-
PDF
- Split View
-
Views
-
Cite
Cite
Kanna Shinkawa, Satomi Yoshida, Tomotsugu Seki, Motoko Yanagita, Koji Kawakami, Risk factors for venous thromboembolism in patients with nephrotic syndrome: a retrospective cohort study, Nephrology Dialysis Transplantation, Volume 36, Issue 7, July 2021, Pages 1199–1206, https://doi.org/10.1093/ndt/gfaa134
Close - Share Icon Share
Abstract
Nephrotic syndrome is associated with an increased risk of venous thromboembolism (VTE). However, the risk factors of VTE in nephrotic syndrome, other than hypoalbuminemia and severe proteinuria, are not well established. Therefore we aimed to investigate the risk factors of VTE in patients with nephrotic syndrome.
This retrospective cohort study used data from a Japanese nationwide claims database. We identified patients ≥18 years of age hospitalized with nephrotic syndrome. Through multivariable logistic regression, we determined the risk factors of VTE in patients with nephrotic syndrome during hospitalization.
Of the 7473 hospitalized patients with nephrotic syndrome without VTE, 221 (3.0%) developed VTE. In the VTE group, 14 (6.3%), 11 (5.0%) and 198 (89.6%) patients developed pulmonary embolism, renal vein thrombosis and deep vein thrombosis, respectively. We found that female sex {odds ratio [OR] 1.39 [95% confidence interval (CI) 1.05–1.85]}, body mass index (BMI) ≥30 [OR 2.01 (95% CI 1.35–2.99)], acute kidney injury [AKI; OR 1.67 (95% CI 1.07–2.62)], sepsis [OR 2.85 (95% CI 1.37–5.93)], lupus nephritis [OR 3.64 (95% CI 1.58–8.37)] and intravenous corticosteroids use [OR 2.40 (95% CI 1.52–3.80)] were associated with a significantly higher risk of developing VTE.
In patients with nephrotic syndrome, female sex, BMI ≥30, AKI, sepsis, lupus nephritis and intravenous corticosteroid use may help evaluate the risk of VTE.
What is already known about this subject?
Venous thromboembolism (VTE), including deep vein thrombosis, renal vein thrombosis and pulmonary embolism, is a common complication in patients with nephrotic syndrome. Low serum albumin, high urinary protein and membranous nephropathy are known risk factors for VTE in nephrotic syndrome. Other factors related to VTE, such as underlying disease or medication, have not been fully explored.
What this study adds?
This study reveals previously unknown potential risk factors of VTE in nephrotic syndrome. Female sex, body mass index ≥30, acute kidney injury, sepsis, lupus nephritis and intravenous corticosteroid use were associated with a higher risk of VTE in patients with nephrotic syndrome.
What impact this may have on practice or policy?
These factors may assist in risk evaluation of VTE in patients with nephrotic syndrome in clinical practice.
INTRODUCTION
Venous thromboembolism (VTE), including deep vein thrombosis (DVT), renal vein thrombosis (RVT) and pulmonary embolism (PE), is a well-known complication in patients with nephrotic syndrome [1–4]. Nephrotic syndrome induces imbalances of prothrombotic and antithrombotic function as well as decreased thrombolytic activity [5, 6]. Among the general Japanese population, the annual incidence of PE and DVT was 61.9 and 115.5, respectively, per 1 000 000 people [7], which is as low as one-eighth and one-quarter of the incidence reported in the Western population, respectively [8, 9]. In nephrotic syndrome, the reported annual incidence rate of VTE was 1.02% [10], which is much higher than that reported in the general population.
Some studies have assessed the risk factors of VTE in patients with nephrotic syndrome. With regard to nephritis, the risk was high for membranous nephropathy, focal segmental glomerulosclerosis and membranoproliferative glomerulonephritis [11–16]. As with other risk factors, low serum albumin, high urinary protein, low antithrombin III activity, high ionized calcium and high D-dimer value are related to VTE [14, 17–20]. However, few studies have explored other risks of VTE, such as underlying disease or medication.
To the best of our knowledge, no large previous studies have investigated VTE in Asian nephrotic patients. Therefore, in this study, using a Japanese administrative database, we investigated the proportion of VTE occurrence and the risk factors of VTE in patients with nephrotic syndrome during hospitalization.
MATERIALS AND METHODS
Study design and data source
This is a retrospective cohort study using a hospital-based database provided by Medical Data Vision (Tokyo, Japan). The database contains electronic health records of administrative claims data and discharge summaries in Japan. It also contains information on ∼18 million patients from 298 acute care hospitals using a diagnosis procedure combination (DPC)-based payment system from April 2008 to October 2017. This database includes anonymized patient identifiers, age, sex, body mass index (BMI), smoking status, diagnostic information using the International Classification of Diseases, 10th revision (ICD-10) codes, and treatment or diagnostic test information using Japanese procedural codes and claims codes. Anatomical Therapeutic Chemical codes are registered to identify the category of prescription drugs. The database records up to four comorbidities on admission and complications during hospitalization [21–23].
Study population
We extracted data from the database on patients >18 years of age who were hospitalized with nephrotic syndrome from April 2008 to October 2017. If the same patient was hospitalized more than once, we identified the first hospitalization during the period. The ICD-10 code that we used to identify nephrotic syndrome was N04.x [4]. We excluded patients with VTE with ICD-10 codes (PE: I26.0, I26.9; RVT: I82.3; DVT: I80.1, I80.2, I80.3, I80.8, I80.9, I82.8, I82.9, O22.3, O22.9 and O87.1) as comorbidities on admission.
Ethical approval
Informed consent from individual patients was waived based on the local institutional ethical guidelines for epidemiological research since this study was a secondary analysis of an anonymous patient database. This study was approved by the Kyoto University Graduate School and Faculty of Medicine Ethics Committee (R1226), following the Strengthening the Reporting of Observational Studies in Epidemiology statement guidelines [24].
Outcomes and variables
Cases of VTE were defined according to the diagnoses of PE, RVT or DVT during hospitalization (ICD-10 codes as listed above) [4, 25]. Because the identification of ICD codes with imaging procedure codes was considered highly sensitive and specific for VTE detection compared with the use of diagnostic ICD codes alone [25], we extracted data on patients who had imaging procedure codes during hospitalization with the disease codes for PE, RVT or DVT. Computed tomography (CT), magnetic resonance imaging (MRI) or ventilation–perfusion scans were used in patients with codes for PE. CT, MRI or compression duplex ultrasound was used in patients with codes for RVT and DVT.
We collected data on age, sex, BMI, smoking status, activity of daily living score (Barthel index) and the following prescriptions on the day of admission: oral corticosteroids, intravenous corticosteroids, immunosuppressive agents, diuretics, anticoagulant drugs and estrogen preparations. We noted comorbidities on admission for the following chronic diseases: hypertension, diabetes mellitus, chronic kidney disease, hyperlipidemia, ischemic heart disease, atrial fibrillation, cerebrovascular disease, peripheral artery disease, connective tissue disease, systemic lupus erythematosus (SLE), antiphospholipid syndrome and malignancy. We also collected data on the following acute disease codes recorded on the day of admission: acute kidney injury (AKI), lower limb fracture, sepsis, urinary tract infection, pneumonia and gastrointestinal infection. We included not only comorbidities, but also primary diagnoses, to collect data on these diseases, because acute diseases were usually not registered as comorbidities in the DPC database. Kidney disease was found to be the most recent diagnosis within 60 days after discharge since renal biopsy is often conducted during hospitalization and results may have been obtained after discharge. To show the clinical course in both groups, we described the following variables during hospitalization: length of hospital stay, intensive care unit (ICU) stay, percutaneous needle biopsy, death and prescriptions during hospitalization (Supplementary data, Table S1).
Statistical analysis
Continuous variables are presented as the mean [standard deviation (SD)] or the median [interquartile range (IQR)]. Both univariable and multivariable logistic regression analyses were used to investigate the odds ratios (ORs) and 95% confidence intervals (CIs) to evaluate the association between each variable and VTE. We conducted a complete case analysis for any missing variable. The multivariable model included the following variables: age, sex, BMI, hypertension, diabetes mellitus, chronic kidney disease, hyperlipidemia, ischemic heart disease, atrial fibrillation, cerebrovascular disease, peripheral artery disease, connective tissue disease, malignancy, AKI, lower limb fracture, sepsis, urinary tract infection, pneumonia, gastrointestinal infection, primary disease of nephrotic syndrome (minimal change nephrotic syndrome, focal glomerular sclerosis, membranous nephropathy, membranoproliferative glomerulonephritis, immunoglobulin A (IgA) nephropathy, diabetic nephropathy, lupus nephritis), oral corticosteroids, intravenous corticosteroids, immunosuppressive agents and diuretics.
All P-values were two-sided and the alpha level was set at 0.05. All data analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Sensitivity analyses
We excluded patients who were given anticoagulant prescriptions on the day of admission to identify them reliably without VTE on admission. In the excluded population, we conducted a sensitivity analysis (univariable and multivariable logistic regression analyses) using the same variables as in the main analysis.
Because there were missing data (4.6%) for BMI using the multivariable regression analysis, we performed multiple imputations for the missing data on the assumption that it was missing at random [26, 27]. We created 25 imputed datasets using fully conditional specification methods, conducted multivariable logistic regression after the imputation and combined the results of the analyses using Rubin’s rule.
RESULTS
From April 2008 to October 2017, we identified 7661 patients who were hospitalized with nephrotic syndrome. After excluding 188 patients who had VTE as comorbidity on admission, 7473 patients were included in the main analysis cohort (Figure 1).
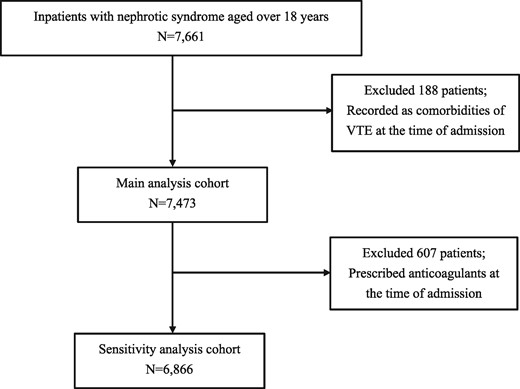
A total of 221 (3.0%) patients developed VTE during hospitalization. In the VTE group, 14 (6.3%), 11 (5.0%) and 198 (89.6%) patients developed PE, RVT and DVT, respectively. The median age was 69.0 (IQR 53.0–79.0) years and 104 (47.1%) patients were male. Ages were similar between both groups. The median age of 39.0 (IQR 26.0–63.0) years in the RVT group was lower than that in the other two groups. In the VTE and non-VTE groups, minimal change nephrotic syndrome was the most common kidney disease, whereas in patients with RVT, lupus nephritis was most common [two patients (18.2%)] (Table 1).
