-
PDF
- Split View
-
Views
-
Cite
Cite
Lily Jakulj, Anneke Kramer, Anders Åsberg, Johan de Meester, Carmen Santiuste de Pablos, Jaakko Helve, Marc H Hemmelder, Alexandre Hertig, Mustafa Arici, Samira Bell, Lucile Mercadal, Carmen Diaz-Corte, Runolfur Palsson, Manuel Benitez Sanchez, Julia Kerschbaum, Frederic Collart, Ziad A Massy, Kitty J Jager, Marlies Noordzij, Recovery of kidney function in patients treated with maintenance dialysis—a report from the ERA-EDTA Registry, Nephrology Dialysis Transplantation, Volume 36, Issue 6, June 2021, Pages 1078–1087, https://doi.org/10.1093/ndt/gfaa368
Close - Share Icon Share
Abstract
Literature on recovery of kidney function (RKF) in patients with end-stage kidney disease treated with maintenance dialysis (i.e. >90 days) is limited. We assessed the incidence of RKF and its associated characteristics in a European cohort of dialysis patients.
We included adult patients from the European Renal Association–European Dialysis and Transplant Association Registry who started maintenance dialysis in 1997–2016. Sustained RKF was defined as permanent discontinuation of dialysis. Temporary discontinuation of ≥30 days (non-sustained RKF) was also evaluated. Factors associated with RKF adjusted for potential confounders were studied using Cox regression analyses.
RKF occurred in 7657 (1.8%) of 440 996 patients, of whom 71% experienced sustained RKF. Approximately 90% of all recoveries occurred within the first 2 years after Day 91 of dialysis. Of patients with non-sustained RKF, 39% restarted kidney replacement therapy within 1 year. Sustained RKF was strongly associated with the following underlying kidney diseases (as registered by the treating physician): tubular necrosis (irreversible) or cortical necrosis {adjusted hazard ratio [aHR] 20.4 [95% confidence interval (CI) 17.9–23.1]}, systemic sclerosis [aHR 18.5 (95% CI 13.8–24.7)] and haemolytic uremic syndrome [aHR 17.3 (95% CI 13.9–21.6)]. Weaker associations were found for haemodialysis as a first dialysis modality [aHR 1.5 (95% CI 1.4–1.6)] and dialysis initiation at an older age [aHR 1.8 (95% CI 1.6–2.0)] or in a more recent time period [aHR 2.4 (95% CI 2.1–2.7)].
Definitive discontinuation of maintenance dialysis is a rare and not necessarily an early event. Certain clinical characteristics, but mostly the type of underlying kidney disease, are associated with a higher likelihood of RKF.
What is already known about this subject?
The incidence of recovery of kidney function (RKF) in patients treated with maintenance dialysis varies in the literature, possibly due to varying definitions of end-stage kidney disease (ESKD) and RKF.
Hence the incidence might be overestimated due to inclusion of patients with acute kidney injury (AKI) instead of ESKD or by inclusion of patients who could only temporarily discontinue dialysis treatment (i.e. non-sustained RKF).
What this study adds?
This study shows a 1.2% incidence of sustained RKF in patients who have been treated with maintenance dialysis for at least 90 days.
Sustained RKF is not necessarily an early event, as nearly half of the patients experienced sustained RKF after at least 1 year of maintenance dialysis.
Sustained RKF is most prevalent in patients with certain underlying primary kidney diseases, such as tubular necrosis (irreversible) or cortical necrosis, systemic sclerosis and haemolytic uremic syndrome.
What impact this may have on practice or policy?
Clinicians should always be vigilant for RKF in patients treated with maintenance dialysis, not only in the first few months after initiation of the treatment.
This is of particular importance in a patient with an underlying kidney disease that is associated with sustained RKF.
INTRODUCTION
Although end-stage kidney disease (ESKD) generally describes irreversible kidney failure requiring kidney replacement therapy (KRT), a small percentage of patients treated with maintenance dialysis (i.e. dialysis for >90 days) experience recovery of kidney function (RKF). This can result in a reduction of dialysis dose or even permanent discontinuation of dialysis treatment.
Data on the incidence of RKF in patients treated with maintenance dialysis in Europe are limited to small case series [1, 2] and single-centre [3, 4] and single-country studies [5]. Reports from observational studies and registries in other parts of the world show that RKF occurs in 1.0–6.7% of dialysis patients [6–10]. However, these studies express wide heterogeneity regarding the definitions used for ESKD and RKF. Several studies describe RKF as occurring within the first 90 days of dialysis treatment, thereby probably including a substantial number of patients with acute kidney injury (AKI) rather than ESKD. Moreover, previous studies include both patients with sustained recoveries, i.e. permanent discontinuation of KRT and patients who had to recommence KRT after variable, sometimes very short-lived KRT-free intervals. Hence literature on the incidence and associated clinical characteristics of definitive discontinuation of maintenance dialysis in patients with ESKD is currently limited to a report from the Australian and New Zealand Dialysis and Transplant (ANZDATA) Registry published in 2009. In that study, RKF was found to occur in 1% of the ∼40 000 individuals treated with maintenance dialysis between 1963 and 2006 [7]. Therefore we assessed the incidence of sustained as well as non-sustained RKF and its associated demographic and clinical characteristics in a large cohort of patients with ESKD treated with maintenance dialysis in Europe.
MATERIALS AND METHODS
Patient characteristics
Data of patients with ESKD ≥20 years of age who started dialysis as their first KRT modality between 1997 and 2016 and who received maintenance dialysis treatment, i.e. dialysis for at least 90 days, were extracted from the European Renal Association–European Dialysis Transplantation Association (ERA-EDTA) Registry database. Follow-up continued until 31 December 2017.
The ERA-EDTA Registry annually collects individual data of patients starting KRT from national and regional renal registries in Europe. Methods of data collection and processing are described in detail elsewhere [11]. Data from the following 27 registries were included: Austria; Dutch-speaking Belgium; French-speaking Belgium; Bosnia and Herzegovina; Denmark; Finland; France; Greece; Iceland; the Netherlands; Norway; the Spanish regional renal registries of Andalusia, Aragon, Asturias, Basque Country, Cantabria, Castile and León, Castile-La Mancha, Catalonia, Extremadura, Galicia, Community of Madrid, Murcia and Valencia (inclusion until 2015, follow-up until 31 December 2016); Sweden; UK (England, Northern Ireland and Wales) and UK (Scotland). National and regional registries contributing data to the ERA-EDTA Registry complied with national legislation with regard to ethics committee approval. We extracted the following patient characteristics: age, sex, dialysis modality at onset, dialysis modality at Day 91, country of residence and underlying kidney disease, coded as primary renal disease (PRD). The latter was recorded by the responsible physician and classified according to the coding system of the ERA-EDTA [11]. We grouped these PRD codes into 20 PRD categories, as shown in Supplementary data, Table S1. The dataset contained no missing values for month or year of birth, sex or dialysis modality, and in the case of PRD, 0.9% of values were missing.
RKF
RKF was defined as a discontinuation of maintenance dialysis while remaining alive for at least 30 days. Sustained RKF was defined as no return to KRT until death or the end of the study period after at least 30 days of KRT-free survival. Patients who returned to KRT within the follow-up period after a KRT-free interval of at least 30 days were considered to have experienced non-sustained RKF.
Follow-up
Patients were followed from Day 91 of dialysis treatment until any of the following events: RKF, kidney transplantation, loss to follow-up, death or end of the study period (31 December 2017). As patients were included from December 1996 until 31 December 2015, potential follow-up was at least 2 years.
Statistical analyses
Data are presented as percentages for categorical variables or as means with standard deviation or median with interquartile range (IQR; 25th–75th percentile) for continuous variables. To study differences between groups, P-values were calculated using chi-squared tests for categorical variables and Wilcoxon tests for continuous variables.
We used the cumulative incidence competing risk approach to study the time from Day 91 of dialysis to RKF, in which kidney transplantation, loss to follow-up, death and end of the study period were taken into account as competing events [12]. Unadjusted and adjusted Cox regression analyses were used to determine factors associated with RKF. The variables included in the adjusted models were age, sex, year of onset of dialysis, dialysis modality at Day 91, underlying kidney disease and country of residence. In addition, we used the cumulative incidence competing risk approach to study time from Day 31 after RKF to death, restart of dialysis or kidney transplantation. In all analyses, follow-up time was censored at 5 years or at the end of the study period (31 December 2017).
To explore trends over time in the occurrence of RKF, the incidence of RKF was evaluated by dividing the number of RKF cases among patients starting dialysis in each year by the total number of incident dialysis patients in that year. As in our dataset, 90% of recoveries occurred within 2 years after the start of maintenance dialysis, we ceased inclusion on 31 December 2015 to allow sufficient follow-up time for RKF to occur. For these analyses, we only included data from registries with available data for the complete time period of 1997–2015. The slope of the trends, i.e. the annual percentage change (APC), was computed using Poisson regression as provided by the Joinpoint regression programme version 4.0.4 (National Cancer Institute, Bethesda, MD, USA). Details of this method have been previously described [13]. Statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA) or Joinpoint 4.0.4. P-values <0.05 were considered statistically significant.
RESULTS
Patient characteristics
Our cohort comprised 440 996 patients who started maintenance dialysis (i.e. dialysis for >90 days) between 1997 and 2016 (Table 1). A total of 7657 patients (1.8%) were reported to have experienced RKF that lasted for at least 30 days. The majority of these patients [n = 5465 (71%)] experienced sustained RKF. Their characteristics, as well as those of patients with a non-sustained RKF, are listed in Table 1.
