-
PDF
- Split View
-
Views
-
Cite
Cite
Mathieu Legendre, Elise Marechal, Jean-Michel Rebibou, Emilie Gaiffe, Cecile Courivaud, Jamal Bamoulid, Thomas Crepin, Didier Ducloux, Genetic determinant of thymopoiesis and clinical outcomes in renal transplantation, Nephrology Dialysis Transplantation, Volume 36, Issue 12, December 2021, Pages 2345–2347, https://doi.org/10.1093/ndt/gfab180
Close - Share Icon Share
A premature decline of thymic output in patients with chronic kidney disease (CKD) is associated with a greater risk of infectious and neoplastic events [1, 2], while its evolution after transplantation has an impact on cancer, infections and rejection occurrences [3–5]. In addition, low pre-transplant thymic function is predictive of death after transplantation [6, 7]. Recently a common intergenic variant located in the TCRA/TCRD locus was linked to interindividual variability of thymic output [8]. In the general population, GG homozygous (wild allele) carriers of the rs2204985 polymorphism had better thymopoiesis than AA homozygous (variant) patients. Our work aimed to analyse the allele impact on thymopoiesis in a cohort of CKD patients receiving a kidney transplant.
This work involved 550 patients from the ORLY-EST (Influence de l'Orientation de la Re’ponse LYmphocytaire dans l'athe ’rosclerose post-trans-plantation) prospective study [5] whose lymphocytic DNAs on the day of transplantation were available. Patients were divided into three groups according to their genotype (AA, AG or GG) for the rs2204985 variant. The genotyping of rs2204985 was performed on peripheral blood mononuclear cells with specific Taqman probes (C_2609811_10; Thermo Fisher Scientific, Waltham, MA, USA) in real-time polymerase chain reaction.
Thymopoiesis was evaluated by CD4+ recent thymic emigrant (RTE) count. CD4+ RTEs were defined in flow cytometry as CD45RA+CD31+CD4+ T cells. T cell phenotypes were performed at day 0 and at 12 (n = 471) and 36 months (n = 128) after transplantation. Follow-up procedures were published previously [5].
The collection of biological and clinical data associated with this study was approved by the French Ministry of Health (authorization DC-2008-713, 11 June 2009) and by the ethics committee of Franche-Comté (2008) and was in accordance with the Helsinki Declaration of 1975 as revised in 2013. Each included patient had previously given oral and written consent.
Data are expressed as mean ± standard deviation for continuous variables and percentage (number) for frequencies. Comparisons of quantitative values between three groups were conducted using analysis of variance (ANOVA) or Kruskal–Wallis tests, depending on whether the normality conditions were met or not. Factors associated with RTE variation were evaluated in multiple linear regression models. Log-rank tests and Cox models were used for survival analyses. All statistical tests were carried out using SPSS version 23 (IBM, Armonk, NY, USA) and GraphPad (GraphPad Software, San Diego, CA, USA). Detailed methods are described in Supplementary data, File S1. Data are available upon request.
Among included patients, 26.9% (n = 148) were AA homozygotes, 23.5% (n = 129) were GG homozygotes and 49.6% (n = 273) were AG heterozygous. There was no difference in clinical and demographic data between the three groups at day 0 and at 12 and 36 months after transplantation (Supplementary data, Tables S1–S3). The RTE counts the day of transplantation were not significantly different between groups (2.12 ± 0.38, 2.11 ± 0.41 and 2.14 ± 0.36 log10/mm3 in the AA, AG and GG groups, respectively; P = 0.7) (Figure 1A). Three years after transplantation, GG carriers had a higher RTE count than A carriers (2.01 ± 0.53, 2.03 ± 0.39 and 2.25 ± 0.35 log10/mm3 in the AA, AG and GG groups, respectively; P = 0.04). Of note, the difference was not significant at 1 year post-transplant (Figure 1B and C). Secondary analyses of patients who received a first transplant and/or received anti-thymocyte globulins (ATGs) showed equivalent results.
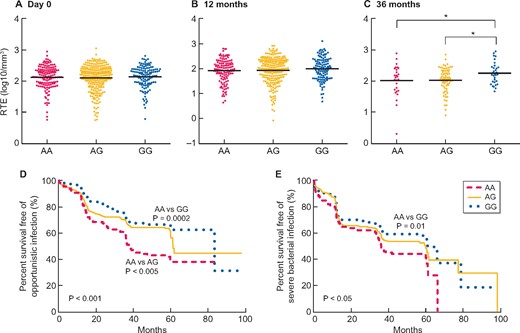
Evaluation of the number of CD4+ RTEs regarding the allele polymorphism (A) the day of transplantation and (B) 12 months and (C) 36 months after transplantation. Survival curves of (D) opportunistic infections and (E) severe bacterial infections according to the allelic polymorphism. Comparisons of RTE counts between two groups were assessed with unpaired t-tests. Horizontal lines represent the mean RTE count per group. *P < 0.05. Log-rank tests were used for survival curves. Significance was taken at the 5% level.