Baseline characteristics on the admission of inpatients with nephrotic syndrome with VTE or without VTE
| . | VTE negative . | VTE positive . | PE positive . | RVT positive . | DVT positive . |
|---|---|---|---|---|---|
| Characteristics . | (n = 7252) . | (n = 221) . | (n = 14) . | (n = 11) . | (n = 198) . |
| Age (years), median (IQR) | 68.0 (54.0, 78.0) | 69.0 (53.0, 79.0) | 72.5 (59.0, 78.0) | 39.0 (26.0, 63.0) | 69.5 (54.0, 80.0) |
| Age (years), n (%) | |||||
| <55 | 1846 (25.5) | 60 (27.2) | 2 (14.3) | 8 (72.7) | 50 (25.3) |
| 55–70 | 2087 (28.8) | 57 (25.8) | 4 (28.6) | 1 (9.1) | 53 (26.8) |
| ≥71 | 3319 (45.8) | 104 (47.1) | 8 (57.1) | 2 (18.2) | 95 (48.0) |
| Male sex, n (%) | 4082 (56.3) | 104 (47.1) | 8 (57.1) | 5 (45.6) | 92 (46.5) |
| BMI, n (%) | |||||
| <25 | 4122 (56.8) | 121 (54.8) | 8 (57.1) | 6 (54.5) | 108 (54.5) |
| 25–29 | 2048 (28.2) | 58 (26.2) | 4 (28.6) | 3 (27.3) | 52 (26.3) |
| ≥30 | 743 (10.2) | 36 (16.3) | 1 (7.1) | 2 (18.2) | 33 (16.7) |
| Missing | 339 (4.7) | 6 (2.7) | 1 (7.1) | 0 | 5 (2.5) |
| Smoking history, n (%) | 2300 (31.7) | 67 (30.3) | 2 (14.2) | 4 (36.4) | 61 (30.8) |
| Missing | 878 (12.1) | 21 (9.5) | 2 (14.2) | 0 | 20 (10.1) |
| Barthel indexa, n (%) | 1375 (19.0) | 41 (18.6) | 4 (28.6) | 1 (9.1) | 37 (18.7) |
| Missing | 786 (10.8) | 29 (13.1) | 3 (21.4) | 0 | 26 (13.1) |
| Comorbidities, n (%) | |||||
| Hypertension | 3703 (51.1) | 93 (42.1) | 4 (28.6) | 4 (36.4) | 86 (43.4) |
| Diabetes mellitus | 2177 (30.0) | 51 (23.1) | 2 (14.3) | 2 (18.2) | 48 (24.2) |
| Chronic kidney disease | 791 (10.9) | 18 (8.1) | 1 (7.1) | 0 | 17 (8.6) |
| Hyperlipidemia | 2169 (29.9) | 62 (28.1) | 5 (35.7) | 4 (36.4) | 53 (26.8) |
| Ischemic heart disease | 443 (6.1) | 11 (5.0) | 1 (7.1) | 1 (9.1) | 9 (4.6) |
| Atrial fibrillation | 189 (2.6) | 5 (2.3) | 0 | 0 | 5 (2.5) |
| Cerebrovascular disease | 471 (6.5) | 13 (5.9) | 2 (14.3) | 0 | 11 (5.6) |
| Peripheral artery disease | 134 (1.9) | 4 (1.8) | 0 | 1 (9.1) | 3 (1.5) |
| Connective tissue disease | 166 (2.3) | 6 (2.7) | 0 | 2 (18.2) | 4 (2.0) |
| SLE | 71 (1.0) | 6 (2.7) | 0 | 2 (18.2) | 4 (2.0) |
| Antiphospholipid syndrome | 4 (0.1) | 0 | 0 | 0 | 0 |
| Malignancy | 484 (6.7) | 17 (7.7) | 0 | 0 | 17 (8.6) |
| AKI | 421 (5.8) | 27 (12.2) | 0 | 1 (9.1) | 26 (13.1) |
| Lower limb fracture | 20 (0.3) | 4 (1.8) | 0 | 0 | 4 (2.0) |
| Sepsis | 92 (1.3) | 10 (4.5) | 0 | 0 | 10 (5.1) |
| Urinary tract infection | 341 (4.7) | 16 (7.2) | 1 (7.1) | 0 | 15 (7.6) |
| Pneumonia | 229 (3.2) | 10 (4.5) | 0 | 0 | 10 (5.1) |
| Gastrointestinal infection | 202 (2.8) | 8 (3.6) | 0 | 2 (18.2) | 6 (3.0) |
| Primary disease of nephrotic syndrome, n (%) | |||||
| Minimal change nephrotic syndrome | 1272 (17.5) | 43 (19.5) | 1 (7.1) | 2 (18.2) | 40 (20.2) |
| Focal glomerular sclerosis | 172 (2.4) | 9 (4.1) | 0 | 0 | 9 (4.6) |
| Membranous nephropathy | 799 (11.0) | 30 (13.6) | 1 (7.1) | 1 (9.1) | 28 (14.1) |
| Membranoproliferative glomerulonephritis | 69 (1.0) | 4 (1.8) | 0 | 0 | 4 (2.0) |
| IgA nephropathy | 203 (2.8) | 10 (4.5) | 2 (14.3) | 0 | 9 (4.6) |
| Diabetic nephropathy | 1046 (14) | 18 (8.1) | 0 | 2 (18.2) | 16 (8.1) |
| Lupus nephritis | 104 (1.4) | 9 (4.1) | 0 | 2 (18.2) | 7 (3.5) |
| Medication on the day of admission, n (%) | |||||
| Oral corticosteroids, aloneb | 452 (6.2) | 11 (5.0) | 1 (7.1) | 2 (18.2) | 9 (4.6) |
| Intravenous corticosteroids | 351 (4.8) | 27 (12.2) | 3 (21.4) | 1 (9.1) | 24 (12.1) |
| Immunosuppressive agents | 145 (2.0) | 6 (2.7) | 0 | 0 | 6 (3.0) |
| Diuretics | 2087 (28.8) | 71 (32.1) | 7 (50.0) | 1 (9.1) | 63 (31.8) |
| Anticoagulant drugs | 569 (7.9) | 38 (17.2) | 5 (35.7) | 1 (9.1) | 33 (16.7) |
| Estrogen preparation | 1 (0.01) | 0 | 0 | 0 | 0 |
| . | VTE negative . | VTE positive . | PE positive . | RVT positive . | DVT positive . |
|---|---|---|---|---|---|
| Characteristics . | (n = 7252) . | (n = 221) . | (n = 14) . | (n = 11) . | (n = 198) . |
| Age (years), median (IQR) | 68.0 (54.0, 78.0) | 69.0 (53.0, 79.0) | 72.5 (59.0, 78.0) | 39.0 (26.0, 63.0) | 69.5 (54.0, 80.0) |
| Age (years), n (%) | |||||
| <55 | 1846 (25.5) | 60 (27.2) | 2 (14.3) | 8 (72.7) | 50 (25.3) |
| 55–70 | 2087 (28.8) | 57 (25.8) | 4 (28.6) | 1 (9.1) | 53 (26.8) |
| ≥71 | 3319 (45.8) | 104 (47.1) | 8 (57.1) | 2 (18.2) | 95 (48.0) |
| Male sex, n (%) | 4082 (56.3) | 104 (47.1) | 8 (57.1) | 5 (45.6) | 92 (46.5) |
| BMI, n (%) | |||||
| <25 | 4122 (56.8) | 121 (54.8) | 8 (57.1) | 6 (54.5) | 108 (54.5) |
| 25–29 | 2048 (28.2) | 58 (26.2) | 4 (28.6) | 3 (27.3) | 52 (26.3) |
| ≥30 | 743 (10.2) | 36 (16.3) | 1 (7.1) | 2 (18.2) | 33 (16.7) |
| Missing | 339 (4.7) | 6 (2.7) | 1 (7.1) | 0 | 5 (2.5) |
| Smoking history, n (%) | 2300 (31.7) | 67 (30.3) | 2 (14.2) | 4 (36.4) | 61 (30.8) |
| Missing | 878 (12.1) | 21 (9.5) | 2 (14.2) | 0 | 20 (10.1) |
| Barthel indexa, n (%) | 1375 (19.0) | 41 (18.6) | 4 (28.6) | 1 (9.1) | 37 (18.7) |
| Missing | 786 (10.8) | 29 (13.1) | 3 (21.4) | 0 | 26 (13.1) |
| Comorbidities, n (%) | |||||
| Hypertension | 3703 (51.1) | 93 (42.1) | 4 (28.6) | 4 (36.4) | 86 (43.4) |
| Diabetes mellitus | 2177 (30.0) | 51 (23.1) | 2 (14.3) | 2 (18.2) | 48 (24.2) |
| Chronic kidney disease | 791 (10.9) | 18 (8.1) | 1 (7.1) | 0 | 17 (8.6) |
| Hyperlipidemia | 2169 (29.9) | 62 (28.1) | 5 (35.7) | 4 (36.4) | 53 (26.8) |
| Ischemic heart disease | 443 (6.1) | 11 (5.0) | 1 (7.1) | 1 (9.1) | 9 (4.6) |
| Atrial fibrillation | 189 (2.6) | 5 (2.3) | 0 | 0 | 5 (2.5) |
| Cerebrovascular disease | 471 (6.5) | 13 (5.9) | 2 (14.3) | 0 | 11 (5.6) |
| Peripheral artery disease | 134 (1.9) | 4 (1.8) | 0 | 1 (9.1) | 3 (1.5) |
| Connective tissue disease | 166 (2.3) | 6 (2.7) | 0 | 2 (18.2) | 4 (2.0) |
| SLE | 71 (1.0) | 6 (2.7) | 0 | 2 (18.2) | 4 (2.0) |
| Antiphospholipid syndrome | 4 (0.1) | 0 | 0 | 0 | 0 |
| Malignancy | 484 (6.7) | 17 (7.7) | 0 | 0 | 17 (8.6) |
| AKI | 421 (5.8) | 27 (12.2) | 0 | 1 (9.1) | 26 (13.1) |
| Lower limb fracture | 20 (0.3) | 4 (1.8) | 0 | 0 | 4 (2.0) |
| Sepsis | 92 (1.3) | 10 (4.5) | 0 | 0 | 10 (5.1) |
| Urinary tract infection | 341 (4.7) | 16 (7.2) | 1 (7.1) | 0 | 15 (7.6) |
| Pneumonia | 229 (3.2) | 10 (4.5) | 0 | 0 | 10 (5.1) |
| Gastrointestinal infection | 202 (2.8) | 8 (3.6) | 0 | 2 (18.2) | 6 (3.0) |
| Primary disease of nephrotic syndrome, n (%) | |||||
| Minimal change nephrotic syndrome | 1272 (17.5) | 43 (19.5) | 1 (7.1) | 2 (18.2) | 40 (20.2) |
| Focal glomerular sclerosis | 172 (2.4) | 9 (4.1) | 0 | 0 | 9 (4.6) |
| Membranous nephropathy | 799 (11.0) | 30 (13.6) | 1 (7.1) | 1 (9.1) | 28 (14.1) |
| Membranoproliferative glomerulonephritis | 69 (1.0) | 4 (1.8) | 0 | 0 | 4 (2.0) |
| IgA nephropathy | 203 (2.8) | 10 (4.5) | 2 (14.3) | 0 | 9 (4.6) |
| Diabetic nephropathy | 1046 (14) | 18 (8.1) | 0 | 2 (18.2) | 16 (8.1) |
| Lupus nephritis | 104 (1.4) | 9 (4.1) | 0 | 2 (18.2) | 7 (3.5) |
| Medication on the day of admission, n (%) | |||||
| Oral corticosteroids, aloneb | 452 (6.2) | 11 (5.0) | 1 (7.1) | 2 (18.2) | 9 (4.6) |
| Intravenous corticosteroids | 351 (4.8) | 27 (12.2) | 3 (21.4) | 1 (9.1) | 24 (12.1) |
| Immunosuppressive agents | 145 (2.0) | 6 (2.7) | 0 | 0 | 6 (3.0) |
| Diuretics | 2087 (28.8) | 71 (32.1) | 7 (50.0) | 1 (9.1) | 63 (31.8) |
| Anticoagulant drugs | 569 (7.9) | 38 (17.2) | 5 (35.7) | 1 (9.1) | 33 (16.7) |
| Estrogen preparation | 1 (0.01) | 0 | 0 | 0 | 0 |
Proportion of patients with Barthel index <100 points.
