the individual cluster. Transcript abundance ranges from low (purple) to high (yellow). (F) Violin plot of selected marker genes that identified the clusters generated by UMAP plotting. It was colored by different cell subtypes.
Abbreviations were as follows: PT, proximal tubule cells; LOH, loop of Henle cells PC, principal cells; IC, intercalated cells; DT, distal tubule cells; EC, endothelial cells; Pod, podocytes; MC, mesangial cell; DC, dendritic cells; Mac, macrophages; Mono, monocytes; Fib, fibroblasts; Per, pericyte.
- apoptosis
- cytokine
- proteinuria
- tumor necrosis factors
- signal transduction
- transcription, genetic
- endothelial cells
- fibroblasts
- autoimmune diseases
- cell lineage
- color
- dendritic cells
- genes
- membranous glomerulonephritis
- infectious mononucleosis
- interferon type i
- interleukin-17
- kidney glomerulus
- kidney tubules, proximal
- henle's loop
- loss of heterozygosity
- macrophages
- mitogen-activated protein kinases
- monocytes
- pericytes
- protein processing, post-translational
- rna, small cytoplasmic
- sequence analysis, rna
- suspensions
- kidney
- renal biopsy
- rna
- epithelial cells
- signal pathway
- signal transduction pathways
- medical devices
- cytolysis
- mesangial cells
- podocytes
- bar codes
- nod-like receptor signaling pathway
- nlr proteins
- ischemic monomelic neuropathy





Comments