Characteristics of patients on maintenance dialysis who started treatment between 1997 and 2016 and those who experienced RKF
| Characteristics . | Maintenance dialysis (n = 440 996) . | Sustained RKF (n = 5465) . | Non-sustained RKF (n = 2192) . | P-value . |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Females | 163 188 (37.0) | 2239 (41.0) | 761 (34.7) | |
| Males | 277 808 (63.0) | 3226 (59.0) | 1431 (65.3) | <0.001 |
| Age at onset (years), median (IQR) | 67.9 (55.8–76.5) | 69.7 (59.6–77.0) | 64.2 (52.0–72.2) | <0.001 |
| Age categories (years), n (%) | ||||
| 20–39 | 33 878 (7.7) | 260 (4.8) | 239 (10.9) | <0.001 |
| 40–59 | 10 8671 (24.6) | 1131 (20.7) | 628 (28.6) | <0.001 |
| 60–79 | 232 786 (52.8) | 3215 (58.8) | 1189 (54.2) | <0.001 |
| 80–100 | 65 661 (14.9) | 859 (15.7) | 136 (6.2) | <0.001 |
| PRD group, n (%) | ||||
| Diabetes mellitus types 1 and 2 | 106 100 (24.1) | 603 (11.0) | 374 (17.1) | <0.001 |
| Cause unknown or missing | 87 772 (19.9) | 1247 (22.8) | 325 (14.8) | <0.001 |
| Hypertension | 63 366 (14.4) | 530 (9.7) | 299 (13.6) | <0.001 |
| Cystic kidney disease | 28 925 (6.6) | 17 (0.3) | 40 (1.8) | <0.001 |
| Glomerulonephritis | 28 570 (6.5) | 442 (8.1) | 211 (9.6) | 0.029 |
| Pyelonephritis/infectious | 24 718 (5.6) | 295 (5.4) | 136 (6.2) | 0.166 |
| Renovascular disease | 18 553 (4.2) | 291 (5.3) | 101 (4.6) | 0.198 |
| IgA nephropathy | 13 300 (3.0) | 120 (2.2) | 53 (2.4) | 0.554 |
| Paraproteinaemia | 11 461 (2.6) | 451 (8.3) | 107 (4.9) | <0.001 |
| TIN and drug-induced | 11 277 (2.6) | 246 (4.5) | 81 (3.7) | 0.115 |
| Vasculitis | 9514 (2.2) | 295 (5.4) | 164 (7.5) | <0.001 |
| FSGS with nephrotic syndrome | 7456 (1.7) | 40 (0.7) | 29 (1.3) | 0.013 |
| Ischaemic renal disease/cholesterol embolism | 6440 (1.5) | 158 (2.9) | 53 (2.4) | 0.253 |
| Kidney tumour/trauma/loss | 5652 (1.3) | 53 (1.0) | 15 (0.7) | 0.229 |
| Congenital kidney disease | 4926 (1.1) | 14 (0.3) | 24 (1.1) | <0.001 |
| Membraneous nephropathy | 4114 (0.9) | 44 (0.8) | 25 (1.1) | 0.160 |
| Miscellaneous | 3689 (0.8) | 70 (1.3) | 32 (1.5) | 0.537 |
| Tubular necrosis (irreversible) or cortical necrosis | 3450 (0.8) | 404 (7.4) | 86 (3.9) | <0.001 |
| HUS | 1167 (0.3) | 95 (1.7) | 30 (1.4) | 0.249 |
| Systemic sclerosis | 546 (0.1) | 50 (0.9) | 7 (0.3) | 0.006 |
| KRT modality at Day 91, n (%) | ||||
| HD | 361 549 (82.0) | 4821 (88.2) | 1707 (77.9) | <0.001 |
| PD | 79 447 (18.0) | 644 (11.8) | 485 (22.1) | <0.001 |
| KRT modality at RKF, n (%) | ||||
| HD | – | 4702 (86.0) | 1680 (76.6) | <0.001 |
| PD | – | 753 (13.8) | 508 (23.2) | <0.001 |
| Unknown | – | 10 (0.2) | 4 (0.2) | 0.996 |
| Duration of RKF, n (%) | ||||
| 30–90 days | – | – | 264 (12.0) | – |
| 90–365 days | – | – | 590 (26.9) | – |
| 1–2 years | – | – | 455 (20.8) | – |
| 2–5 years | – | – | 614 (28.0) | – |
| >5 years | – | – | 269 (12.3) | – |
| KRT modality after non-sustained RKF, n (%) | ||||
| HD | – | – | 1749 (79.8) | – |
| PD | – | – | 347 (15.8) | – |
| Transplantation | – | – | 96 (4.4) | – |
| Characteristics . | Maintenance dialysis (n = 440 996) . | Sustained RKF (n = 5465) . | Non-sustained RKF (n = 2192) . | P-value . |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Females | 163 188 (37.0) | 2239 (41.0) | 761 (34.7) | |
| Males | 277 808 (63.0) | 3226 (59.0) | 1431 (65.3) | <0.001 |
| Age at onset (years), median (IQR) | 67.9 (55.8–76.5) | 69.7 (59.6–77.0) | 64.2 (52.0–72.2) | <0.001 |
| Age categories (years), n (%) | ||||
| 20–39 | 33 878 (7.7) | 260 (4.8) | 239 (10.9) | <0.001 |
| 40–59 | 10 8671 (24.6) | 1131 (20.7) | 628 (28.6) | <0.001 |
| 60–79 | 232 786 (52.8) | 3215 (58.8) | 1189 (54.2) | <0.001 |
| 80–100 | 65 661 (14.9) | 859 (15.7) | 136 (6.2) | <0.001 |
| PRD group, n (%) | ||||
| Diabetes mellitus types 1 and 2 | 106 100 (24.1) | 603 (11.0) | 374 (17.1) | <0.001 |
| Cause unknown or missing | 87 772 (19.9) | 1247 (22.8) | 325 (14.8) | <0.001 |
| Hypertension | 63 366 (14.4) | 530 (9.7) | 299 (13.6) | <0.001 |
| Cystic kidney disease | 28 925 (6.6) | 17 (0.3) | 40 (1.8) | <0.001 |
| Glomerulonephritis | 28 570 (6.5) | 442 (8.1) | 211 (9.6) | 0.029 |
| Pyelonephritis/infectious | 24 718 (5.6) | 295 (5.4) | 136 (6.2) | 0.166 |
| Renovascular disease | 18 553 (4.2) | 291 (5.3) | 101 (4.6) | 0.198 |
| IgA nephropathy | 13 300 (3.0) | 120 (2.2) | 53 (2.4) | 0.554 |
| Paraproteinaemia | 11 461 (2.6) | 451 (8.3) | 107 (4.9) | <0.001 |
| TIN and drug-induced | 11 277 (2.6) | 246 (4.5) | 81 (3.7) | 0.115 |
| Vasculitis | 9514 (2.2) | 295 (5.4) | 164 (7.5) | <0.001 |
| FSGS with nephrotic syndrome | 7456 (1.7) | 40 (0.7) | 29 (1.3) | 0.013 |
| Ischaemic renal disease/cholesterol embolism | 6440 (1.5) | 158 (2.9) | 53 (2.4) | 0.253 |
| Kidney tumour/trauma/loss | 5652 (1.3) | 53 (1.0) | 15 (0.7) | 0.229 |
| Congenital kidney disease | 4926 (1.1) | 14 (0.3) | 24 (1.1) | <0.001 |
| Membraneous nephropathy | 4114 (0.9) | 44 (0.8) | 25 (1.1) | 0.160 |
| Miscellaneous | 3689 (0.8) | 70 (1.3) | 32 (1.5) | 0.537 |
| Tubular necrosis (irreversible) or cortical necrosis | 3450 (0.8) | 404 (7.4) | 86 (3.9) | <0.001 |
| HUS | 1167 (0.3) | 95 (1.7) | 30 (1.4) | 0.249 |
| Systemic sclerosis | 546 (0.1) | 50 (0.9) | 7 (0.3) | 0.006 |
| KRT modality at Day 91, n (%) | ||||
| HD | 361 549 (82.0) | 4821 (88.2) | 1707 (77.9) | <0.001 |
| PD | 79 447 (18.0) | 644 (11.8) | 485 (22.1) | <0.001 |
| KRT modality at RKF, n (%) | ||||
| HD | – | 4702 (86.0) | 1680 (76.6) | <0.001 |
| PD | – | 753 (13.8) | 508 (23.2) | <0.001 |
| Unknown | – | 10 (0.2) | 4 (0.2) | 0.996 |
| Duration of RKF, n (%) | ||||
| 30–90 days | – | – | 264 (12.0) | – |
| 90–365 days | – | – | 590 (26.9) | – |
| 1–2 years | – | – | 455 (20.8) | – |
| 2–5 years | – | – | 614 (28.0) | – |
| >5 years | – | – | 269 (12.3) | – |
| KRT modality after non-sustained RKF, n (%) | ||||
| HD | – | – | 1749 (79.8) | – |
| PD | – | – | 347 (15.8) | – |
| Transplantation | – | – | 96 (4.4) | – |
FSGS, focal segmental glomerulosclerosis; IgA, immunoglobulin A; TIN, tubulo-interstitial nephritis.
Paraproteinaemia includes multiple myeloma, light chain nephropathy and amyloidosis.
For categorical variables, P-values were calculated with chi-squared tests, for continuous variables the Wilcoxon test was applied. Percentages are column percentages. P-values comparing sustained and non-sustained RKF.