Patients who used intravenous corticosteroids were not included.
Baseline characteristics on the admission of inpatients with nephrotic syndrome with VTE or without VTE
| . | VTE negative . | VTE positive . | PE positive . | RVT positive . | DVT positive . |
|---|---|---|---|---|---|
| Characteristics . | (n = 7252) . | (n = 221) . | (n = 14) . | (n = 11) . | (n = 198) . |
| Age (years), median (IQR) | 68.0 (54.0, 78.0) | 69.0 (53.0, 79.0) | 72.5 (59.0, 78.0) | 39.0 (26.0, 63.0) | 69.5 (54.0, 80.0) |
| Age (years), n (%) | |||||
| <55 | 1846 (25.5) | 60 (27.2) | 2 (14.3) | 8 (72.7) | 50 (25.3) |
| 55–70 | 2087 (28.8) | 57 (25.8) | 4 (28.6) | 1 (9.1) | 53 (26.8) |
| ≥71 | 3319 (45.8) | 104 (47.1) | 8 (57.1) | 2 (18.2) | 95 (48.0) |
| Male sex, n (%) | 4082 (56.3) | 104 (47.1) | 8 (57.1) | 5 (45.6) | 92 (46.5) |
| BMI, n (%) | |||||
| <25 | 4122 (56.8) | 121 (54.8) | 8 (57.1) | 6 (54.5) | 108 (54.5) |
| 25–29 | 2048 (28.2) | 58 (26.2) | 4 (28.6) | 3 (27.3) | 52 (26.3) |
| ≥30 | 743 (10.2) | 36 (16.3) | 1 (7.1) | 2 (18.2) | 33 (16.7) |
| Missing | 339 (4.7) | 6 (2.7) | 1 (7.1) | 0 | 5 (2.5) |
| Smoking history, n (%) | 2300 (31.7) | 67 (30.3) | 2 (14.2) | 4 (36.4) | 61 (30.8) |
| Missing | 878 (12.1) | 21 (9.5) | 2 (14.2) | 0 | 20 (10.1) |
| Barthel indexa, n (%) | 1375 (19.0) | 41 (18.6) | 4 (28.6) | 1 (9.1) | 37 (18.7) |
| Missing | 786 (10.8) | 29 (13.1) | 3 (21.4) | 0 | 26 (13.1) |
| Comorbidities, n (%) | |||||
| Hypertension | 3703 (51.1) | 93 (42.1) | 4 (28.6) | 4 (36.4) | 86 (43.4) |
| Diabetes mellitus | 2177 (30.0) | 51 (23.1) | 2 (14.3) | 2 (18.2) | 48 (24.2) |
| Chronic kidney disease | 791 (10.9) | 18 (8.1) | 1 (7.1) | 0 | 17 (8.6) |
| Hyperlipidemia | 2169 (29.9) | 62 (28.1) | 5 (35.7) | 4 (36.4) | 53 (26.8) |
| Ischemic heart disease | 443 (6.1) | 11 (5.0) | 1 (7.1) | 1 (9.1) | 9 (4.6) |
| Atrial fibrillation | 189 (2.6) | 5 (2.3) | 0 | 0 | 5 (2.5) |
| Cerebrovascular disease | 471 (6.5) | 13 (5.9) | 2 (14.3) | 0 | 11 (5.6) |
| Peripheral artery disease | 134 (1.9) | 4 (1.8) | 0 | 1 (9.1) | 3 (1.5) |
| Connective tissue disease | 166 (2.3) | 6 (2.7) | 0 | 2 (18.2) | 4 (2.0) |
| SLE | 71 (1.0) | 6 (2.7) | 0 | 2 (18.2) | 4 (2.0) |
| Antiphospholipid syndrome | 4 (0.1) | 0 | 0 | 0 | 0 |
| Malignancy | 484 (6.7) | 17 (7.7) | 0 | 0 | 17 (8.6) |
| AKI | 421 (5.8) | 27 (12.2) | 0 | 1 (9.1) | 26 (13.1) |
| Lower limb fracture | 20 (0.3) | 4 (1.8) | 0 | 0 | 4 (2.0) |
| Sepsis | 92 (1.3) | 10 (4.5) | 0 | 0 | 10 (5.1) |
| Urinary tract infection | 341 (4.7) | 16 (7.2) | 1 (7.1) | 0 | 15 (7.6) |
| Pneumonia | 229 (3.2) | 10 (4.5) | 0 | 0 | 10 (5.1) |
| Gastrointestinal infection | 202 (2.8) | 8 (3.6) | 0 | 2 (18.2) | 6 (3.0) |
| Primary disease of nephrotic syndrome, n (%) | |||||
| Minimal change nephrotic syndrome | 1272 (17.5) | 43 (19.5) | 1 (7.1) | 2 (18.2) | 40 (20.2) |
| Focal glomerular sclerosis | 172 (2.4) | 9 (4.1) | 0 | 0 | 9 (4.6) |
| Membranous nephropathy | 799 (11.0) | 30 (13.6) | 1 (7.1) | 1 (9.1) | 28 (14.1) |
| Membranoproliferative glomerulonephritis | 69 (1.0) | 4 (1.8) | 0 | 0 | 4 (2.0) |
| IgA nephropathy | 203 (2.8) | 10 (4.5) | 2 (14.3) | 0 | 9 (4.6) |
| Diabetic nephropathy | 1046 (14) | 18 (8.1) | 0 | 2 (18.2) | 16 (8.1) |
| Lupus nephritis | 104 (1.4) | 9 (4.1) | 0 | 2 (18.2) | 7 (3.5) |
| Medication on the day of admission, n (%) | |||||
| Oral corticosteroids, aloneb | 452 (6.2) | 11 (5.0) | 1 (7.1) | 2 (18.2) | 9 (4.6) |
| Intravenous corticosteroids | 351 (4.8) | 27 (12.2) | 3 (21.4) | 1 (9.1) | 24 (12.1) |
| Immunosuppressive agents | 145 (2.0) | 6 (2.7) | 0 | 0 | 6 (3.0) |
| Diuretics | 2087 (28.8) | 71 (32.1) | 7 (50.0) | 1 (9.1) | 63 (31.8) |
| Anticoagulant drugs | 569 (7.9) | 38 (17.2) | 5 (35.7) | 1 (9.1) | 33 (16.7) |
| Estrogen preparation | 1 (0.01) | 0 | 0 | 0 | 0 |
| . | VTE negative . | VTE positive . | PE positive . | RVT positive . | DVT positive . |
|---|---|---|---|---|---|
| Characteristics . | (n = 7252) . | (n = 221) . | (n = 14) . | (n = 11) . | (n = 198) . |
| Age (years), median (IQR) | 68.0 (54.0, 78.0) | 69.0 (53.0, 79.0) | 72.5 (59.0, 78.0) | 39.0 (26.0, 63.0) | 69.5 (54.0, 80.0) |
| Age (years), n (%) | |||||
| <55 | 1846 (25.5) | 60 (27.2) | 2 (14.3) | 8 (72.7) | 50 (25.3) |
| 55–70 | 2087 (28.8) | 57 (25.8) | 4 (28.6) | 1 (9.1) | 53 (26.8) |
| ≥71 | 3319 (45.8) | 104 (47.1) | 8 (57.1) | 2 (18.2) | 95 (48.0) |
| Male sex, n (%) | 4082 (56.3) | 104 (47.1) | 8 (57.1) | 5 (45.6) | 92 (46.5) |
| BMI, n (%) | |||||
| <25 | 4122 (56.8) | 121 (54.8) | 8 (57.1) | 6 (54.5) | 108 (54.5) |
| 25–29 | 2048 (28.2) | 58 (26.2) | 4 (28.6) | 3 (27.3) | 52 (26.3) |
| ≥30 | 743 (10.2) | 36 (16.3) | 1 (7.1) | 2 (18.2) | 33 (16.7) |
| Missing | 339 (4.7) | 6 (2.7) | 1 (7.1) | 0 | 5 (2.5) |
| Smoking history, n (%) | 2300 (31.7) | 67 (30.3) | 2 (14.2) | 4 (36.4) | 61 (30.8) |
| Missing | 878 (12.1) | 21 (9.5) | 2 (14.2) | 0 | 20 (10.1) |
| Barthel indexa, n (%) | 1375 (19.0) | 41 (18.6) | 4 (28.6) | 1 (9.1) | 37 (18.7) |
| Missing | 786 (10.8) | 29 (13.1) | 3 (21.4) | 0 | 26 (13.1) |
| Comorbidities, n (%) | |||||
| Hypertension | 3703 (51.1) | 93 (42.1) | 4 (28.6) | 4 (36.4) | 86 (43.4) |
| Diabetes mellitus | 2177 (30.0) | 51 (23.1) | 2 (14.3) | 2 (18.2) | 48 (24.2) |
| Chronic kidney disease | 791 (10.9) | 18 (8.1) | 1 (7.1) | 0 | 17 (8.6) |
| Hyperlipidemia | 2169 (29.9) | 62 (28.1) | 5 (35.7) | 4 (36.4) | 53 (26.8) |
| Ischemic heart disease | 443 (6.1) | 11 (5.0) | 1 (7.1) | 1 (9.1) | 9 (4.6) |
| Atrial fibrillation | 189 (2.6) | 5 (2.3) | 0 | 0 | 5 (2.5) |
| Cerebrovascular disease | 471 (6.5) | 13 (5.9) | 2 (14.3) | 0 | 11 (5.6) |
| Peripheral artery disease | 134 (1.9) | 4 (1.8) | 0 | 1 (9.1) | 3 (1.5) |
| Connective tissue disease | 166 (2.3) | 6 (2.7) | 0 | 2 (18.2) | 4 (2.0) |
| SLE | 71 (1.0) | 6 (2.7) | 0 | 2 (18.2) | 4 (2.0) |
| Antiphospholipid syndrome | 4 (0.1) | 0 | 0 | 0 | 0 |
| Malignancy | 484 (6.7) | 17 (7.7) | 0 | 0 | 17 (8.6) |
| AKI | 421 (5.8) | 27 (12.2) | 0 | 1 (9.1) | 26 (13.1) |
| Lower limb fracture | 20 (0.3) | 4 (1.8) | 0 | 0 | 4 (2.0) |
| Sepsis | 92 (1.3) | 10 (4.5) | 0 | 0 | 10 (5.1) |
| Urinary tract infection | 341 (4.7) | 16 (7.2) | 1 (7.1) | 0 | 15 (7.6) |
| Pneumonia | 229 (3.2) | 10 (4.5) | 0 | 0 | 10 (5.1) |
| Gastrointestinal infection | 202 (2.8) | 8 (3.6) | 0 | 2 (18.2) | 6 (3.0) |
| Primary disease of nephrotic syndrome, n (%) | |||||
| Minimal change nephrotic syndrome | 1272 (17.5) | 43 (19.5) | 1 (7.1) | 2 (18.2) | 40 (20.2) |
| Focal glomerular sclerosis | 172 (2.4) | 9 (4.1) | 0 | 0 | 9 (4.6) |
| Membranous nephropathy | 799 (11.0) | 30 (13.6) | 1 (7.1) | 1 (9.1) | 28 (14.1) |
| Membranoproliferative glomerulonephritis | 69 (1.0) | 4 (1.8) | 0 | 0 | 4 (2.0) |
| IgA nephropathy | 203 (2.8) | 10 (4.5) | 2 (14.3) | 0 | 9 (4.6) |
| Diabetic nephropathy | 1046 (14) | 18 (8.1) | 0 | 2 (18.2) | 16 (8.1) |
| Lupus nephritis | 104 (1.4) | 9 (4.1) | 0 | 2 (18.2) | 7 (3.5) |
| Medication on the day of admission, n (%) | |||||
| Oral corticosteroids, aloneb | 452 (6.2) | 11 (5.0) | 1 (7.1) | 2 (18.2) | 9 (4.6) |
| Intravenous corticosteroids | 351 (4.8) | 27 (12.2) | 3 (21.4) | 1 (9.1) | 24 (12.1) |
| Immunosuppressive agents | 145 (2.0) | 6 (2.7) | 0 | 0 | 6 (3.0) |
| Diuretics | 2087 (28.8) | 71 (32.1) | 7 (50.0) | 1 (9.1) | 63 (31.8) |
| Anticoagulant drugs | 569 (7.9) | 38 (17.2) | 5 (35.7) | 1 (9.1) | 33 (16.7) |
| Estrogen preparation | 1 (0.01) | 0 | 0 | 0 | 0 |
Proportion of patients with Barthel index <100 points.
