-
PDF
- Split View
-
Views
-
Cite
Cite
Xenophon Kassianides, Adil Hazara, Philip A Kalra, Iain Macdougall, Sunil Bhandari, MO538
THE SHORT-TERM IMPACT OF HIGH DOSE INTRAVENOUS IRON USE ON RENAL FUNCTION IN PATIENTS WITH CHRONIC KIDNEY DISEASE AND IRON DEFICIENCY WITHOUT ANAEMIA - A POST-HOC ANALYSIS OF A MULTICENTRE RANDOMIZED CONTROLLED TRIAL*, Nephrology Dialysis Transplantation, Volume 36, Issue Supplement_1, May 2021, gfab085.001, https://doi.org/10.1093/ndt/gfab085.001Close - Share Icon Share
Abstract
High dose intravenous (IV) iron is commonly used in patients with chronic kidney disease (CKD) but it remains unclear whether any short or long term impact on renal function exists. Studies using iron sucrose, a second generation IV iron compound suggest effects on proteinuria while evidence with third generation iron products revealed no impact on estimated glomerular filtration rate (eGFR). These newer iron compounds have compact iron-carbohydrate cores, potentially limiting the nephrotoxic effects of labile free iron.
As a part of the Iron & Heart study, we examined the impact of high dose ferric derisomaltose (FDI), a third generation IV iron product, in patients with non-dialysis dependent CKD and iron deficiency on markers of renal injury and function using both established (serum creatinine, eGFR, 24-hour excretion of protein) and novel methods (Cystatin C, Neutrophil gelatinase-associated lipocalin (NGAL)).
In addition, correlations between the different markers of renal dysfunction were examined alongside the impact of various confounders including age and diabetes mellitus on the reliability of such markers.
This was a multicentre randomized double-blinded placebo-controlled study involving three tertiary renal centres in the United Kingdom. Patients with CKD stages 3b-5 (non-dialysis), a serum ferritin <100 micrograms/L and/or transferrin saturation <20% and a haemoglobin value of 110 – 150 g/L were enrolled. The participants were randomized 1:1 to receive either 1000 mg of FDI or placebo. Cystatin C, NGAL, serum creatinine eGFR and 24-hour urinary excretion of protein were measured at baseline and then repeated at 1- and 3- months. Changes in the levels of these were analysed both in terms of their absolute values and percentage change from baseline. Pearson’s coefficient (r) was calculated as a measure of correlation between changes in the follow-up values, and the level of statistical significance was set at less than 0.05.
54 patients were randomized; 26 to FDI and 28 to placebo. Patients in the two treatment arms were similar in age, gender, the prevalence of diabetes, baseline eGFR and urinary protein excretion (200mg vs 350mg/24hr in the FDI and placebo groups respectively, p = 0.2713). Compared to baseline levels, serum creatinine, cystatin C and NGAL did not change significantly in either arm (figure 1). There were no significant changes in urinary protein excretion both within and between groups (median change in urinary protein excretion: FDI: -10mg and -39mg/24hr; placebo: 0mg and 0mg/24hr at 1- and 3- months respectively, p>0.05) There was a significant correlation between changes in cystatin C levels and serum creatinine (r = 0.6994, p<0.0001) during follow-up. This correlation persisted when patients were stratified by an age of 65 years and presence of diabetes (figure 2). Changes in Cystatin C levels did not correlate well with changes in NGAL.
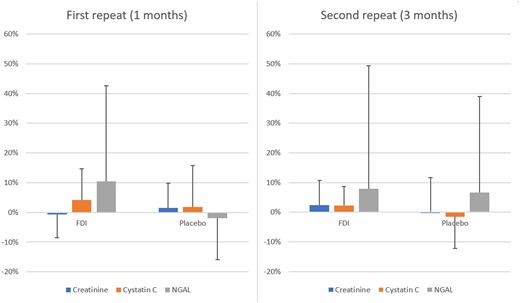
Percentage change in Creatinine, Cystatin C and NGAL relative to baseline at 1 and 3 months. Bar heights represent mean change and error bars represent standard deviations of the sample. FDI: Ferric derisomaltose.
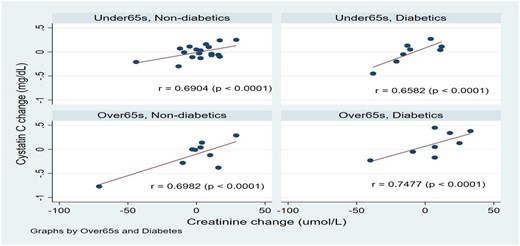
Change in cystatin C and creatinine relative to baseline in the two treatment arms, stratified by age and diabetes mellitus.
This post-hoc analysis of data from the Iron & Heart study indicates that high dose FDI did not cause any significant detriment in the short-term to renal function compared to placebo. This complements the safety profile of high dose third generation IV iron products and improves our understanding of their use in patients with CKD at their approved doses. There was a good correlation between cystatin C and creatinine, which was not affected by various sub-groups such as age or presence of diabetes mellitus. The analysis confirms the role of cystatin C as a robust biomarker of measuring renal function at least when compared to other established methods.
- anemia
- proteinuria
- hemodialysis
- iron
- diabetes mellitus
- excretory function
- renal function
- kidney failure, chronic
- carbohydrates
- creatinine
- kidney failure
- hemoglobin
- biological markers
- follow-up
- iron compounds
- safety
- urinary tract
- arm
- dialysis procedure
- heart
- kidney
- gender
- iron deficiency
- serum ferritin level result
- iron sucrose
- urine protein test
- renal trauma
- creatinine tests, serum
- cystatin c measurement
- transferrin saturation measurement
- glomerular filtration rate, estimated
- lipocalin-2 protein
- impact





Comments