Characteristics of patients on maintenance dialysis who started treatment between 1997 and 2016 and those who experienced RKF
| Characteristics . | Maintenance dialysis (n = 440 996) . | Sustained RKF (n = 5465) . | Non-sustained RKF (n = 2192) . | P-value . |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Females | 163 188 (37.0) | 2239 (41.0) | 761 (34.7) | |
| Males | 277 808 (63.0) | 3226 (59.0) | 1431 (65.3) | <0.001 |
| Age at onset (years), median (IQR) | 67.9 (55.8–76.5) | 69.7 (59.6–77.0) | 64.2 (52.0–72.2) | <0.001 |
| Age categories (years), n (%) | ||||
| 20–39 | 33 878 (7.7) | 260 (4.8) | 239 (10.9) | <0.001 |
| 40–59 | 10 8671 (24.6) | 1131 (20.7) | 628 (28.6) | <0.001 |
| 60–79 | 232 786 (52.8) | 3215 (58.8) | 1189 (54.2) | <0.001 |
| 80–100 | 65 661 (14.9) | 859 (15.7) | 136 (6.2) | <0.001 |
| PRD group, n (%) | ||||
| Diabetes mellitus types 1 and 2 | 106 100 (24.1) | 603 (11.0) | 374 (17.1) | <0.001 |
| Cause unknown or missing | 87 772 (19.9) | 1247 (22.8) | 325 (14.8) | <0.001 |
| Hypertension | 63 366 (14.4) | 530 (9.7) | 299 (13.6) | <0.001 |
| Cystic kidney disease | 28 925 (6.6) | 17 (0.3) | 40 (1.8) | <0.001 |
| Glomerulonephritis | 28 570 (6.5) | 442 (8.1) | 211 (9.6) | 0.029 |
| Pyelonephritis/infectious | 24 718 (5.6) | 295 (5.4) | 136 (6.2) | 0.166 |
| Renovascular disease | 18 553 (4.2) | 291 (5.3) | 101 (4.6) | 0.198 |
| IgA nephropathy | 13 300 (3.0) | 120 (2.2) | 53 (2.4) | 0.554 |
| Paraproteinaemia | 11 461 (2.6) | 451 (8.3) | 107 (4.9) | <0.001 |
| TIN and drug-induced | 11 277 (2.6) | 246 (4.5) | 81 (3.7) | 0.115 |
| Vasculitis | 9514 (2.2) | 295 (5.4) | 164 (7.5) | <0.001 |
| FSGS with nephrotic syndrome | 7456 (1.7) | 40 (0.7) | 29 (1.3) | 0.013 |
| Ischaemic renal disease/cholesterol embolism | 6440 (1.5) | 158 (2.9) | 53 (2.4) | 0.253 |
| Kidney tumour/trauma/loss | 5652 (1.3) | 53 (1.0) | 15 (0.7) | 0.229 |
| Congenital kidney disease | 4926 (1.1) | 14 (0.3) | 24 (1.1) | <0.001 |
| Membraneous nephropathy | 4114 (0.9) | 44 (0.8) | 25 (1.1) | 0.160 |
| Miscellaneous | 3689 (0.8) | 70 (1.3) | 32 (1.5) | 0.537 |
| Tubular necrosis (irreversible) or cortical necrosis | 3450 (0.8) | 404 (7.4) | 86 (3.9) | <0.001 |
| HUS | 1167 (0.3) | 95 (1.7) | 30 (1.4) | 0.249 |
| Systemic sclerosis | 546 (0.1) | 50 (0.9) | 7 (0.3) | 0.006 |
| KRT modality at Day 91, n (%) | ||||
| HD | 361 549 (82.0) | 4821 (88.2) | 1707 (77.9) | <0.001 |
| PD | 79 447 (18.0) | 644 (11.8) | 485 (22.1) | <0.001 |
| KRT modality at RKF, n (%) | ||||
| HD | – | 4702 (86.0) | 1680 (76.6) | <0.001 |
| PD | – | 753 (13.8) | 508 (23.2) | <0.001 |
| Unknown | – | 10 (0.2) | 4 (0.2) | 0.996 |
| Duration of RKF, n (%) | ||||
| 30–90 days | – | – | 264 (12.0) | – |
| 90–365 days | – | – | 590 (26.9) | – |
| 1–2 years | – | – | 455 (20.8) | – |
| 2–5 years | – | – | 614 (28.0) | – |
| >5 years | – | – | 269 (12.3) | – |
| KRT modality after non-sustained RKF, n (%) | ||||
| HD | – | – | 1749 (79.8) | – |
| PD | – | – | 347 (15.8) | – |
| Transplantation | – | – | 96 (4.4) | – |
| Characteristics . | Maintenance dialysis (n = 440 996) . | Sustained RKF (n = 5465) . | Non-sustained RKF (n = 2192) . | P-value . |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Females | 163 188 (37.0) | 2239 (41.0) | 761 (34.7) | |
| Males | 277 808 (63.0) | 3226 (59.0) | 1431 (65.3) | <0.001 |
| Age at onset (years), median (IQR) | 67.9 (55.8–76.5) | 69.7 (59.6–77.0) | 64.2 (52.0–72.2) | <0.001 |
| Age categories (years), n (%) | ||||
| 20–39 | 33 878 (7.7) | 260 (4.8) | 239 (10.9) | <0.001 |
| 40–59 | 10 8671 (24.6) | 1131 (20.7) | 628 (28.6) | <0.001 |
| 60–79 | 232 786 (52.8) | 3215 (58.8) | 1189 (54.2) | <0.001 |
| 80–100 | 65 661 (14.9) | 859 (15.7) | 136 (6.2) | <0.001 |
| PRD group, n (%) | ||||
| Diabetes mellitus types 1 and 2 | 106 100 (24.1) | 603 (11.0) | 374 (17.1) | <0.001 |
| Cause unknown or missing | 87 772 (19.9) | 1247 (22.8) | 325 (14.8) | <0.001 |
| Hypertension | 63 366 (14.4) | 530 (9.7) | 299 (13.6) | <0.001 |
| Cystic kidney disease | 28 925 (6.6) | 17 (0.3) | 40 (1.8) | <0.001 |
| Glomerulonephritis | 28 570 (6.5) | 442 (8.1) | 211 (9.6) | 0.029 |
| Pyelonephritis/infectious | 24 718 (5.6) | 295 (5.4) | 136 (6.2) | 0.166 |
| Renovascular disease | 18 553 (4.2) | 291 (5.3) | 101 (4.6) | 0.198 |
| IgA nephropathy | 13 300 (3.0) | 120 (2.2) | 53 (2.4) | 0.554 |
| Paraproteinaemia | 11 461 (2.6) | 451 (8.3) | 107 (4.9) | <0.001 |
| TIN and drug-induced | 11 277 (2.6) | 246 (4.5) | 81 (3.7) | 0.115 |
| Vasculitis | 9514 (2.2) | 295 (5.4) | 164 (7.5) | <0.001 |
| FSGS with nephrotic syndrome | 7456 (1.7) | 40 (0.7) | 29 (1.3) | 0.013 |
| Ischaemic renal disease/cholesterol embolism | 6440 (1.5) | 158 (2.9) | 53 (2.4) | 0.253 |
| Kidney tumour/trauma/loss | 5652 (1.3) | 53 (1.0) | 15 (0.7) | 0.229 |
| Congenital kidney disease | 4926 (1.1) | 14 (0.3) | 24 (1.1) | <0.001 |
| Membraneous nephropathy | 4114 (0.9) | 44 (0.8) | 25 (1.1) | 0.160 |
| Miscellaneous | 3689 (0.8) | 70 (1.3) | 32 (1.5) | 0.537 |
| Tubular necrosis (irreversible) or cortical necrosis | 3450 (0.8) | 404 (7.4) | 86 (3.9) | <0.001 |
| HUS | 1167 (0.3) | 95 (1.7) | 30 (1.4) | 0.249 |
| Systemic sclerosis | 546 (0.1) | 50 (0.9) | 7 (0.3) | 0.006 |
| KRT modality at Day 91, n (%) | ||||
| HD | 361 549 (82.0) | 4821 (88.2) | 1707 (77.9) | <0.001 |
| PD | 79 447 (18.0) | 644 (11.8) | 485 (22.1) | <0.001 |
| KRT modality at RKF, n (%) | ||||
| HD | – | 4702 (86.0) | 1680 (76.6) | <0.001 |
| PD | – | 753 (13.8) | 508 (23.2) | <0.001 |
| Unknown | – | 10 (0.2) | 4 (0.2) | 0.996 |
| Duration of RKF, n (%) | ||||
| 30–90 days | – | – | 264 (12.0) | – |
| 90–365 days | – | – | 590 (26.9) | – |
| 1–2 years | – | – | 455 (20.8) | – |
| 2–5 years | – | – | 614 (28.0) | – |
| >5 years | – | – | 269 (12.3) | – |
| KRT modality after non-sustained RKF, n (%) | ||||
| HD | – | – | 1749 (79.8) | – |
| PD | – | – | 347 (15.8) | – |
| Transplantation | – | – | 96 (4.4) | – |
FSGS, focal segmental glomerulosclerosis; IgA, immunoglobulin A; TIN, tubulo-interstitial nephritis.
Paraproteinaemia includes multiple myeloma, light chain nephropathy and amyloidosis.
For categorical variables, P-values were calculated with chi-squared tests, for continuous variables the Wilcoxon test was applied. Percentages are column percentages. P-values comparing sustained and non-sustained RKF.
Patients with sustained RKF were older, more often female and more frequently initiated KRT with haemodialysis (HD) as compared with patients with non-sustained RKF (Table 1). Patients with non-sustained RKF recommenced KRT after various KRT-free intervals; 39% of patients restarted KRT within 1 year and 60% within 2 years after discontinuation of maintenance dialysis (Table 1).