Patients who used intravenous corticosteroids were not included.
The length of hospital stay was 58 (IQR 32–85) days in the VTE group and 24 (IQR 13–44) days in the non-VTE group. Seven (3.2%) patients in the VTE group and 57 (0.8%) in the non-VTE group had experienced an ICU stay. Percutaneous needle biopsies were performed in 51.1% of the VTE group and 45.6% of the non-VTE group. During hospitalization, 4 (28.6%) patients with PE and 16 (8.1%) patients with DVT died, whereas no patients with RVT died. We evaluated prescriptions during hospitalization (from admission to discharge) and 87.3% of the VTE group and 26.4% of the non-VTE group had been prescribed anticoagulant drugs (including oral administration and injectables) during hospitalization (Table 2).
| . | VTE negative . | VTE positive . | PE positive . | RVT positive . | DVT positive . |
|---|---|---|---|---|---|
| Characteristics . | (n = 7252) . | (n = 221) . | (n = 14) . | (n = 11) . | (n = 198) . |
| Hospital stay (days), median (IQR) | 24 (13, 44) | 58 (32, 85) | 32 (12, 60) | 51 (26, 96) | 59 (33, 85) |
| ICU stay during hospitalization, n (%) | 57 (0.8) | 7 (3.2) | 0 | 0 | 7 (3.5) |
| Percutaneous needle biopsy, n (%)a | 3305 (45.6) | 113 (51.1) | 4 (28.6) | 6 (54.6) | 104 (52.5) |
| Death, n (%) | 261 (3.6) | 19 (8.6) | 4 (28.6) | 0 | 16 (8.1) |
| Medication during hospitalization, n (%)b | |||||
| Oral corticosteroids, alonec | 2072 (28.6) | 66 (29.9) | 3 (21.4) | 2 (18.2) | 62 (31.3) |
| Intravenous corticosteroids | 1562 (21.5) | 121 (54.8) | 9 (64.3) | 6 (54.6) | 107 (54.0) |
| Immunosuppressive agents | 1074 (14.8) | 85 (38.5) | 2 (14.3) | 6 (54.6) | 77 (38.9) |
| Anticoagulant drugs | 1915 (26.4) | 193 (87.3) | 13 (92.9) | 11 (100.0) | 171 (86.4) |
| Heparin | 1437 (19.8) | 127 (57.5) | 13 (92.9) | 6 (54.6) | 110 (55.6) |
| Low molecular weight heparin | 99 (1.4) | 12 (5.4) | 1 (7.1) | 0 | 11 (5.6) |
| Antiplatelet drugs | 1836 (25.3) | 61 (27.6) | 4 (28.6) | 3 (27.3) | 55 (27.8) |
| Antihypertensive drugs | 4766 (65.7) | 150 (67.9) | 6 (42.9) | 8 (72.7) | 137 (69.2) |
| Ras inhibitors | 3774 (52.0) | 114 (51.6) | 4 (28.6) | 7 (63.6) | 103 (52.0) |
| Calcium blockers | 3582 (49.4) | 108 (48.9) | 5 (35.7) | 4 (36.4) | 100 (50.5) |
| Antihyperlipidemic drugs | 3446 (47.5) | 146 (66.1) | 6 (42.9) | 9 (81.8) | 131 (66.2) |
| Statin | 3239 (44.7) | 138 (62.4) | 6 (42.9) | 8 (72.7) | 124 (62.6) |
| Antidiabetic drugs | 2595 (35.8) | 104 (47.1) | 2 (14.3) | 3 (27.3) | 100 (50.5) |
| Diuretics | 4919 (67.8) | 188 (85.1) | 11 (78.6) | 7 (63.6) | 170 (85.9) |
| Albumin preparation | 1205 (16.6) | 95 (43.0) | 5 (35.7) | 2 (18.2) | 89 (45.0) |
| . | VTE negative . | VTE positive . | PE positive . | RVT positive . | DVT positive . |
|---|---|---|---|---|---|
| Characteristics . | (n = 7252) . | (n = 221) . | (n = 14) . | (n = 11) . | (n = 198) . |
| Hospital stay (days), median (IQR) | 24 (13, 44) | 58 (32, 85) | 32 (12, 60) | 51 (26, 96) | 59 (33, 85) |
| ICU stay during hospitalization, n (%) | 57 (0.8) | 7 (3.2) | 0 | 0 | 7 (3.5) |
| Percutaneous needle biopsy, n (%)a | 3305 (45.6) | 113 (51.1) | 4 (28.6) | 6 (54.6) | 104 (52.5) |
| Death, n (%) | 261 (3.6) | 19 (8.6) | 4 (28.6) | 0 | 16 (8.1) |
| Medication during hospitalization, n (%)b | |||||
| Oral corticosteroids, alonec | 2072 (28.6) | 66 (29.9) | 3 (21.4) | 2 (18.2) | 62 (31.3) |
| Intravenous corticosteroids | 1562 (21.5) | 121 (54.8) | 9 (64.3) | 6 (54.6) | 107 (54.0) |
| Immunosuppressive agents | 1074 (14.8) | 85 (38.5) | 2 (14.3) | 6 (54.6) | 77 (38.9) |
| Anticoagulant drugs | 1915 (26.4) | 193 (87.3) | 13 (92.9) | 11 (100.0) | 171 (86.4) |
| Heparin | 1437 (19.8) | 127 (57.5) | 13 (92.9) | 6 (54.6) | 110 (55.6) |
| Low molecular weight heparin | 99 (1.4) | 12 (5.4) | 1 (7.1) | 0 | 11 (5.6) |
| Antiplatelet drugs | 1836 (25.3) | 61 (27.6) | 4 (28.6) | 3 (27.3) | 55 (27.8) |
| Antihypertensive drugs | 4766 (65.7) | 150 (67.9) | 6 (42.9) | 8 (72.7) | 137 (69.2) |
| Ras inhibitors | 3774 (52.0) | 114 (51.6) | 4 (28.6) | 7 (63.6) | 103 (52.0) |
| Calcium blockers | 3582 (49.4) | 108 (48.9) | 5 (35.7) | 4 (36.4) | 100 (50.5) |
| Antihyperlipidemic drugs | 3446 (47.5) | 146 (66.1) | 6 (42.9) | 9 (81.8) | 131 (66.2) |
| Statin | 3239 (44.7) | 138 (62.4) | 6 (42.9) | 8 (72.7) | 124 (62.6) |
| Antidiabetic drugs | 2595 (35.8) | 104 (47.1) | 2 (14.3) | 3 (27.3) | 100 (50.5) |
| Diuretics | 4919 (67.8) | 188 (85.1) | 11 (78.6) | 7 (63.6) | 170 (85.9) |
| Albumin preparation | 1205 (16.6) | 95 (43.0) | 5 (35.7) | 2 (18.2) | 89 (45.0) |
Percutaneous needle biopsy including other than kidney during hospitalization.
Including prescription from admission date to discharge date.