In multiple linear regression, the GG allele was not associated with the RTE count at transplant or at 1 year post-transplant but was associated with the RTE count 3 years post-transplant {β coefficient 0.22 [95% confidence interval (CI) 0.128–0.315], P < 0.001}. Factors associated with the RTE count at transplant were age, female gender, past history of neoplasia and diabetes. At 1 year post-transplant, the RTE count was related to age, pre-transplant dialysis, pre-transplant RTE count, ATG induction and the use of tacrolimus maintenance. At 3 years post-transplant, the RTE count was associated with age, pre-transplant RTE count, ATG induction and GG allele group.
During a follow-up of 43.5 ± 24.0 months, 9.4% (n = 52) of patients died, 13.8% (n = 76) had to start dialysis, 46.2% (n = 254) had a severe bacterial infection, 36.5% (n = 201) experienced opportunistic infection and 20.3% (n = 112) were treated for acute rejection. Overall survival and kidney prognosis were not statistically different between groups. In contrast, in univariate and multivariate analyses, GG carriers were less prone to suffer from opportunistic infections than A carriers [hazard ratio 0.69 (95% CI 0.48–0.98), P = 0.04]. We also observed a trend towards less frequent severe bacterial infections in GG carriers (Figure 1D and E and Supplementary data, Table S4).
CKD is associated with a premature decline of thymic function that induces a decrease in the CD4+ RTE count [1, 2]. However, great interindividual variability in the RTE count was observed in both CKD patients and healthy populations [9]. Recently the polymorphism of rs2204985 has been proposed as an explanation for these interindividual differences. rs2204985 is in an intergenic region of decondensed chromatin within the TCRA/TCRD locus. It participates in the encoding of the T cell receptor and its rearrangement during the double-positive stage of thymic maturation [8, 9].
We observed a similar distribution of the variant in CKD patients as in two independent recently reported French cohorts [8]. While GG homozygous healthy patients free of CKD had better thymopoiesis, we did not observe any difference in CKD patients. Nevertheless, this difference reappeared at 3 years post-transplant. This suggests that the uraemic environment could conceal the effect of the genetic variant, which could be restored after transplantation. Of note, the population with an RTE count evaluation at 3 years post-transplant accounted for less than a quarter of the original one and the difference in the RTE count between the AA and GG groups was rather low. Although we did not find any confounding factors, such as initial induction or maintenance therapies on the day of transplantation and 12 and 36 months later, these results should be analysed with caution. Interestingly, better thymopoiesis in GG carriers who received a kidney transplant was associated with a lesser risk of opportunistic infections and a marginal reduction in bacterial infections. Immunosuppressive protocols were available at transplant but not during the complete follow-up. However, because modifications in immunosuppressive drugs are not based on the RTE count, a difference in strategy in the three groups is unlikely.
It should be noted that thymopoiesis was evaluated by the CD4+ RTE count, while Clave et al. [8] used the number of T cell excision circles. Although these evaluations are not equivalent, they are surrogate markers of thymopoiesis [1, 8, 10, 11].
The rs2204985 polymorphism could be associated with better thymic function and a reduced risk of infections in kidney transplant patients. These results must be confirmed in a replication cohort.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
ACKNOWLEDGEMENTS
The authors would like to thank all the investigators of the ORLY-EST study.
FUNDING
This work was supported by grants from the Fondation Transplantation, the PHRC 2005 and 2011 (to D.D.), the Fondation de France (Appel d’offre ‘Maladies Cardiovasculaires’ 2007 #2007 001859 to P.S.), the DHOS/INSERM/INCa (Appel d’offre Recherche Translationnelle 2008 to D.D.) and the APICHU 2010 (to J.B.). J.B., C.C. and T.C. received financial support from the Fondation Transplantation (ET-031211 and ET-050320, respectively). This work was also sponsored by the Fédération hospitalo-universitaire INCREASE (INtegrated Centre for Research in Inflammatory DisEASEs).
AUTHORS’ CONTRIBUTIONS
M.L. and E.M. prepared the manuscript and performed the statistical analysis. C.C., J.B., T.C. and D.D. planned the research, coordinated the study and collected the clinical data. M.L., E.M., T.C. and E.G. performed most of the biological tests and analysed the data. E.G. participated in the design of the study. All authors revised the manuscript. M.L., E.M. and D.D. authors contributed equally to this work as first and last authors.
CONFLICT OF INTEREST STATEMENT
The results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
Author notes
Mathieu Legendre, Elise Marechal, Didier Ducloux contributed equally to this work as first and last authors.





Comments