Characteristics associated with RKF
Underlying kidney disease
After adjustment for age at the start of dialysis, sex, treatment modality at Day 91, country of residence and time period of dialysis initiation, patients with the following PRD codes had the highest likelihood of experiencing sustained RKF as compared with patients with diabetes mellitus (reference group): tubular necrosis (irreversible) or cortical necrosis {adjusted hazard ratio [aHR] 20.35 [95% confidence interval (CI) 17.89–23.14]}, systemic sclerosis [aHR 18.50 (95% CI 13.84–24.72)], haemolytic uraemic syndrome [HUS; aHR 17.31 (95% CI 13.88–21.59)], paraproteinaemia [aHR 7.96 (95% CI 7.04–9.01)], vasculitis [aHR 5.72 (95% CI 4.96–6.59)] and ischaemic renal disease/cholesterol embolism [aHR 4.15 (95% CI 3.46–4.98)]. The lowest likelihood of sustained RKF was found in patients with cystic kidney disease [aHR 0.11 (95% CI 0.07–0.19)] and congenital kidney disease [aHR 0.67 (95% CI 0.39–1.14)]. The findings were consistent when patients with non-sustained RKF were included (Table 2).
| . | . | All RKF . | Sustained RKF . | ||||
|---|---|---|---|---|---|---|---|
| Characteristics . | At risk, n . | RKF, n (%) . | Unadjusted HR (95% CI) . | aHR (95% CI) . | RKF, n (%) . | Unadjusted HR (95% CI) . | aHR (95% CI) . |
| Sex | |||||||
| Male | 277 808 | 4607 (1.7) | 1 (Ref) | 1 (Ref) | 3185 (1.1) | 1 (Ref) | 1 (Ref) |
| Female | 163 188 | 2954 (1.8) | 1.08 (1.03–1.13) | 1.04 (1–1.09) | 2201 (1.3) | 1.17 (1.11–1.23) | 1.13 (1.07–1.19) |
| KRT modality at Day 91 | |||||||
| HD | 361 549 | 6450 (1.8) | 1.27 (1.19–1.35) | 1.21 (1.13–1.29) | 4757 (1.3) | 1.65 (1.52–1.79) | 1.50 (1.38–1.64) |
| PD | 79 447 | 1111 (1.4) | 1 (Ref) | 1 (Ref) | 629 (0.8) | 1 (Ref) | 1 (Ref) |
| Age start (years) | |||||||
| 20–39 | 33 878 | 495 (1.5) | 1 (Ref) | 1 (Ref) | 257 (0.8) | 1 (Ref) | 1 (Ref) |
| 40–59 | 108 671 | 1735 (1.6) | 1.02 (0.93–1.13) | 1.18 (1.07–1.31) | 1111 (1.0) | 1.27 (1.11–1.45) | 1.46 (1.27–1.67) |
| 60–79 | 232 786 | 4342 (1.9) | 1.21 (1.10–1.32) | 1.27 (1.16–1.40) | 3165 (1.4) | 1.70 (1.50–1.93) | 1.77 (1.55–2.02) |
| 80–100 | 65 661 | 989 (1.5) | 1.05 (0.94–1.17) | 1.05 (0.94–1.18) | 853 (1.3) | 1.75 (1.52–2.01) | 1.69 (1.46–1.95) |
| PRD group | |||||||
| Diabetes mellitus types 1 and 2 | 106 100 | 965 (0.9) | 1 (Ref) | 1 (Ref) | 592 (0.6) | 1 (Ref) | 1 (Ref) |
| Cause unknown or missing | 87 772 | 1552 (1.8) | 2.01 (1.86–2.18) | 2.28 (2.10–2.47) | 1230 (1.4) | 2.60 (2.36–2.87) | 2.90 (2.63–3.20) |
| Hypertension | 63 366 | 819 (1.3) | 1.42 (1.29–1.56) | 1.39 (1.27–1.53) | 523 (0.8) | 1.48 (1.31–1.66) | 1.45 (1.29–1.63) |
| Cystic kidney disease | 28 925 | 57 (0.2) | 0.22 (0.17–0.28) | 0.22 (0.17–0.29) | 17 (0.1) | 0.10 (0.06–0.17) | 0.11 (0.07–0.19) |
| Glomerulonephritis | 28 570 | 650 (2.3) | 2.51 (2.27–2.77) | 2.75 (2.48–3.04) | 441 (1.5) | 2.78 (2.46–3.14) | 3.25 (2.87–3.68) |
| Pyelonephritis/infectious | 24 718 | 419 (1.7) | 1.85 (1.65–2.07) | 2.07 (1.84–2.32) | 284 (1.1) | 2.04 (1.77–2.35) | 2.31 (2.01–2.66) |
| Renovascular disease | 18 553 | 381 (2.1) | 2.33 (2.07–2.63) | 2.09 (1.85–2.36) | 283 (1.5) | 2.82 (2.45–3.25) | 2.42 (2.09–2.80) |
| IgA nephropathy | 13 300 | 171 (1.3) | 1.50 (1.28–1.77) | 1.61 (1.36–1.89) | 118 (0.9) | 1.69 (1.39–2.06) | 2.00 (1.64–2.44) |
| Paraproteinaemia | 11 461 | 553 (4.8) | 6.37 (5.73–7.07) | 6.22 (5.60–6.91) | 447 (3.9) | 8.35 (7.39–9.45) | 7.96 (7.04–9.01) |
| TIN and drug-induced | 11 277 | 326 (2.9) | 3.25 (2.86–3.68) | 3.03 (2.67–3.43) | 245 (2.2) | 3.98 (3.43–4.62) | 3.77 (3.24–4.37) |
| Vasculitis | 9514 | 454 (4.8) | 5.38 (4.81–6.01) | 5.37 (4.80–6.01) | 291 (3.1) | 5.62 (4.88–6.47) | 5.72 (4.96–6.59) |
| FSGS with nephrotic syndrome | 7456 | 69 (0.9) | 1.01 (0.79–1.30) | 1.06 (0.83–1.36) | 40 (0.5) | 0.96 (0.70–1.32) | 1.07 (0.77–1.47) |
| Ischaemic renal disease/cholesterol embolism | 6440 | 207 (3.2) | 3.69 (3.18–4.29) | 3.51 (3.00–4.09) | 154 (2.4) | 4.47 (3.74–5.34) | 4.15 (3.46–4.98) |
| Kidney tumour/trauma/loss | 5652 | 67 (1.2) | 1.34 (1.05–1.72) | 1.27 (0.99–1.63) | 52 (0.9) | 1.70 (1.28–2.26) | 1.56 (1.17–2.07) |
| Congenital kidney disease | 4926 | 38 (0.8) | 0.87 (0.63–1.20) | 0.96 (0.70–1.34) | 14 (0.3) | 0.52 (0.31–0.89) | 0.67 (0.39–1.14) |
| Membraneous nephropathy | 4114 | 67 (1.6) | 1.75 (1.37–2.24) | 1.91 (1.49–2.45) | 43 (1.0) | 1.83 (1.35–2.50) | 2.02 (1.48–2.76) |
| Miscellaneous | 3689 | 100 (2.7) | 3.06 (2.49–3.76) | 3.14 (2.56–3.86) | 68 (1.8) | 3.39 (2.64–4.35) | 3.63 (2.82–4.67) |
| Tubular necrosis (irreversible) or cortical necrosis | 3450 | 485 (14.1) | 18.44 (16.54–20.57) | 15.87 (14.21–17.73) | 400 (11.6) | 24.71 (21.76–28.05) | 20.35 (17.89–23.14) |
| HUS | 1167 | 124 (10.6) | 12.52 (10.39–15.10) | 12.44 (10.29–15.04) | 94 (8.1) | 15.46 (12.43–19.21) | 17.31 (13.88–21.59) |
| Systemic sclerosis | 546 | 57 (10.4) | 12.92 (9.89–16.88) | 12.76 (9.76–16.69) | 50 (9.2) | 18.43 (13.81–24.59) | 18.50 (13.84–24.72) |
| Onset of KRT | |||||||
| 1997–2001 | 59 042 | 777 (1.3) | 1 (Ref) | 1 (Ref) | 428 (0.7) | 1 (Ref) | 1 (Ref) |
| 2002–2006 | 98 103 | 1576 (1.6) | 1.2 (1.10–1.31) | 1.37 (1.26–1.49) | 1026 (1.0) | 1.42 (1.27–1.59) | 1.57 (1.40–1.76) |
| 2007–2011 | 133 838 | 2578 (1.9) | 1.42 (1.31–1.54) | 1.68 (1.55–1.83) | 1782 (1.3) | 1.79 (1.61–1.99) | 2.04 (1.83–2.27) |
| 2012–2016 | 150 013 | 2630 (1.8) | 1.40 (1.29–1.51) | 1.67 (1.53–1.81) | 2150 (1.4) | 2.07 (1.87–2.30) | 2.38 (2.14–2.65) |
| . | . | All RKF . | Sustained RKF . | ||||
|---|---|---|---|---|---|---|---|
| Characteristics . | At risk, n . | RKF, n (%) . | Unadjusted HR (95% CI) . | aHR (95% CI) . | RKF, n (%) . | Unadjusted HR (95% CI) . | aHR (95% CI) . |
| Sex | |||||||
| Male | 277 808 | 4607 (1.7) | 1 (Ref) | 1 (Ref) | 3185 (1.1) | 1 (Ref) | 1 (Ref) |
| Female | 163 188 | 2954 (1.8) | 1.08 (1.03–1.13) | 1.04 (1–1.09) | 2201 (1.3) | 1.17 (1.11–1.23) | 1.13 (1.07–1.19) |
| KRT modality at Day 91 | |||||||
| HD | 361 549 | 6450 (1.8) | 1.27 (1.19–1.35) | 1.21 (1.13–1.29) | 4757 (1.3) | 1.65 (1.52–1.79) | 1.50 (1.38–1.64) |
| PD | 79 447 | 1111 (1.4) | 1 (Ref) | 1 (Ref) | 629 (0.8) | 1 (Ref) | 1 (Ref) |
| Age start (years) | |||||||
| 20–39 | 33 878 | 495 (1.5) | 1 (Ref) | 1 (Ref) | 257 (0.8) | 1 (Ref) | 1 (Ref) |
| 40–59 | 108 671 | 1735 (1.6) | 1.02 (0.93–1.13) | 1.18 (1.07–1.31) | 1111 (1.0) | 1.27 (1.11–1.45) | 1.46 (1.27–1.67) |
| 60–79 | 232 786 | 4342 (1.9) | 1.21 (1.10–1.32) | 1.27 (1.16–1.40) | 3165 (1.4) | 1.70 (1.50–1.93) | 1.77 (1.55–2.02) |