Patients who used intravenous corticosteroids were not included.
| . | VTE negative . | VTE positive . | PE positive . | RVT positive . | DVT positive . |
|---|---|---|---|---|---|
| Characteristics . | (n = 7252) . | (n = 221) . | (n = 14) . | (n = 11) . | (n = 198) . |
| Hospital stay (days), median (IQR) | 24 (13, 44) | 58 (32, 85) | 32 (12, 60) | 51 (26, 96) | 59 (33, 85) |
| ICU stay during hospitalization, n (%) | 57 (0.8) | 7 (3.2) | 0 | 0 | 7 (3.5) |
| Percutaneous needle biopsy, n (%)a | 3305 (45.6) | 113 (51.1) | 4 (28.6) | 6 (54.6) | 104 (52.5) |
| Death, n (%) | 261 (3.6) | 19 (8.6) | 4 (28.6) | 0 | 16 (8.1) |
| Medication during hospitalization, n (%)b | |||||
| Oral corticosteroids, alonec | 2072 (28.6) | 66 (29.9) | 3 (21.4) | 2 (18.2) | 62 (31.3) |
| Intravenous corticosteroids | 1562 (21.5) | 121 (54.8) | 9 (64.3) | 6 (54.6) | 107 (54.0) |
| Immunosuppressive agents | 1074 (14.8) | 85 (38.5) | 2 (14.3) | 6 (54.6) | 77 (38.9) |
| Anticoagulant drugs | 1915 (26.4) | 193 (87.3) | 13 (92.9) | 11 (100.0) | 171 (86.4) |
| Heparin | 1437 (19.8) | 127 (57.5) | 13 (92.9) | 6 (54.6) | 110 (55.6) |
| Low molecular weight heparin | 99 (1.4) | 12 (5.4) | 1 (7.1) | 0 | 11 (5.6) |
| Antiplatelet drugs | 1836 (25.3) | 61 (27.6) | 4 (28.6) | 3 (27.3) | 55 (27.8) |
| Antihypertensive drugs | 4766 (65.7) | 150 (67.9) | 6 (42.9) | 8 (72.7) | 137 (69.2) |
| Ras inhibitors | 3774 (52.0) | 114 (51.6) | 4 (28.6) | 7 (63.6) | 103 (52.0) |
| Calcium blockers | 3582 (49.4) | 108 (48.9) | 5 (35.7) | 4 (36.4) | 100 (50.5) |
| Antihyperlipidemic drugs | 3446 (47.5) | 146 (66.1) | 6 (42.9) | 9 (81.8) | 131 (66.2) |
| Statin | 3239 (44.7) | 138 (62.4) | 6 (42.9) | 8 (72.7) | 124 (62.6) |
| Antidiabetic drugs | 2595 (35.8) | 104 (47.1) | 2 (14.3) | 3 (27.3) | 100 (50.5) |
| Diuretics | 4919 (67.8) | 188 (85.1) | 11 (78.6) | 7 (63.6) | 170 (85.9) |
| Albumin preparation | 1205 (16.6) | 95 (43.0) | 5 (35.7) | 2 (18.2) | 89 (45.0) |
| . | VTE negative . | VTE positive . | PE positive . | RVT positive . | DVT positive . |
|---|---|---|---|---|---|
| Characteristics . | (n = 7252) . | (n = 221) . | (n = 14) . | (n = 11) . | (n = 198) . |
| Hospital stay (days), median (IQR) | 24 (13, 44) | 58 (32, 85) | 32 (12, 60) | 51 (26, 96) | 59 (33, 85) |
| ICU stay during hospitalization, n (%) | 57 (0.8) | 7 (3.2) | 0 | 0 | 7 (3.5) |
| Percutaneous needle biopsy, n (%)a | 3305 (45.6) | 113 (51.1) | 4 (28.6) | 6 (54.6) | 104 (52.5) |
| Death, n (%) | 261 (3.6) | 19 (8.6) | 4 (28.6) | 0 | 16 (8.1) |
| Medication during hospitalization, n (%)b | |||||
| Oral corticosteroids, alonec | 2072 (28.6) | 66 (29.9) | 3 (21.4) | 2 (18.2) | 62 (31.3) |
| Intravenous corticosteroids | 1562 (21.5) | 121 (54.8) | 9 (64.3) | 6 (54.6) | 107 (54.0) |
| Immunosuppressive agents | 1074 (14.8) | 85 (38.5) | 2 (14.3) | 6 (54.6) | 77 (38.9) |
| Anticoagulant drugs | 1915 (26.4) | 193 (87.3) | 13 (92.9) | 11 (100.0) | 171 (86.4) |
| Heparin | 1437 (19.8) | 127 (57.5) | 13 (92.9) | 6 (54.6) | 110 (55.6) |
| Low molecular weight heparin | 99 (1.4) | 12 (5.4) | 1 (7.1) | 0 | 11 (5.6) |
| Antiplatelet drugs | 1836 (25.3) | 61 (27.6) | 4 (28.6) | 3 (27.3) | 55 (27.8) |
| Antihypertensive drugs | 4766 (65.7) | 150 (67.9) | 6 (42.9) | 8 (72.7) | 137 (69.2) |
| Ras inhibitors | 3774 (52.0) | 114 (51.6) | 4 (28.6) | 7 (63.6) | 103 (52.0) |
| Calcium blockers | 3582 (49.4) | 108 (48.9) | 5 (35.7) | 4 (36.4) | 100 (50.5) |
| Antihyperlipidemic drugs | 3446 (47.5) | 146 (66.1) | 6 (42.9) | 9 (81.8) | 131 (66.2) |
| Statin | 3239 (44.7) | 138 (62.4) | 6 (42.9) | 8 (72.7) | 124 (62.6) |
| Antidiabetic drugs | 2595 (35.8) | 104 (47.1) | 2 (14.3) | 3 (27.3) | 100 (50.5) |
| Diuretics | 4919 (67.8) | 188 (85.1) | 11 (78.6) | 7 (63.6) | 170 (85.9) |
| Albumin preparation | 1205 (16.6) | 95 (43.0) | 5 (35.7) | 2 (18.2) | 89 (45.0) |
Percutaneous needle biopsy including other than kidney during hospitalization.
Including prescription from admission date to discharge date.
Patients who used intravenous corticosteroids were not included.
On multivariable logistic regression, female sex [OR 1.39 (95% CI 1.05–1.85)], BMI ≥30 [OR 2.01 (95% CI 1.35–2.99)], AKI [OR 1.67 (95% CI 1.07–2.62)], lower limb fracture [OR 7.43 (95% CI 2.43–22.7)], sepsis [OR 2.85 (95% CI 1.37–5.93)], lupus nephritis [OR 3.64 (95% CI 1.58–8.37)] and intravenous corticosteroids use [OR 2.40 (95% CI 1.52–3.80) were associated with a significantly higher risk of VTE (Table 3). Membranous nephropathy had a borderline significantly higher risk of VTE [OR 1.54 (95% CI 1.00–2.37)]. The association between malignancy and VTE was not significant [OR 1.58 (95% CI 0.92–2.70)] (Table 3).
Variables on admission associated with VTE in patients with nephrotic syndrome
| Variables . | Univariable OR (95% CI) . | Multivariable OR (95% CI) . |
|---|---|---|
| Age (years) | ||
| 55–70 (versus <55) | 0.84 (0.58–1.21) | 1.08 (0.73–1.62) |
| ≥71 (versus <55) | 0.96 (0.70–1.33) | 1.18 (0.81–1.72) |
| Female sex (versus male) | 1.45 (1.11–1.89) | 1.39 (1.05–1.85) |
| BMI | ||
| 25–29 (versus <25) | 0.97 (0.70–1.33) | 1.07 (0.77–1.48) |
| ≥30 (versus <25) | 1.65 (1.13–2.41) | 2.01 (1.35–2.99) |
| Comorbidities on admission | ||
| Hypertension | 0.70 (0.53–0.91) | 0.75 (0.56–0.99) |
| Diabetes mellitus | 0.70 (0.51–0.96) | 0.84 (0.59–1.21) |
| Chronic kidney disease | 0.73 (0.45–1.18) | 0.78 (0.47–1.31) |
| Hyperlipidemia | 0.91 (0.68–1.23) | 0.93 (0.68–1.28) |
| Ischemic heart disease | 0.81 (0.44–1.49) | 0.90 (0.48–1.68) |
| Atrial fibrillation | 0.87 (0.35–2.12) | 0.99 (0.40–2.45) |
| Cerebrovascular disease | 0.90 (0.51–1.59) | 1.03 (0.57–1.84) |
| Peripheral artery disease | 0.98 (0.36–2.67) | 1.04 (0.38–2.88) |
| Connective tissue disease | 1.19 (0.52–2.72) | 0.61 (0.24–1.58) |
| Malignancy | 1.60 (0.97–2.65) | 1.58 (0.92–2.70) |
| AKI | 2.26 (1.49–3.42) | 1.67 (1.07–2.62) |
| Lower limb fracture | 6.67 (2.26–19.7) | 7.43 (2.43–22.7) |
| Sepsis | 3.69 (1.89–7.19) | 2.85 (1.37–5.93) |
| Urinary tract infection | 1.80 (1.07–3.04) | 1.59 (0.93–2.72) |
| Pneumonia | 1.52 (0.79–2.90) | 1.33 (0.68–2.61) |
| Gastrointestinal infection | 1.51 (0.73–3.11) | 1.45 (0.69–3.01) |
| Primary disease of nephrotic syndrome | ||
| Minimal change nephrotic syndrome | 1.14 (0.81–1.59) | 1.09 (0.73–1.63) |
| Focal glomerular sclerosis | 1.75 (0.88–3.46) | 1.93 (0.94–3.94) |
| Membranous nephropathy | 1.27 (0.86–1.88) | 1.54 (1.00–2.37) |
| Membranoproliferative glomerulonephritis | 1.92 (0.69–5.31) | 2.56 (0.90–7.27) |
| IgA nephropathy | 1.65 (0.86–3.15) | 1.87 (0.94–3.70) |
| Diabetic nephropathy | 0.53 (0.32–0.86) | 0.69 (0.40–1.21) |
| Lupus nephritis | 2.92 (1.46–5.84) | 3.64 (1.58–8.37) |
| Medication on the day of admission | ||
| Oral corticosteroids, alonea | 0.79 (0.43–1.46) | 0.76 (0.39–1.46) |
| Intravenous corticosteroids | 2.74 (1.80–4.15) | 2.40 (1.52–3.80) |
| Immunosuppressive agents | 1.37 (0.60–3.13) | 1.21 (0.50–2.92) |
| Diuretics | 1.17 (0.88–1.56) | 1.15 (0.84–1.56) |
| Variables . | Univariable OR (95% CI) . | Multivariable OR (95% CI) . |
|---|---|---|
| Age (years) | ||
| 55–70 (versus <55) | 0.84 (0.58–1.21) | 1.08 (0.73–1.62) |
| ≥71 (versus <55) | 0.96 (0.70–1.33) | 1.18 (0.81–1.72) |
| Female sex (versus male) | 1.45 (1.11–1.89) | 1.39 (1.05–1.85) |
| BMI | ||
| 25–29 (versus <25) | 0.97 (0.70–1.33) | 1.07 (0.77–1.48) |
| ≥30 (versus <25) | 1.65 (1.13–2.41) | 2.01 (1.35–2.99) |
| Comorbidities on admission | ||
| Hypertension | 0.70 (0.53–0.91) | 0.75 (0.56–0.99) |
| Diabetes mellitus | 0.70 (0.51–0.96) | 0.84 (0.59–1.21) |
| Chronic kidney disease | 0.73 (0.45–1.18) | 0.78 (0.47–1.31) |
| Hyperlipidemia | 0.91 (0.68–1.23) | 0.93 (0.68–1.28) |
| Ischemic heart disease | 0.81 (0.44–1.49) | 0.90 (0.48–1.68) |
| Atrial fibrillation | 0.87 (0.35–2.12) | 0.99 (0.40–2.45) |
| Cerebrovascular disease | 0.90 (0.51–1.59) | 1.03 (0.57–1.84) |
| Peripheral artery disease | 0.98 (0.36–2.67) | 1.04 (0.38–2.88) |
| Connective tissue disease | 1.19 (0.52–2.72) | 0.61 (0.24–1.58) |
| Malignancy | 1.60 (0.97–2.65) | 1.58 (0.92–2.70) |
| AKI | 2.26 (1.49–3.42) | 1.67 (1.07–2.62) |
| Lower limb fracture | 6.67 (2.26–19.7) | 7.43 (2.43–22.7) |
| Sepsis | 3.69 (1.89–7.19) | 2.85 (1.37–5.93) |
| Urinary tract infection | 1.80 (1.07–3.04) | 1.59 (0.93–2.72) |
| Pneumonia | 1.52 (0.79–2.90) | 1.33 (0.68–2.61) |
| Gastrointestinal infection | 1.51 (0.73–3.11) | 1.45 (0.69–3.01) |
| Primary disease of nephrotic syndrome | ||
| Minimal change nephrotic syndrome | 1.14 (0.81–1.59) | 1.09 (0.73–1.63) |
| Focal glomerular sclerosis | 1.75 (0.88–3.46) | 1.93 (0.94–3.94) |
| Membranous nephropathy | 1.27 (0.86–1.88) | 1.54 (1.00–2.37) |
| Membranoproliferative glomerulonephritis | 1.92 (0.69–5.31) | 2.56 (0.90–7.27) |
| IgA nephropathy | 1.65 (0.86–3.15) | 1.87 (0.94–3.70) |
| Diabetic nephropathy | 0.53 (0.32–0.86) | 0.69 (0.40–1.21) |
| Lupus nephritis | 2.92 (1.46–5.84) | 3.64 (1.58–8.37) |
| Medication on the day of admission | ||
| Oral corticosteroids, alonea | 0.79 (0.43–1.46) | 0.76 (0.39–1.46) |
| Intravenous corticosteroids | 2.74 (1.80–4.15) | 2.40 (1.52–3.80) |
| Immunosuppressive agents | 1.37 (0.60–3.13) | 1.21 (0.50–2.92) |
| Diuretics | 1.17 (0.88–1.56) | 1.15 (0.84–1.56) |
Patients who used intravenous corticosteroids were not included.
Variables on admission associated with VTE in patients with nephrotic syndrome
| Variables . | Univariable OR (95% CI) . | Multivariable OR (95% CI) . |
|---|---|---|
| Age (years) | ||
| 55–70 (versus <55) | 0.84 (0.58–1.21) | 1.08 (0.73–1.62) |
| ≥71 (versus <55) | 0.96 (0.70–1.33) | 1.18 (0.81–1.72) |
| Female sex (versus male) | 1.45 (1.11–1.89) | 1.39 (1.05–1.85) |
| BMI | ||
| 25–29 (versus <25) | 0.97 (0.70–1.33) | 1.07 (0.77–1.48) |
| ≥30 (versus <25) | 1.65 (1.13–2.41) | 2.01 (1.35–2.99) |
| Comorbidities on admission | ||
| Hypertension | 0.70 (0.53–0.91) | 0.75 (0.56–0.99) |
| Diabetes mellitus | 0.70 (0.51–0.96) | 0.84 (0.59–1.21) |
| Chronic kidney disease | 0.73 (0.45–1.18) | 0.78 (0.47–1.31) |
| Hyperlipidemia | 0.91 (0.68–1.23) | 0.93 (0.68–1.28) |
| Ischemic heart disease | 0.81 (0.44–1.49) | 0.90 (0.48–1.68) |
| Atrial fibrillation | 0.87 (0.35–2.12) | 0.99 (0.40–2.45) |
| Cerebrovascular disease | 0.90 (0.51–1.59) | 1.03 (0.57–1.84) |
| Peripheral artery disease | 0.98 (0.36–2.67) | 1.04 (0.38–2.88) |
| Connective tissue disease | 1.19 (0.52–2.72) | 0.61 (0.24–1.58) |
| Malignancy | 1.60 (0.97–2.65) | 1.58 (0.92–2.70) |
| AKI | 2.26 (1.49–3.42) | 1.67 (1.07–2.62) |
| Lower limb fracture | 6.67 (2.26–19.7) | 7.43 (2.43–22.7) |
| Sepsis | 3.69 (1.89–7.19) | 2.85 (1.37–5.93) |
| Urinary tract infection | 1.80 (1.07–3.04) | 1.59 (0.93–2.72) |
| Pneumonia | 1.52 (0.79–2.90) | 1.33 (0.68–2.61) |
| Gastrointestinal infection | 1.51 (0.73–3.11) | 1.45 (0.69–3.01) |
| Primary disease of nephrotic syndrome | ||
| Minimal change nephrotic syndrome | 1.14 (0.81–1.59) | 1.09 (0.73–1.63) |
| Focal glomerular sclerosis | 1.75 (0.88–3.46) | 1.93 (0.94–3.94) |
| Membranous nephropathy | 1.27 (0.86–1.88) | 1.54 (1.00–2.37) |
| Membranoproliferative glomerulonephritis | 1.92 (0.69–5.31) | 2.56 (0.90–7.27) |
| IgA nephropathy | 1.65 (0.86–3.15) | 1.87 (0.94–3.70) |
| Diabetic nephropathy | 0.53 (0.32–0.86) | 0.69 (0.40–1.21) |
| Lupus nephritis | 2.92 (1.46–5.84) | 3.64 (1.58–8.37) |
| Medication on the day of admission | ||
| Oral corticosteroids, alonea | 0.79 (0.43–1.46) | 0.76 (0.39–1.46) |
| Intravenous corticosteroids | 2.74 (1.80–4.15) | 2.40 (1.52–3.80) |
| Immunosuppressive agents | 1.37 (0.60–3.13) | 1.21 (0.50–2.92) |
| Diuretics | 1.17 (0.88–1.56) | 1.15 (0.84–1.56) |
| Variables . | Univariable OR (95% CI) . | Multivariable OR (95% CI) . |
|---|---|---|
| Age (years) | ||
| 55–70 (versus <55) | 0.84 (0.58–1.21) | 1.08 (0.73–1.62) |
| ≥71 (versus <55) | 0.96 (0.70–1.33) | 1.18 (0.81–1.72) |
| Female sex (versus male) | 1.45 (1.11–1.89) | 1.39 (1.05–1.85) |
| BMI | ||
| 25–29 (versus <25) | 0.97 (0.70–1.33) | 1.07 (0.77–1.48) |
| ≥30 (versus <25) | 1.65 (1.13–2.41) | 2.01 (1.35–2.99) |
| Comorbidities on admission | ||
| Hypertension | 0.70 (0.53–0.91) | 0.75 (0.56–0.99) |
| Diabetes mellitus | 0.70 (0.51–0.96) | 0.84 (0.59–1.21) |
| Chronic kidney disease | 0.73 (0.45–1.18) | 0.78 (0.47–1.31) |
| Hyperlipidemia | 0.91 (0.68–1.23) | 0.93 (0.68–1.28) |
| Ischemic heart disease | 0.81 (0.44–1.49) | 0.90 (0.48–1.68) |
| Atrial fibrillation | 0.87 (0.35–2.12) | 0.99 (0.40–2.45) |
| Cerebrovascular disease | 0.90 (0.51–1.59) | 1.03 (0.57–1.84) |
| Peripheral artery disease | 0.98 (0.36–2.67) | 1.04 (0.38–2.88) |
| Connective tissue disease | 1.19 (0.52–2.72) | 0.61 (0.24–1.58) |
| Malignancy | 1.60 (0.97–2.65) | 1.58 (0.92–2.70) |
| AKI | 2.26 (1.49–3.42) | 1.67 (1.07–2.62) |
| Lower limb fracture | 6.67 (2.26–19.7) | 7.43 (2.43–22.7) |
| Sepsis | 3.69 (1.89–7.19) | 2.85 (1.37–5.93) |
| Urinary tract infection | 1.80 (1.07–3.04) | 1.59 (0.93–2.72) |
| Pneumonia | 1.52 (0.79–2.90) | 1.33 (0.68–2.61) |
| Gastrointestinal infection | 1.51 (0.73–3.11) | 1.45 (0.69–3.01) |
| Primary disease of nephrotic syndrome | ||
| Minimal change nephrotic syndrome | 1.14 (0.81–1.59) | 1.09 (0.73–1.63) |
| Focal glomerular sclerosis | 1.75 (0.88–3.46) | 1.93 (0.94–3.94) |
| Membranous nephropathy | 1.27 (0.86–1.88) | 1.54 (1.00–2.37) |
| Membranoproliferative glomerulonephritis | 1.92 (0.69–5.31) | 2.56 (0.90–7.27) |
| IgA nephropathy | 1.65 (0.86–3.15) | 1.87 (0.94–3.70) |
| Diabetic nephropathy | 0.53 (0.32–0.86) | 0.69 (0.40–1.21) |
| Lupus nephritis | 2.92 (1.46–5.84) | 3.64 (1.58–8.37) |
| Medication on the day of admission | ||
| Oral corticosteroids, alonea | 0.79 (0.43–1.46) | 0.76 (0.39–1.46) |
| Intravenous corticosteroids | 2.74 (1.80–4.15) | 2.40 (1.52–3.80) |
| Immunosuppressive agents | 1.37 (0.60–3.13) | 1.21 (0.50–2.92) |
| Diuretics | 1.17 (0.88–1.56) | 1.15 (0.84–1.56) |
Patients who used intravenous corticosteroids were not included.
In sensitivity analyses, after excluding 607 patients who were prescribed anticoagulants on admission, 6866 patients were included in the cohort. On multivariable logistic regression, female sex, BMI ≥30, AKI, sepsis, lupus nephritis and intravenous corticosteroid use were consistent with the results of the main analyses. The significant association between lower limb fracture and VTE disappeared. Focal glomerular sclerosis and membranous nephropathy were associated with a significantly higher risk of VTE in the sensitivity analysis (Table 4). In an analysis using multiple imputations, the significant association between membranous nephropathy and VTE disappeared (Supplementary data, Table S2).