| 80–100 | 65 661 | 989 (1.5) | 1.05 (0.94–1.17) | 1.05 (0.94–1.18) | 853 (1.3) | 1.75 (1.52–2.01) | 1.69 (1.46–1.95) |
| PRD group | |||||||
| Diabetes mellitus types 1 and 2 | 106 100 | 965 (0.9) | 1 (Ref) | 1 (Ref) | 592 (0.6) | 1 (Ref) | 1 (Ref) |
| Cause unknown or missing | 87 772 | 1552 (1.8) | 2.01 (1.86–2.18) | 2.28 (2.10–2.47) | 1230 (1.4) | 2.60 (2.36–2.87) | 2.90 (2.63–3.20) |
| Hypertension | 63 366 | 819 (1.3) | 1.42 (1.29–1.56) | 1.39 (1.27–1.53) | 523 (0.8) | 1.48 (1.31–1.66) | 1.45 (1.29–1.63) |
| Cystic kidney disease | 28 925 | 57 (0.2) | 0.22 (0.17–0.28) | 0.22 (0.17–0.29) | 17 (0.1) | 0.10 (0.06–0.17) | 0.11 (0.07–0.19) |
| Glomerulonephritis | 28 570 | 650 (2.3) | 2.51 (2.27–2.77) | 2.75 (2.48–3.04) | 441 (1.5) | 2.78 (2.46–3.14) | 3.25 (2.87–3.68) |
| Pyelonephritis/infectious | 24 718 | 419 (1.7) | 1.85 (1.65–2.07) | 2.07 (1.84–2.32) | 284 (1.1) | 2.04 (1.77–2.35) | 2.31 (2.01–2.66) |
| Renovascular disease | 18 553 | 381 (2.1) | 2.33 (2.07–2.63) | 2.09 (1.85–2.36) | 283 (1.5) | 2.82 (2.45–3.25) | 2.42 (2.09–2.80) |
| IgA nephropathy | 13 300 | 171 (1.3) | 1.50 (1.28–1.77) | 1.61 (1.36–1.89) | 118 (0.9) | 1.69 (1.39–2.06) | 2.00 (1.64–2.44) |
| Paraproteinaemia | 11 461 | 553 (4.8) | 6.37 (5.73–7.07) | 6.22 (5.60–6.91) | 447 (3.9) | 8.35 (7.39–9.45) | 7.96 (7.04–9.01) |
| TIN and drug-induced | 11 277 | 326 (2.9) | 3.25 (2.86–3.68) | 3.03 (2.67–3.43) | 245 (2.2) | 3.98 (3.43–4.62) | 3.77 (3.24–4.37) |
| Vasculitis | 9514 | 454 (4.8) | 5.38 (4.81–6.01) | 5.37 (4.80–6.01) | 291 (3.1) | 5.62 (4.88–6.47) | 5.72 (4.96–6.59) |
| FSGS with nephrotic syndrome | 7456 | 69 (0.9) | 1.01 (0.79–1.30) | 1.06 (0.83–1.36) | 40 (0.5) | 0.96 (0.70–1.32) | 1.07 (0.77–1.47) |
| Ischaemic renal disease/cholesterol embolism | 6440 | 207 (3.2) | 3.69 (3.18–4.29) | 3.51 (3.00–4.09) | 154 (2.4) | 4.47 (3.74–5.34) | 4.15 (3.46–4.98) |
| Kidney tumour/trauma/loss | 5652 | 67 (1.2) | 1.34 (1.05–1.72) | 1.27 (0.99–1.63) | 52 (0.9) | 1.70 (1.28–2.26) | 1.56 (1.17–2.07) |
| Congenital kidney disease | 4926 | 38 (0.8) | 0.87 (0.63–1.20) | 0.96 (0.70–1.34) | 14 (0.3) | 0.52 (0.31–0.89) | 0.67 (0.39–1.14) |
| Membraneous nephropathy | 4114 | 67 (1.6) | 1.75 (1.37–2.24) | 1.91 (1.49–2.45) | 43 (1.0) | 1.83 (1.35–2.50) | 2.02 (1.48–2.76) |
| Miscellaneous | 3689 | 100 (2.7) | 3.06 (2.49–3.76) | 3.14 (2.56–3.86) | 68 (1.8) | 3.39 (2.64–4.35) | 3.63 (2.82–4.67) |
| Tubular necrosis (irreversible) or cortical necrosis | 3450 | 485 (14.1) | 18.44 (16.54–20.57) | 15.87 (14.21–17.73) | 400 (11.6) | 24.71 (21.76–28.05) | 20.35 (17.89–23.14) |
| HUS | 1167 | 124 (10.6) | 12.52 (10.39–15.10) | 12.44 (10.29–15.04) | 94 (8.1) | 15.46 (12.43–19.21) | 17.31 (13.88–21.59) |
| Systemic sclerosis | 546 | 57 (10.4) | 12.92 (9.89–16.88) | 12.76 (9.76–16.69) | 50 (9.2) | 18.43 (13.81–24.59) | 18.50 (13.84–24.72) |
| Onset of KRT | |||||||
| 1997–2001 | 59 042 | 777 (1.3) | 1 (Ref) | 1 (Ref) | 428 (0.7) | 1 (Ref) | 1 (Ref) |
| 2002–2006 | 98 103 | 1576 (1.6) | 1.2 (1.10–1.31) | 1.37 (1.26–1.49) | 1026 (1.0) | 1.42 (1.27–1.59) | 1.57 (1.40–1.76) |
| 2007–2011 | 133 838 | 2578 (1.9) | 1.42 (1.31–1.54) | 1.68 (1.55–1.83) | 1782 (1.3) | 1.79 (1.61–1.99) | 2.04 (1.83–2.27) |
| 2012–2016 | 150 013 | 2630 (1.8) | 1.40 (1.29–1.51) | 1.67 (1.53–1.81) | 2150 (1.4) | 2.07 (1.87–2.30) | 2.38 (2.14–2.65) |
FSGS, focal segmental glomerulosclerosis; IgA, immunoglobulin A; TIN, tubulo-interstitial nephritis. R adjusted for age at onset of dialysis, sex, year of dialysis onset, treatment modality at Day 91, underlying kidney disease (PRD) and country of residence.
Patient numbers differ slightly from those shown in Table 1, as the Cox regression analyses included only patients with RKF within 5 years after initiation of dialysis. Percentages are row percentages.
| . | . | All RKF . | Sustained RKF . | ||||
|---|---|---|---|---|---|---|---|
| Characteristics . | At risk, n . | RKF, n (%) . | Unadjusted HR (95% CI) . | aHR (95% CI) . | RKF, n (%) . | Unadjusted HR (95% CI) . | aHR (95% CI) . |
| Sex | |||||||
| Male | 277 808 | 4607 (1.7) | 1 (Ref) | 1 (Ref) | 3185 (1.1) | 1 (Ref) | 1 (Ref) |
| Female | 163 188 | 2954 (1.8) | 1.08 (1.03–1.13) | 1.04 (1–1.09) | 2201 (1.3) | 1.17 (1.11–1.23) | 1.13 (1.07–1.19) |
| KRT modality at Day 91 | |||||||
| HD | 361 549 | 6450 (1.8) | 1.27 (1.19–1.35) | 1.21 (1.13–1.29) | 4757 (1.3) | 1.65 (1.52–1.79) | 1.50 (1.38–1.64) |
| PD | 79 447 | 1111 (1.4) | 1 (Ref) | 1 (Ref) | 629 (0.8) | 1 (Ref) | 1 (Ref) |
| Age start (years) | |||||||
| 20–39 | 33 878 | 495 (1.5) | 1 (Ref) | 1 (Ref) | 257 (0.8) | 1 (Ref) | 1 (Ref) |
| 40–59 | 108 671 | 1735 (1.6) | 1.02 (0.93–1.13) | 1.18 (1.07–1.31) | 1111 (1.0) | 1.27 (1.11–1.45) | 1.46 (1.27–1.67) |
| 60–79 | 232 786 | 4342 (1.9) | 1.21 (1.10–1.32) | 1.27 (1.16–1.40) | 3165 (1.4) | 1.70 (1.50–1.93) | 1.77 (1.55–2.02) |
| 80–100 | 65 661 | 989 (1.5) | 1.05 (0.94–1.17) | 1.05 (0.94–1.18) | 853 (1.3) | 1.75 (1.52–2.01) | 1.69 (1.46–1.95) |
| PRD group | |||||||
| Diabetes mellitus types 1 and 2 | 106 100 | 965 (0.9) | 1 (Ref) | 1 (Ref) | 592 (0.6) | 1 (Ref) | 1 (Ref) |
| Cause unknown or missing | 87 772 | 1552 (1.8) | 2.01 (1.86–2.18) | 2.28 (2.10–2.47) | 1230 (1.4) | 2.60 (2.36–2.87) | 2.90 (2.63–3.20) |
| Hypertension | 63 366 | 819 (1.3) | 1.42 (1.29–1.56) | 1.39 (1.27–1.53) | 523 (0.8) | 1.48 (1.31–1.66) | 1.45 (1.29–1.63) |
| Cystic kidney disease | 28 925 | 57 (0.2) | 0.22 (0.17–0.28) | 0.22 (0.17–0.29) | 17 (0.1) | 0.10 (0.06–0.17) | 0.11 (0.07–0.19) |
| Glomerulonephritis | 28 570 | 650 (2.3) | 2.51 (2.27–2.77) | 2.75 (2.48–3.04) | 441 (1.5) | 2.78 (2.46–3.14) | 3.25 (2.87–3.68) |
| Pyelonephritis/infectious | 24 718 | 419 (1.7) | 1.85 (1.65–2.07) | 2.07 (1.84–2.32) | 284 (1.1) | 2.04 (1.77–2.35) | 2.31 (2.01–2.66) |
| Renovascular disease | 18 553 | 381 (2.1) | 2.33 (2.07–2.63) | 2.09 (1.85–2.36) | 283 (1.5) | 2.82 (2.45–3.25) | 2.42 (2.09–2.80) |
| IgA nephropathy | 13 300 | 171 (1.3) | 1.50 (1.28–1.77) | 1.61 (1.36–1.89) | 118 (0.9) | 1.69 (1.39–2.06) | 2.00 (1.64–2.44) |
| Paraproteinaemia | 11 461 | 553 (4.8) | 6.37 (5.73–7.07) | 6.22 (5.60–6.91) | 447 (3.9) | 8.35 (7.39–9.45) | 7.96 (7.04–9.01) |
| TIN and drug-induced | 11 277 | 326 (2.9) | 3.25 (2.86–3.68) | 3.03 (2.67–3.43) | 245 (2.2) | 3.98 (3.43–4.62) | 3.77 (3.24–4.37) |
| Vasculitis | 9514 | 454 (4.8) | 5.38 (4.81–6.01) | 5.37 (4.80–6.01) | 291 (3.1) | 5.62 (4.88–6.47) | 5.72 (4.96–6.59) |