Variables on admission associated with VTE in the population after excluding patients who were prescribed anticoagulants on admission
| Variables . | Univariable OR (95% CI) . | Multivariable OR (95% CI) . |
|---|---|---|
| Age (years) | ||
| 55–70 (versus <55) | 0.95 (0.63–1.43) | 1.13 (0.73–1.75) |
| ≥71 (versus <55) | 1.04 (0.72–1.49) | 1.17 (0.77–1.78) |
| Female sex (versus male) | 1.43 (1.07–1.92) | 1.43 (1.05–1.94) |
| BMI | ||
| 25–29 (versus <25) | 1.05 (0.75–1.47) | 1.15 (0.82–1.63) |
| ≥30 (versus <25) | 1.47 (0.95–2.27) | 1.77(1.12–2.80) |
| Comorbidities on admission | ||
| Hypertension | 0.71 (0.53–0.96) | 0.77 (0.56–1.06) |
| Diabetes mellitus | 0.74 (0.52–1.04) | 0.89 (0.60–1.32) |
| Chronic kidney disease | 0.73 (0.43–1.24) | 0.79 (0.45–1.38) |
| Hyperlipidemia | 0.78 (0.56–1.10) | 0.78 (0.54–1.11) |
| Ischemic heart disease | 0.84 (0.43–1.66) | 0.95 (0.48–1.90) |
| Atrial fibrillation | 1.02 (0.37–2.77) | 1.08 (0.39–3.00) |
| Cerebrovascular disease | 1.03 (0.57–1.86) | 1.15 (0.62–2.12) |
| Peripheral artery disease | 0.97 (0.31–3.08) | 1.05 (0.33–3.37) |
| Connective tissue disease | 1.45 (0.63–3.32) | 0.73 (0.28–1.90) |
| Malignancy | 1.67 (0.97–2.86) | 1.52 (0.85–2.70) |
| AKI | 2.68 (1.72–4.16) | 2.15 (1.34–3.46) |
| Lower limb fracture | 3.68 (0.85–15.9) | 4.30 (0.97–19.1) |
| Sepsis | 3.68 (1.75–7.72) | 2.49 (1.09–5.66) |
| Urinary tract infection | 1.49 (0.90–2.77) | 1.28 (0.68–2.41) |
| Pneumonia | 1.76 (0.89–3.49) | 1.51 (0.74–3.07) |
| Gastrointestinal infection | 1.41 (0.62–3.23) | 1.34 (0.58–3.11) |
| Primary disease of nephrotic syndrome | ||
| Minimal change nephrotic syndrome | 1.12 (0.77–1.64) | 1.24 (0.80–1.92) |
| Focal glomerular sclerosis | 2.21 (1.11–4.40) | 2.49 (1.20–5.14) |
| Membranous nephropathy | 1.30 (0.85–1.97) | 1.62 (1.02–2.57) |
| Membranoproliferative glomerulonephritis | 1.67 (0.52–5.37) | 2.29 (0.69–7.53) |
| IgA nephropathy | 1.15 (0.50–2.63) | 1.33 (0.56–3.15) |
| Diabetic nephropathy | 0.56 (0.33–0.93) | 0.74 (0.41–1.35) |
| Lupus nephritis | 3.63 (1.80–7.30) | 4.16 (1.78–9.73) |
| Medication on the day of admission | ||
| Oral corticosteroids, alonea | 0.56 (0.25–1.28) | 0.51 (0.22–1.22) |
| Intravenous corticosteroids | 2.29 (1.31–4.02) | 2.01 (1.11–3.62) |
| Immunosuppressive agents | 1.27 (0.46–3.47) | 1.41 (0.48–4.13) |
| Diuretics | 1.07 (0.78–1.49) | 1.07 (0.76–1.52) |
| Variables . | Univariable OR (95% CI) . | Multivariable OR (95% CI) . |
|---|---|---|
| Age (years) | ||
| 55–70 (versus <55) | 0.95 (0.63–1.43) | 1.13 (0.73–1.75) |
| ≥71 (versus <55) | 1.04 (0.72–1.49) | 1.17 (0.77–1.78) |
| Female sex (versus male) | 1.43 (1.07–1.92) | 1.43 (1.05–1.94) |
| BMI | ||
| 25–29 (versus <25) | 1.05 (0.75–1.47) | 1.15 (0.82–1.63) |
| ≥30 (versus <25) | 1.47 (0.95–2.27) | 1.77(1.12–2.80) |
| Comorbidities on admission | ||
| Hypertension | 0.71 (0.53–0.96) | 0.77 (0.56–1.06) |
| Diabetes mellitus | 0.74 (0.52–1.04) | 0.89 (0.60–1.32) |
| Chronic kidney disease | 0.73 (0.43–1.24) | 0.79 (0.45–1.38) |
| Hyperlipidemia | 0.78 (0.56–1.10) | 0.78 (0.54–1.11) |
| Ischemic heart disease | 0.84 (0.43–1.66) | 0.95 (0.48–1.90) |
| Atrial fibrillation | 1.02 (0.37–2.77) | 1.08 (0.39–3.00) |
| Cerebrovascular disease | 1.03 (0.57–1.86) | 1.15 (0.62–2.12) |
| Peripheral artery disease | 0.97 (0.31–3.08) | 1.05 (0.33–3.37) |
| Connective tissue disease | 1.45 (0.63–3.32) | 0.73 (0.28–1.90) |
| Malignancy | 1.67 (0.97–2.86) | 1.52 (0.85–2.70) |
| AKI | 2.68 (1.72–4.16) | 2.15 (1.34–3.46) |
| Lower limb fracture | 3.68 (0.85–15.9) | 4.30 (0.97–19.1) |
| Sepsis | 3.68 (1.75–7.72) | 2.49 (1.09–5.66) |
| Urinary tract infection | 1.49 (0.90–2.77) | 1.28 (0.68–2.41) |
| Pneumonia | 1.76 (0.89–3.49) | 1.51 (0.74–3.07) |
| Gastrointestinal infection | 1.41 (0.62–3.23) | 1.34 (0.58–3.11) |
| Primary disease of nephrotic syndrome | ||
| Minimal change nephrotic syndrome | 1.12 (0.77–1.64) | 1.24 (0.80–1.92) |
| Focal glomerular sclerosis | 2.21 (1.11–4.40) | 2.49 (1.20–5.14) |
| Membranous nephropathy | 1.30 (0.85–1.97) | 1.62 (1.02–2.57) |
| Membranoproliferative glomerulonephritis | 1.67 (0.52–5.37) | 2.29 (0.69–7.53) |
| IgA nephropathy | 1.15 (0.50–2.63) | 1.33 (0.56–3.15) |
| Diabetic nephropathy | 0.56 (0.33–0.93) | 0.74 (0.41–1.35) |
| Lupus nephritis | 3.63 (1.80–7.30) | 4.16 (1.78–9.73) |
| Medication on the day of admission | ||
| Oral corticosteroids, alonea | 0.56 (0.25–1.28) | 0.51 (0.22–1.22) |
| Intravenous corticosteroids | 2.29 (1.31–4.02) | 2.01 (1.11–3.62) |
| Immunosuppressive agents | 1.27 (0.46–3.47) | 1.41 (0.48–4.13) |
| Diuretics | 1.07 (0.78–1.49) | 1.07 (0.76–1.52) |
Patients who used intravenous corticosteroids were not included.
Variables on admission associated with VTE in the population after excluding patients who were prescribed anticoagulants on admission
| Variables . | Univariable OR (95% CI) . | Multivariable OR (95% CI) . |
|---|---|---|
| Age (years) | ||
| 55–70 (versus <55) | 0.95 (0.63–1.43) | 1.13 (0.73–1.75) |
| ≥71 (versus <55) | 1.04 (0.72–1.49) | 1.17 (0.77–1.78) |
| Female sex (versus male) | 1.43 (1.07–1.92) | 1.43 (1.05–1.94) |
| BMI | ||
| 25–29 (versus <25) | 1.05 (0.75–1.47) | 1.15 (0.82–1.63) |
| ≥30 (versus <25) | 1.47 (0.95–2.27) | 1.77(1.12–2.80) |
| Comorbidities on admission | ||
| Hypertension | 0.71 (0.53–0.96) | 0.77 (0.56–1.06) |
| Diabetes mellitus | 0.74 (0.52–1.04) | 0.89 (0.60–1.32) |
| Chronic kidney disease | 0.73 (0.43–1.24) | 0.79 (0.45–1.38) |
| Hyperlipidemia | 0.78 (0.56–1.10) | 0.78 (0.54–1.11) |
| Ischemic heart disease | 0.84 (0.43–1.66) | 0.95 (0.48–1.90) |
| Atrial fibrillation | 1.02 (0.37–2.77) | 1.08 (0.39–3.00) |
| Cerebrovascular disease | 1.03 (0.57–1.86) | 1.15 (0.62–2.12) |
| Peripheral artery disease | 0.97 (0.31–3.08) | 1.05 (0.33–3.37) |
| Connective tissue disease | 1.45 (0.63–3.32) | 0.73 (0.28–1.90) |
| Malignancy | 1.67 (0.97–2.86) | 1.52 (0.85–2.70) |
| AKI | 2.68 (1.72–4.16) | 2.15 (1.34–3.46) |
| Lower limb fracture | 3.68 (0.85–15.9) | 4.30 (0.97–19.1) |
| Sepsis | 3.68 (1.75–7.72) | 2.49 (1.09–5.66) |
| Urinary tract infection | 1.49 (0.90–2.77) | 1.28 (0.68–2.41) |
| Pneumonia | 1.76 (0.89–3.49) | 1.51 (0.74–3.07) |
| Gastrointestinal infection | 1.41 (0.62–3.23) | 1.34 (0.58–3.11) |
| Primary disease of nephrotic syndrome | ||
| Minimal change nephrotic syndrome | 1.12 (0.77–1.64) | 1.24 (0.80–1.92) |
| Focal glomerular sclerosis | 2.21 (1.11–4.40) | 2.49 (1.20–5.14) |
| Membranous nephropathy | 1.30 (0.85–1.97) | 1.62 (1.02–2.57) |
| Membranoproliferative glomerulonephritis | 1.67 (0.52–5.37) | 2.29 (0.69–7.53) |
| IgA nephropathy | 1.15 (0.50–2.63) | 1.33 (0.56–3.15) |
| Diabetic nephropathy | 0.56 (0.33–0.93) | 0.74 (0.41–1.35) |
| Lupus nephritis | 3.63 (1.80–7.30) | 4.16 (1.78–9.73) |
| Medication on the day of admission | ||
| Oral corticosteroids, alonea | 0.56 (0.25–1.28) | 0.51 (0.22–1.22) |
| Intravenous corticosteroids | 2.29 (1.31–4.02) | 2.01 (1.11–3.62) |
| Immunosuppressive agents | 1.27 (0.46–3.47) | 1.41 (0.48–4.13) |
| Diuretics | 1.07 (0.78–1.49) | 1.07 (0.76–1.52) |
| Variables . | Univariable OR (95% CI) . | Multivariable OR (95% CI) . |
|---|---|---|
| Age (years) | ||
| 55–70 (versus <55) | 0.95 (0.63–1.43) | 1.13 (0.73–1.75) |
| ≥71 (versus <55) | 1.04 (0.72–1.49) | 1.17 (0.77–1.78) |
| Female sex (versus male) | 1.43 (1.07–1.92) | 1.43 (1.05–1.94) |
| BMI | ||
| 25–29 (versus <25) | 1.05 (0.75–1.47) | 1.15 (0.82–1.63) |
| ≥30 (versus <25) | 1.47 (0.95–2.27) | 1.77(1.12–2.80) |
| Comorbidities on admission | ||
| Hypertension | 0.71 (0.53–0.96) | 0.77 (0.56–1.06) |
| Diabetes mellitus | 0.74 (0.52–1.04) | 0.89 (0.60–1.32) |
| Chronic kidney disease | 0.73 (0.43–1.24) | 0.79 (0.45–1.38) |
| Hyperlipidemia | 0.78 (0.56–1.10) | 0.78 (0.54–1.11) |
| Ischemic heart disease | 0.84 (0.43–1.66) | 0.95 (0.48–1.90) |
| Atrial fibrillation | 1.02 (0.37–2.77) | 1.08 (0.39–3.00) |
| Cerebrovascular disease | 1.03 (0.57–1.86) | 1.15 (0.62–2.12) |
| Peripheral artery disease | 0.97 (0.31–3.08) | 1.05 (0.33–3.37) |
| Connective tissue disease | 1.45 (0.63–3.32) | 0.73 (0.28–1.90) |
| Malignancy | 1.67 (0.97–2.86) | 1.52 (0.85–2.70) |
| AKI | 2.68 (1.72–4.16) | 2.15 (1.34–3.46) |
| Lower limb fracture | 3.68 (0.85–15.9) | 4.30 (0.97–19.1) |
| Sepsis | 3.68 (1.75–7.72) | 2.49 (1.09–5.66) |
| Urinary tract infection | 1.49 (0.90–2.77) | 1.28 (0.68–2.41) |
| Pneumonia | 1.76 (0.89–3.49) | 1.51 (0.74–3.07) |
| Gastrointestinal infection | 1.41 (0.62–3.23) | 1.34 (0.58–3.11) |
| Primary disease of nephrotic syndrome | ||
| Minimal change nephrotic syndrome | 1.12 (0.77–1.64) | 1.24 (0.80–1.92) |
| Focal glomerular sclerosis | 2.21 (1.11–4.40) | 2.49 (1.20–5.14) |
| Membranous nephropathy | 1.30 (0.85–1.97) | 1.62 (1.02–2.57) |
| Membranoproliferative glomerulonephritis | 1.67 (0.52–5.37) | 2.29 (0.69–7.53) |
| IgA nephropathy | 1.15 (0.50–2.63) | 1.33 (0.56–3.15) |
| Diabetic nephropathy | 0.56 (0.33–0.93) | 0.74 (0.41–1.35) |
| Lupus nephritis | 3.63 (1.80–7.30) | 4.16 (1.78–9.73) |
| Medication on the day of admission | ||
| Oral corticosteroids, alonea | 0.56 (0.25–1.28) | 0.51 (0.22–1.22) |
| Intravenous corticosteroids | 2.29 (1.31–4.02) | 2.01 (1.11–3.62) |
| Immunosuppressive agents | 1.27 (0.46–3.47) | 1.41 (0.48–4.13) |
| Diuretics | 1.07 (0.78–1.49) | 1.07 (0.76–1.52) |
Patients who used intravenous corticosteroids were not included.