| FSGS with nephrotic syndrome | 7456 | 69 (0.9) | 1.01 (0.79–1.30) | 1.06 (0.83–1.36) | 40 (0.5) | 0.96 (0.70–1.32) | 1.07 (0.77–1.47) |
| Ischaemic renal disease/cholesterol embolism | 6440 | 207 (3.2) | 3.69 (3.18–4.29) | 3.51 (3.00–4.09) | 154 (2.4) | 4.47 (3.74–5.34) | 4.15 (3.46–4.98) |
| Kidney tumour/trauma/loss | 5652 | 67 (1.2) | 1.34 (1.05–1.72) | 1.27 (0.99–1.63) | 52 (0.9) | 1.70 (1.28–2.26) | 1.56 (1.17–2.07) |
| Congenital kidney disease | 4926 | 38 (0.8) | 0.87 (0.63–1.20) | 0.96 (0.70–1.34) | 14 (0.3) | 0.52 (0.31–0.89) | 0.67 (0.39–1.14) |
| Membraneous nephropathy | 4114 | 67 (1.6) | 1.75 (1.37–2.24) | 1.91 (1.49–2.45) | 43 (1.0) | 1.83 (1.35–2.50) | 2.02 (1.48–2.76) |
| Miscellaneous | 3689 | 100 (2.7) | 3.06 (2.49–3.76) | 3.14 (2.56–3.86) | 68 (1.8) | 3.39 (2.64–4.35) | 3.63 (2.82–4.67) |
| Tubular necrosis (irreversible) or cortical necrosis | 3450 | 485 (14.1) | 18.44 (16.54–20.57) | 15.87 (14.21–17.73) | 400 (11.6) | 24.71 (21.76–28.05) | 20.35 (17.89–23.14) |
| HUS | 1167 | 124 (10.6) | 12.52 (10.39–15.10) | 12.44 (10.29–15.04) | 94 (8.1) | 15.46 (12.43–19.21) | 17.31 (13.88–21.59) |
| Systemic sclerosis | 546 | 57 (10.4) | 12.92 (9.89–16.88) | 12.76 (9.76–16.69) | 50 (9.2) | 18.43 (13.81–24.59) | 18.50 (13.84–24.72) |
| Onset of KRT | |||||||
| 1997–2001 | 59 042 | 777 (1.3) | 1 (Ref) | 1 (Ref) | 428 (0.7) | 1 (Ref) | 1 (Ref) |
| 2002–2006 | 98 103 | 1576 (1.6) | 1.2 (1.10–1.31) | 1.37 (1.26–1.49) | 1026 (1.0) | 1.42 (1.27–1.59) | 1.57 (1.40–1.76) |
| 2007–2011 | 133 838 | 2578 (1.9) | 1.42 (1.31–1.54) | 1.68 (1.55–1.83) | 1782 (1.3) | 1.79 (1.61–1.99) | 2.04 (1.83–2.27) |
| 2012–2016 | 150 013 | 2630 (1.8) | 1.40 (1.29–1.51) | 1.67 (1.53–1.81) | 2150 (1.4) | 2.07 (1.87–2.30) | 2.38 (2.14–2.65) |
| . | . | All RKF . | Sustained RKF . | ||||
|---|---|---|---|---|---|---|---|
| Characteristics . | At risk, n . | RKF, n (%) . | Unadjusted HR (95% CI) . | aHR (95% CI) . | RKF, n (%) . | Unadjusted HR (95% CI) . | aHR (95% CI) . |
| Sex | |||||||
| Male | 277 808 | 4607 (1.7) | 1 (Ref) | 1 (Ref) | 3185 (1.1) | 1 (Ref) | 1 (Ref) |
| Female | 163 188 | 2954 (1.8) | 1.08 (1.03–1.13) | 1.04 (1–1.09) | 2201 (1.3) | 1.17 (1.11–1.23) | 1.13 (1.07–1.19) |
| KRT modality at Day 91 | |||||||
| HD | 361 549 | 6450 (1.8) | 1.27 (1.19–1.35) | 1.21 (1.13–1.29) | 4757 (1.3) | 1.65 (1.52–1.79) | 1.50 (1.38–1.64) |
| PD | 79 447 | 1111 (1.4) | 1 (Ref) | 1 (Ref) | 629 (0.8) | 1 (Ref) | 1 (Ref) |
| Age start (years) | |||||||
| 20–39 | 33 878 | 495 (1.5) | 1 (Ref) | 1 (Ref) | 257 (0.8) | 1 (Ref) | 1 (Ref) |
| 40–59 | 108 671 | 1735 (1.6) | 1.02 (0.93–1.13) | 1.18 (1.07–1.31) | 1111 (1.0) | 1.27 (1.11–1.45) | 1.46 (1.27–1.67) |
| 60–79 | 232 786 | 4342 (1.9) | 1.21 (1.10–1.32) | 1.27 (1.16–1.40) | 3165 (1.4) | 1.70 (1.50–1.93) | 1.77 (1.55–2.02) |
| 80–100 | 65 661 | 989 (1.5) | 1.05 (0.94–1.17) | 1.05 (0.94–1.18) | 853 (1.3) | 1.75 (1.52–2.01) | 1.69 (1.46–1.95) |
| PRD group | |||||||
| Diabetes mellitus types 1 and 2 | 106 100 | 965 (0.9) | 1 (Ref) | 1 (Ref) | 592 (0.6) | 1 (Ref) | 1 (Ref) |
| Cause unknown or missing | 87 772 | 1552 (1.8) | 2.01 (1.86–2.18) | 2.28 (2.10–2.47) | 1230 (1.4) | 2.60 (2.36–2.87) | 2.90 (2.63–3.20) |
| Hypertension | 63 366 | 819 (1.3) | 1.42 (1.29–1.56) | 1.39 (1.27–1.53) | 523 (0.8) | 1.48 (1.31–1.66) | 1.45 (1.29–1.63) |
| Cystic kidney disease | 28 925 | 57 (0.2) | 0.22 (0.17–0.28) | 0.22 (0.17–0.29) | 17 (0.1) | 0.10 (0.06–0.17) | 0.11 (0.07–0.19) |
| Glomerulonephritis | 28 570 | 650 (2.3) | 2.51 (2.27–2.77) | 2.75 (2.48–3.04) | 441 (1.5) | 2.78 (2.46–3.14) | 3.25 (2.87–3.68) |
| Pyelonephritis/infectious | 24 718 | 419 (1.7) | 1.85 (1.65–2.07) | 2.07 (1.84–2.32) | 284 (1.1) | 2.04 (1.77–2.35) | 2.31 (2.01–2.66) |
| Renovascular disease | 18 553 | 381 (2.1) | 2.33 (2.07–2.63) | 2.09 (1.85–2.36) | 283 (1.5) | 2.82 (2.45–3.25) | 2.42 (2.09–2.80) |
| IgA nephropathy | 13 300 | 171 (1.3) | 1.50 (1.28–1.77) | 1.61 (1.36–1.89) | 118 (0.9) | 1.69 (1.39–2.06) | 2.00 (1.64–2.44) |
| Paraproteinaemia | 11 461 | 553 (4.8) | 6.37 (5.73–7.07) | 6.22 (5.60–6.91) | 447 (3.9) | 8.35 (7.39–9.45) | 7.96 (7.04–9.01) |
| TIN and drug-induced | 11 277 | 326 (2.9) | 3.25 (2.86–3.68) | 3.03 (2.67–3.43) | 245 (2.2) | 3.98 (3.43–4.62) | 3.77 (3.24–4.37) |
| Vasculitis | 9514 | 454 (4.8) | 5.38 (4.81–6.01) | 5.37 (4.80–6.01) | 291 (3.1) | 5.62 (4.88–6.47) | 5.72 (4.96–6.59) |
| FSGS with nephrotic syndrome | 7456 | 69 (0.9) | 1.01 (0.79–1.30) | 1.06 (0.83–1.36) | 40 (0.5) | 0.96 (0.70–1.32) | 1.07 (0.77–1.47) |
| Ischaemic renal disease/cholesterol embolism | 6440 | 207 (3.2) | 3.69 (3.18–4.29) | 3.51 (3.00–4.09) | 154 (2.4) | 4.47 (3.74–5.34) | 4.15 (3.46–4.98) |
| Kidney tumour/trauma/loss | 5652 | 67 (1.2) | 1.34 (1.05–1.72) | 1.27 (0.99–1.63) | 52 (0.9) | 1.70 (1.28–2.26) | 1.56 (1.17–2.07) |
| Congenital kidney disease | 4926 | 38 (0.8) | 0.87 (0.63–1.20) | 0.96 (0.70–1.34) | 14 (0.3) | 0.52 (0.31–0.89) | 0.67 (0.39–1.14) |
| Membraneous nephropathy | 4114 | 67 (1.6) | 1.75 (1.37–2.24) | 1.91 (1.49–2.45) | 43 (1.0) | 1.83 (1.35–2.50) | 2.02 (1.48–2.76) |
| Miscellaneous | 3689 | 100 (2.7) | 3.06 (2.49–3.76) | 3.14 (2.56–3.86) | 68 (1.8) | 3.39 (2.64–4.35) | 3.63 (2.82–4.67) |
| Tubular necrosis (irreversible) or cortical necrosis | 3450 | 485 (14.1) | 18.44 (16.54–20.57) | 15.87 (14.21–17.73) | 400 (11.6) | 24.71 (21.76–28.05) | 20.35 (17.89–23.14) |
| HUS | 1167 | 124 (10.6) | 12.52 (10.39–15.10) | 12.44 (10.29–15.04) | 94 (8.1) | 15.46 (12.43–19.21) | 17.31 (13.88–21.59) |
| Systemic sclerosis | 546 | 57 (10.4) | 12.92 (9.89–16.88) | 12.76 (9.76–16.69) | 50 (9.2) | 18.43 (13.81–24.59) | 18.50 (13.84–24.72) |
| Onset of KRT | |||||||
| 1997–2001 | 59 042 | 777 (1.3) | 1 (Ref) | 1 (Ref) | 428 (0.7) | 1 (Ref) | 1 (Ref) |
| 2002–2006 | 98 103 | 1576 (1.6) | 1.2 (1.10–1.31) | 1.37 (1.26–1.49) | 1026 (1.0) | 1.42 (1.27–1.59) | 1.57 (1.40–1.76) |
| 2007–2011 | 133 838 | 2578 (1.9) | 1.42 (1.31–1.54) | 1.68 (1.55–1.83) | 1782 (1.3) | 1.79 (1.61–1.99) | 2.04 (1.83–2.27) |
| 2012–2016 | 150 013 | 2630 (1.8) | 1.40 (1.29–1.51) | 1.67 (1.53–1.81) | 2150 (1.4) | 2.07 (1.87–2.30) | 2.38 (2.14–2.65) |
FSGS, focal segmental glomerulosclerosis; IgA, immunoglobulin A; TIN, tubulo-interstitial nephritis. R adjusted for age at onset of dialysis, sex, year of dialysis onset, treatment modality at Day 91, underlying kidney disease (PRD) and country of residence.
Patient numbers differ slightly from those shown in Table 1, as the Cox regression analyses included only patients with RKF within 5 years after initiation of dialysis. Percentages are row percentages.
Other characteristics
Female sex [aHR 1.13 (95% CI 1.07–1.19)], onset of dialysis treatment at age 60–79 years when compared with 20–39 years [aHR 1.77 (95% CI 1.55–2.02)], HD as a treatment modality [aHR 1.50 (95% CI 1.38–1.64)] and dialysis initiation in a more recent time period [aHR 2.38 (95% CI 2.14–2.65)] were also significantly associated with a higher likelihood of sustained RKF, although to a lesser extent than the aforementioned underlying kidney diseases (Table 2). These observations were consistent when patients with non-sustained RKF were included, except for patients >80 years of age at the initiation of dialysis (Table 2).
RKF: incidence and trends over time
Figure 1 displays the cumulative incidence of RKF (1.8%) and the competing events death (48.7%), kidney transplantation (22.6%) and loss to follow-up (0.9%) in the 5 years from Day 91 of dialysis. The distribution of time on maintenance dialysis before RKF is depicted in Figure 2. Approximately 90% of all RKF occurred within the first 2 years after Day 91 of dialysis. The median time on maintenance dialysis before non-sustained RKF was 265 days (IQR 153–510) and 237 days (IQR 137–464) for patients with sustained RKF, respectively.
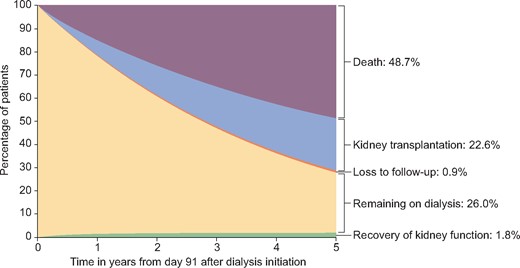
Cumulative incidence of RKF and other competing events during 5 years after initiation of maintenance dialysis treatment.
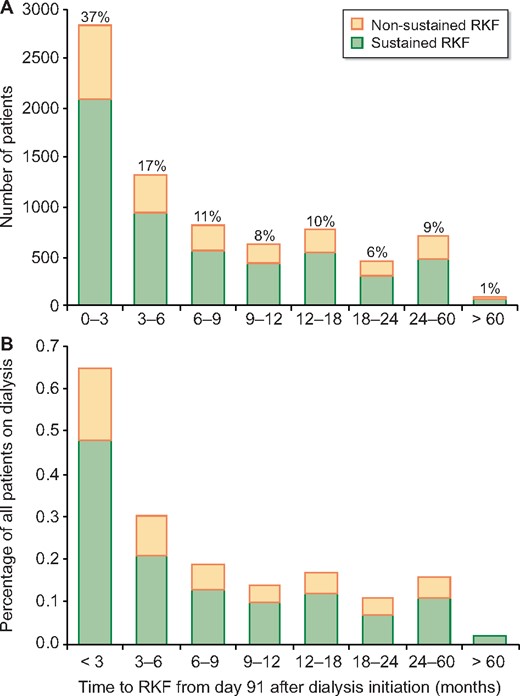
Time between Day 91 of dialysis treatment and sustained and non-sustained RKF displayed as (A) total patient numbers and (B) as a percentage of all patients with RKF.
As presented in Figure 3, we observed a statistically significant increase in the incidence of RKF in the years 1997–2007 with an APC of 6.5 (95% CI 5.1–7.9). Between 2007 and 2012 the incidence of RKF was stable over time and declined in the years 2012–2015 [APC −10.3 (95% CI −17.6 to −2.4)]. The proportion of patients with a kidney disease with a high likelihood of RKF remained stable over the time period 1997–2015 (data not shown).
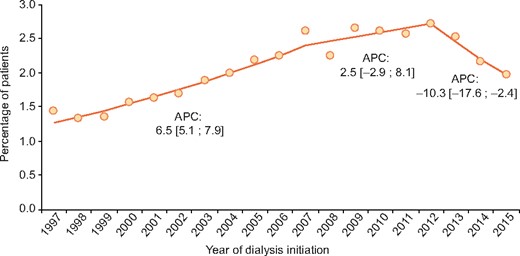
Incidence of RKF with annual percentage change within 2 years after initiation of maintenance dialysis. This includes patients with both sustained and non-sustained RKF. Inclusion was limited to the years 1997–2016 and follow-up to 31 December 2017 in order to ensure sufficient follow-up time for RKF to occur, as 90% of all recoveries occurred within 2 years. Only data from registries providing data for the complete time period were included: Austria; Dutch-speaking Belgium; French-speaking Belgium; Denmark; Finland; Greece; Iceland; the Netherlands; Norway; the Spanish regional renal registries of Andalusia, Asturias, Basque Country, Cantabria and Catalonia; Sweden and UK (Scotland).
The time to RKF varied markedly according to the type of underlying kidney disease (Supplementary data, Figure S1). Across all PRD categories the incidence of RKF was highest in the first 9 months after initiation of maintenance dialysis (Figure 2), although patients with vasculitis and ischaemic renal disease/cholesterol embolism showed a more stable, although very low, incidence of RKF over time.
After RKF: mortality and return to KRT
The cumulative incidence of death and return to KRT in the 5 years after Day 31 of RKF is shown in Figure 4; 55% did not have a subsequent event within the follow-up period and were thus considered as sustained RKF, 15.7% died, 28.1% recommenced dialysis treatment and 1.2% underwent kidney transplantation. Among the underlying kidney diseases with the highest likelihood of RKF, the percentage of patients who did not restart KRT after RKF was 74% tubular necrosis (irreversible) or cortical necrosis, 79% for systemic sclerosis, 73% for HUS and 65% for paraproteinaemia. However, among patients with vasculitis and ischaemic renal disease/cholesterol embolism, the proportion with sustained RKF, 59% and 61%, respectively, was more in line with the entire group of patients (data not shown).
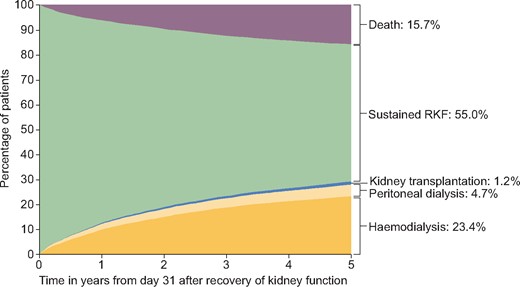
Cumulative incidence of death, kidney transplantation and dialysis as competing events during 5 years after RKF.
DISCUSSION
This study shows that permanent discontinuation of maintenance dialysis occurs in 1.2% of ESKD patients in Europe across a variety of underlying kidney diseases, although patients with PRD categories tubular necrosis (irreversible) or cortical necrosis, systemic sclerosis and HUS have the highest likelihood of RKF. More than half of the sustained RKF occurred within 1 year after Day 91 of dialysis, showing that sustained RKF is not necessarily an early event. Moreover, the time point at which RKF occurred was found to differ between the types of underlying kidney diseases.
Although the observed incidence of RKF is in line with the average of 1% reported in case series and registries in 2000–2013 [9], these former reports display a wide heterogeneity in definitions of ESKD and follow-up time and thereby the observed duration of RKF. A recent study that included 194 000 individuals in the US Medicare ESRD programme found sustained dialysis-independent RKF to occur in >5% of patients, primarily in the first 2 months after initiation of dialysis [8]. That study was limited by a follow-up time of at most 2 years and included all patients who started dialysis instead of only those remaining on dialysis after 91 days, thereby likely comprising a substantial number of patients who potentially became dialysis independent by recovering from AKI [8]. By exclusive inclusion of patients with ESKD as currently defined [14], we have attempted to minimize the possibility of AKI underlying the temporary need for KRT. In other smaller studies in patients with ESKD defined as such, the incidence of RKF varied from 0.8 to 4% [3, 6, 7, 15, 16]. Of these, the study by MacDonald et al. [7] of the ANZDATA Registry was the largest and most comparable with this study, reporting RKF to occur in 1.1% of the 15 912 individuals treated with peritoneal dialysis (PD) and 1.0% of 23 658 patients treated with HD between 1963 and 2006. In that study, RKF occurred after ∼1 year. Twenty-one percent of recovered patients died within 1 year after RKF, 57% returned to KRT and 22% were alive with dialysis-independent kidney function at the end of that study. In contrast, we found a lower mortality of 15.7% after 5 years, with a substantially greater number of recovered patients (55%) who did not return to KRT until the end of follow-up. Moreover, only 28% of recovered patients returned to KRT in our study as compared with 57% in the ANZDATA Registry [7]. Patient characteristics were quite similar between this study and that of the ANZDATA Registry. The observed differences in dialysis-free survival after RKF are not likely to be explained by the longer follow-up time in that study, as in our study only 1–2% of the patients with a non-sustained RKF required restart of KRT after 5 years of dialysis discontinuation. Hence this relatively small number of patients would be insufficient to fully explain the difference. Other potential explanations are geographical differences in treatment strategies or the larger cohort and the more recent era in our study. Although the rate of RKF in the ANZDATA Registry did not change when analyses were restricted to patients starting dialysis from 1996 [7], we did find an increase in the incidence of RKF over time, which was significant for the years 1997–2007. This trend was also observed in the US Renal Data System database [17, 18] and in a smaller single-country study [4]. Whether the observed increase in incidence of RKF over time merely reflects changes in coding practices by physicians, variations over time in the practice of early versus late start of dialysis or true increments by means of improved treatment strategies for certain kidney diseases remains to be established. Finally, we observed a decline in the incidence of RKF in the years 2012–2015, however, additional data over a longer period of time are needed to confirm whether this trend is truly significant.