DISCUSSION
This study investigated the association between risk factors on admission and VTE during hospitalization in patients with nephrotic syndrome using a nationwide administrative database in Japan. In this cohort, 3.0% of patients with nephrotic syndrome developed VTE and 0.19, 0.15 and 2.6% developed PE, RVT and DVT, respectively. Mahmoodi et al. [10] showed that the annual incidence of VTE in nephrotic patients was 1.02% . However, we obtained a higher proportion. The reported annual incidence is calculated based on long-term follow-up (∼10 years) and the annual incidence calculated based on the first 6 months of observation time was high (9.85%). Although our follow-up period is limited to the hospitalization period, the percentage (3.0%) obtained in our study may be lower during the first 6 months. Another reason may be due to the varying VTE detection methods used. The previous study verified symptomatic VTE using imaging findings in medical records, whereas we used diagnostic and procedure codes. The positive predictive value of identifying methods for VTE using an administrative database was reported to be ∼70% [25]. Thus we may have overestimated VTE occurrence. On the other hand, a recent database research study reported that the prevalence of PE and DVT in nephrotic patients was 0.5 and 1.5%, respectively [4]. The percentages in our analyses were slightly lower for PE and somewhat higher for DVT. Since Asians are reported to have a low frequency of VTE, the low proportion of PE could be explained [28]. Differences in the frequency of DVTs might be due to a difference in population size or varying methods of VTE identification. In the previous studies, VTE was identified using only ICD-10 codes, whereas we used a combination of ICD codes and imaging procedures.
In this study, female sex, BMI ≥30, AKI, sepsis, lupus nephritis and intravenous corticosteroids were associated with a higher risk of VTE in patients with nephrotic syndrome on our main and sensitivity analyses. Studies in Western populations suggest that male sex is associated with an increased risk of VTE [29, 30]. However, in our population, women had a higher risk of VTE than men. We checked the use of estrogen preparations in this population, but there was only one patient. Our results were consistent with the general Japanese population, in which women develop VTE more often than men [31]. Higher BMI is associated with an increased risk of VTE in the general population [32]. However, a high BMI in nephrotic syndrome may be due not only to obesity, but also excess in fluid volume related to edema, since nephrotic patients tend to have edema that causes weight gain. Edema is related to hypoalbuminemia, an independent predictor of VTE in nephrotic syndrome [33, 34].
AKI is one complication in patients with nephrotic syndrome. Its cause is multifactorial and may be secondary to factors such as vascular volume depletion and severe hypoalbuminemia, both of which are common risk factors of VTE [34, 35]. In our study, AKI may be identified as a risk factor and may be a surrogate for hypoalbuminemia or vascular volume depletion, neither of which was identified in this database. Patients with sepsis in the general population have a marked prevalence (∼3- to 10-fold higher) of VTE compared with those who were admitted to the ICU for other medical conditions [36]. Although little is known about the association between infection and nephrotic syndrome, one study reported that 11.4% of patients with primary nephrotic syndrome in a hospital had infections and the most common type was pulmonary infections [37]. In our results, urinary tract infections, pneumonia and gastrointestinal infections had no significant association with VTE. Dysregulated hemostasis and coagulation in sepsis [36] may interact synergistically with the hypercoagulable state of nephrotic syndrome.
We found that the use of intravenous corticosteroids was associated with VTE, whereas the use of oral glucocorticoids was not. The risk of VTE was high in patients who used glucocorticoids in the general population [38–40]. A previous study showed that oral glucocorticoids were associated with a higher risk of VTE than injectables, but the risk increased as the cumulative dose increased [39]. The dose, rather than the route of administration, may have been responsible for the occurrence of VTE. In addition, intravenous corticosteroids in nephrotic patients may be given as steroid pulse therapy and may be used as an alternative to oral glucocorticoid therapy in those with severe nephrotic syndrome [41]. Thus the use of intravenous corticosteroids may reflect the severity of the nephrotic syndrome.
In our study, we investigated the association between each renal disease and VTE. It is well known that membranous nephropathy and malignancy are strongly associated with an increased risk of VTE [11, 12, 15, 42]. However, no consistent results were obtained through the main and sensitivity analyses. Lack of power may have caused these results. Although few studies have examined the relationship between VTE and lupus nephritis, SLE and antiphospholipid syndrome are known risk factors of VTE. The incidence rate of VTE in patients with SLE is 25- to 50-fold higher than that in the general population [43]. Lupus nephritis with nephrotic syndrome should be recognized as an independent risk factor of VTE.
The strength of this study, using a nationwide administrative database, was that it investigated various risk factors of VTE in patients with nephrotic syndrome. Although the risk factors that we revealed are already known as risk factors for VTE in the general population, these factors may have additional implications in patients with nephrotic syndrome. This is evident in the necessary consideration of systemic edema in patients with a BMI ≥30, which is considered a risk factor of VTE. Variables such as AKI and sepsis, which cause systemic hemodynamic changes, may individually or simultaneously affect VTE occurrence. Furthermore, to our knowledge, this is the first study to investigate the association between nephrotic syndrome and VTE using a large database in Asia. Thus we could determine the occurrence of PE and RVT, which are considered to be rare.
There are several limitations to this study. First, we could not reliably determine the incidence of VTE during hospitalization. We used DPC codes and performed imaging procedures during hospitalization to identify VTE. We considered adding another criterion, such as an anticoagulant prescription. However, it would lead to a further reduction in sensitivity and negative predictive value [25]. Second, the comorbidities that we evaluated on admission were underestimated because only a maximum of four comorbidities can be registered in the DPC database. Third, we could not evaluate long-term outcomes, as the follow-up period consisted of the hospitalization duration due to the use of the database during this period. It is known that one of the risks of VTE is hospitalization [44]. In our study, there was a difference in the length of hospital stay in the VTE and non-VTE groups. Longer hospital stays in the VTE group may have contributed to the development of VTE. The severity of nephrotic syndrome causing long-term hospitalization may have affected the length of hospital stay. Fourth, we could not distinguish between systemic edema and obesity because we could not evaluate the dry weight in this database. Fifth, the VTE group had a high frequency of anticoagulant drugs prescribed. However, the prophylactic or therapeutic prescription of these drugs could not be distinguished. Finally, we could not obtain clinical data such as results of laboratory examinations (including essential confounding factors such as serum creatinine, serum albumin and urinary protein) and pathological diagnoses that were proven by renal biopsy in this administrative database. In addition, the severity of the nephrotic syndrome and the stage of AKI were difficult to estimate.
CONCLUSION
We found the following risk factors for VTE during hospitalization in patients with nephrotic syndrome using a nationwide administrative database: female sex, BMI ≥30, AKI, sepsis, lupus nephritis and intravenous corticosteroids use. These variables may help evaluate the risk of VTE in patients with nephrotic syndrome. Further studies using a primary database that contains more detailed clinical data are necessary to confirm our findings.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
ACKNOWLEDGEMENTS
We received no funds for this study. The authors thank Medical Data Vision for providing valuable data.
AUTHORS’ CONTRIBUTIONS
K.S. conceived of the study. K.S., S.Y., T.S., M.Y. and K.K. designed the study. K.S. performed the data analysis and wrote the manuscript. All authors revised the manuscript.
CONFLICT OF INTEREST STATEMENT
T.S. received personal fees outside the submitted work from Pfizer. M.Y. received research grants from Astellas, Chugai, Daiichi Sankyo, Fujiyakuhin, Kyowa Hakko Kirin, Mitsubishi Tanabe, MSD, Nippon Boehringer Ingelheim and Torii. K.K. received research funds from Sumitomo Dainippon Pharma, Stella Pharma, CMIC, Suntory Beverage & Food and Bayer Yakuhin; consulting fees or speaker honoraria from Kyowa Hakko Kirin, Kaken Pharmaceutical, Astellas Pharma, Mitsubishi Tanabe Pharma, AbbVie, Santen Pharmaceutical, Daiichi Sankyo, Takeda Pharmaceutical and Boehringer Ingelheim Japan and is a stockholder of Real World Data. There are no patented products under development or marketed products to declare relevant to these companies. K.S. and S.Y. have no conflict of interest to disclose.
(See related article by Lobbes et al. Prevention of venous thromboembolism in nephrotic syndrome: the quest towards precision medicine. Nephrol Dial Transplant 2021; 36: 1151–1154)
REFERENCES
JCS Joint Working Group. et al.
Institute of Social and Preventive Medicine, University of Bern. STROBE Statement: Strengthening the reporting of observational studies in epidemiologyhttp://www.strobe-statement.org/index.php?id=strobe-home (30 July 2019, date last accessed)





Comments