Several studies have shown that the underlying kidney disease is the most important factor associated with RKF [6, 7, 15, 18, 19]. This is in line with our findings, as patients with PRD codes for tubular necrosis (irreversible) or cortical necrosis, systemic sclerosis and HUS had the highest likelihood of RKF, followed by patients with paraproteinaemia, vasculitis and ischaemic renal disease/cholesterol embolism. In contrast, patients with congenital or cystic kidney disease expressed the lowest likelihood of RKF. This also applied to patients with diabetes mellitus. Our finding that the PRD categories systemic sclerosis, tubular necrosis (irreversible) or cortical necrosis, HUS and paraproteinaemia were associated with a higher incidence of RKF is consistent with previous reports [6, 7, 15]. This is also in line with clinical practice experience, as patients with these underlying kidney diseases often present with AKI with possible treatment options to restore kidney function [20]. In a subgroup analysis of autoimmune diseases in the ANZDATA Registry [6, 7], RKF was associated with microscopic polyangiitis, consistent with our observed association between vasculitis and a higher likelihood of RKF. In turn, we could confirm their observed positive association between interstitial nephritis and RKF [7], although in our analyses the association was not as strong as previously reported (data not shown). Multiple myeloma [21, 22], systemic sclerosis [23, 24] and HUS [5] were associated with a higher likelihood of RKF in earlier smaller observational studies, which is also consistent with our current findings.
In this study, female sex, HD, onset of dialysis at an older age and initiation of dialysis in a more recent era were also associated with a higher likelihood of RKF, although to a far lesser extent than the underlying kidney disease. A possible explanation for the observed negative association between age at dialysis onset and the occurrence of RKF is that in younger patients, congenital, cystic and diabetic disease might be overrepresented. These kidney diseases are associated with a low likelihood of RKF. In addition, older patients might be more likely to experience pre-ESKD AKI, due to a higher prevalence of heart failure or cardiorenal syndrome in these older patients. Age and sex have been associated with RKF in observational studies employing different definitions of ESKD and RKF [9, 10]. However, in the ANZDATA Registry, after adjustment for comorbidities, smoking and body mass index, there was no statistically significant independent association of RKF with age, sex or type of comorbidity [6]. Finally, we found a higher likelihood of RKF in patients treated with HD as compared with PD. A possible explanation for this association might be that patients who become dialysis dependent due to AKI, whether or not superimposed on an underlying kidney disease, usually start on HD rather than PD. Existing data comparing RKF in HD and PD are inconclusive [10]. The ANZDATA Registry showed that dialysis modality had no relationship with the rate, timing or durability of the RKF [7]. Hence the difference with our findings is either explained by more extensive adjustment for possible confounders in that study or by geographical and historical differences between our study and those of the ANZDATA Registry [7].
Our study has several limitations. First, information on race; body mass index; comorbidities (heart failure or cardiorenal syndrome in particular); use of medication such as renin–angiotensin–aldosterone system inhibitors, non-steroidal anti-inflammatory drugs or immunosuppressive drugs; type of vascular access; delivered dialysis dose and measurements of residual kidney function at the time of dialysis onset or during maintenance dialysis were unavailable. Due to the lack of these data, we could not adjust our analyses for these potentially relevant factors. Second, although we exclusively included patients with ESKD, it is possible that a considerable number of included patients became maintenance dialysis dependent due to AKI rather than progression of their kidney disease. In a retrospective cohort of 47 341 incident HD patients, 54% had experienced at least one AKI event in the 2 years prior to ESKD [25]. Moreover, in that study, 1-year all-cause mortality was higher in the pre-ESKD AKI group compared with the non-AKI group [adjusted odds ratio 1.36 (95% CI 1.30–1.42)]. Since we do not have data on pre-ESKD AKI events, presence of cardiorenal syndrome, use of nephrotoxic medications or serum creatinine slopes in the time prior to initiation of KRT, it is impossible to investigate to what extent possible pre-ESKD AKI events influenced the observed incidence of RKF or mortality in our study. Furthermore, within the ERA-EDTA Registry we can only rely on the kidney disease as coded by the treating physicians, according to pre-specified, not necessarily histologically proven, PRD codes. Hence it is conceivable that patients, and particularly those registered with the PRD code ‘Tubular necrosis (irreversible) or cortical necrosis’, did indeed experience AKI. Notwithstanding, approximately half of these patients experienced RKF after 1 year of dialysis treatment. This supports the message of this study that AKI, whether superimposed on an underlying PRD or not, might still result in RKF even after a long duration of maintenance dialysis. Third, as information on patients who permanently discontinued KRT might be incomplete, the mortality rate after permanent discontinuation of KRT (sustained RKF) might be underestimated. Yet we consider it unlikely that many patients who were qualified as having experienced sustained RKF discontinued dialysis in order to pursue conservative medical or palliative care. Finally, due to the observational design of this cohort study, it is impossible to study causal inference.
Previous reports on RKF have suggested postponing kidney transplantation in patients on maintenance dialysis with certain types of underlying kidney disease associated with a high likelihood of RKF [3, 5]. Until more specific markers of improvement in kidney function can be identified to guide management, and based on our current findings with the aforementioned limitations or on other available literature on this subject to date, we do not advocate postponing kidney transplantation in patients with kidney disease associated with a high likelihood of RKF.
In conclusion, our study shows that permanent discontinuation of maintenance dialysis in patients with ESKD is an uncommon event that occurs across a variety of underlying kidney pathologies, although patients with certain types of kidney disease have the highest likelihood of RKF in this setting. In addition, sustained RKF is not necessarily an early event. Therefore, although rare, clinicians should be vigilant of the occurrence of RKF, even in patients who have been treated with maintenance dialysis for a longer period of time.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
ACKNOWLEDGEMENTS
We would like to thank the patients and the staff of the dialysis and transplant units for contributing the data via their national and regional renal registries. Furthermore, we gratefully acknowledge the following registries and persons for their contribution of the data: Austrian Dialysis and Transplant Registry (OEDTR) (R. Kramar); Dutch-speaking Belgian Society of Nephrology (NBVN) (M. Couttenye and F. Schroven); French-speaking Belgian Society of Nephrology (GNFB) (J.M. des Grottes); Renal Registry Bosnia and Herzegovina (H. Resić, B.Jakovljevic and M.Tomić); Danish Nephrology Registry (DNS) (J.G. Heaf); Finnish Registry for Kidney Diseases (P. Finne and P.H. Groop); French Renal Epidemiology and Information Network (REIN) (M. Lassalle and C. Couchoud); Hellenic Renal Registry (G. Moustakas); Icelandic End-Stage Renal Disease Registry; Norwegian Renal Registry (A.V. Reisæter); Swedish Renal Registry (SRR) (K.G. Prütz, M. Stendahl, M. Evans, S. Schön, T. Lundgren and M. Segelmark); Dutch Renal Registry (RENINE) (L. Heuveling and S. Vogelaar); UK Renal Registry (all the staff of the UK Renal Registry and of the renal units submitting data); Scottish Renal Registry (SRR) (all of the Scottish renal units); the regional registries of Andalusia (SICATA) [P. Castro de la Nuez (on behalf of all users of SICATA)], Aragon (F. Arribas Monzón, J.M. Abad Diez and J.I. Sanchez Miret), Asturias (P. Beltrán, J.R. Quirós and the RERCA Working Group), Basque Country (UNIPAR) (Á. Magaz, J. Aranzabal, M. Rodrigo and I. Moina), Cantabria (J.C. Ruiz San Millán, O. Garcia Ruiz and C. Piñera Haces), Castile and León (M.A. Palencia García), Castile-La Mancha (G. Gutiérrez Ávila and I. Moreno Alía), Catalonia (RMRC) (E. Arcos, J. Comas and J. Tort), Community of Madrid (M.I. Aparicio de Madre), Extremadura [all the renal units (Nephroloy and Dialysis)], Galicia (E. Bouzas-Caamaño), Renal Registry of the Region of Murcia (I. Marín Sánchez) and Valencia region (REMRENAL) (M. Ferrer Alamar, N. Fuster Camarena and J. Pérez Penadés); and the other ERA-EDTA registry committee members not mentioned above for their advice in the analysis and the drafting of this article: C. Zoccali, P. Ambühl, J. Harambat, L. Mercadal, M. Nordio, S.S. Sørensen and E. Vidal; and R. Boenink in the Academic Medical Center Registry office for data collection and management.
FUNDING
The ERA-EDTA Registry is funded by the ERA-EDTA.
AUTHORS’ CONTRIBUTIONS
This article was written by L.J., A.K., A.Å., J.D.M., C.S.P., J.H., M.H., A.H., M.A., S.B., L.M., C.D.C., R.P., M.B.S., J.K., F.C., Z.A.M., K.J.J. and M.N. on behalf of the ERA-EDTA Registry, which is an official body of the ERA-EDTA.
CONFLICT OF INTEREST STATEMENT
M.A. reports personal fees from Amgen, AstraZeneca, Boehringer Ingelheim, Menarini and Sanofi, all outside the submitted work. J.d.M. reports personal fees from Menarini, outside the submitted work. K.J.J. reports grants from the ERA-EDTA during the conduct of the study.
REFERENCES
ERA-EDTA Registry. ERA-EDTA Registry Annual Report 2016. Amsterdam: Amsterdam UMC, location AMC, Department of Medical Informatics,
Kidney Disease: Improving Global Outcomes CKD Work Group.






Comments