-
PDF
- Split View
-
Views
-
Cite
Cite
Peter Rossing, Jorma Strand, Angelo Avogaro, Michael Becka, Friederike Kanefendt, Christiane Otto, on behalf of the investigators of the CADA DIA trial, Effects of the chymase inhibitor fulacimstat in diabetic kidney disease—results from the CADA DIA trial, Nephrology Dialysis Transplantation, Volume 36, Issue 12, December 2021, Pages 2263–2273, https://doi.org/10.1093/ndt/gfaa299
Close - Share Icon Share
Abstract
The protease chymase generates multiple factors involved in tissue remodelling including angiotensin II (Ang II) and has been implicated in the pathophysiology of diabetic kidney disease (DKD). This study investigated the effects of the chymase inhibitor fulacimstat on albuminuria in patients with Type II diabetes mellitus and a clinical diagnosis of DKD.
In this double-blind, randomized, placebo-controlled trial, patients were on the maximum tolerated dose of either an Ang II receptor blocker or an Ang-converting enzyme inhibitor since at least 3 months before the screening visit. Eligible patients were randomized in a 2:1 ratio to treatment with either 25 mg fulacimstat (n = 99) or placebo (n = 48) twice daily on top of standard of care.
The randomized patients had a mean urine albumin–creatinine ratio (UACR) of 131 mg/g (range: 29–2429 mg) and a mean (standard deviation) estimated glomerular filtration rate of 60.8 ± 16.9 mL/min/1.73 m2 before treatment start. Fulacimstat was safe and well tolerated, and achieved mean total trough concentrations that were ∼9-fold higher than those predicted to be required for minimal therapeutic activity. UACR increased by 27.4% [coefficient of variation (CV) 86%] and 3% (CV 88.9%) after 24 weeks of treatment with placebo or fulacimstat, respectively. Analysis of covariance revealed a least square mean UACR ratio (fulacimstat/placebo) of 0.804 (90% CI 0.627–1.030, P = 0.1477), indicating a statistically non-significant UACR reduction of 19.6% after fulacimstat treatment compared with placebo.
Fulacimstat was safe and well tolerated but did not reduce albuminuria in patients with DKD. These findings do not support a therapeutic role for chymase inhibition in DKD.
What is already known about this subject?
chymase generates mediators of tissue remodelling including angiotensin II (Ang II) that have been implicated in the pathophysiology of diabetic kidney disease (DKD);
in animal experiments, chymase inhibitors ameliorate mesangial cell proliferation, proteinuria and interstitial fibrosis; and
in kidneys from DKD patients, chymase is markedly upregulated with a differential expression pattern compared with Ang-converting enzyme.
What this study adds?
to assess whether chymase inhibition can reduce albuminuria in patients with Type 2 diabetes mellitus and a clinical diagnosis of DKD, we performed a randomized placebo-controlled trial with the potent and selective chymase inhibitor fulacimstat; and
after 24 weeks of treatment, fulacimstat had no statistically significant effect on albuminuria progression and did not reduce baseline albuminuria.
What impact this may have on practice and policy?
although failure to reduce albuminuria does not preclude beneficial clinical effects in general, subgroup and sensitivity analyses do not support a role for chymase inhibition in DKD treatment.
INTRODUCTION
Approximately 50% of patients with Type II diabetes mellitus (T2DM) develop diabetic kidney disease (DKD) [1]. DKD is characterized by a persistently elevated urinary albumin–creatinine ratio (UACR) of ≥30 mg/g and/or a persistent reduction in estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2. Progressive declines in renal function finally lead to end-stage renal disease (ESRD) requiring renal replacement therapy [1]. In addition, DKD patients with albuminuria face an increased cardiovascular risk, which increases with disease progression [2]. As clinical studies have revealed an association between albuminuria reduction and improved cardiovascular outcomes as well as reduced progression to ESRD [3–5], albuminuria reduction is one mainstay of DKD treatment.
Chymase is a chymotrypsin-like serine protease, which is secreted locally from activated mast cells and other cells in response to inflammation and tissue injury. In diseased tissues, chymase generates and activates factors such as angiotensin II (Ang II), transforming growth factor (TGF)-β1 and matrix metalloproteinases, which are implicated in hallmarks of DKD such as albuminuria, mesangial cell expansion and interstitial fibrosis [6]. Mast cell density and chymase expression are increased in kidneys from DKD patients. Marked chymase upregulation—correlating with the degree of hypertension and albuminuria—is observed in mesangial cells and vascular smooth muscle cells [7]. In contrast, Ang-converting enzyme (ACE)—the second Ang II-generating enzyme—seems to be upregulated in the tubulo-interstitium [7]. The differential expression of ACE and chymase in kidneys of DKD patients suggests that chymase inhibition on top of current standard of care [ACE inhibitors (ACEi); Ang II receptor blockers (ARBs)] may provide additional benefits for the treatment of DKD. In vitro studies revealed increased chymase expression and Ang II generation in human mesangial cells cultivated under high glucose conditions [8]. In rat mesangial cells, chymase induced the TGF-β1 pathway in an Ang II-independent pathway, underlining that chymase inhibition can provide additional effects to ACEi or ARB therapy [9]. Several chymase inhibitors ameliorated DKD progression in different diabetic animal models [9–11]. Compared with an ACEi, a specific chymase inhibitor was much more effective in reducing oxidative stress, mesangial cell expansion, TGF-β1 expression and proteinuria in diabetic hamsters without exhibiting any haemodynamic effects [11].
Fulacimstat is a specific chymase inhibitor [12, 13] and was safe and well tolerated in healthy male subjects [14] and in patients suffering either from chronic systolic heart failure [15] or from left-ventricular dysfunction (LVD) after acute myocardial infarction (MI) [16]. To assess whether chymase inhibition could be a novel therapeutic strategy in DKD, we performed the ChymAse inhibitor for DiAbetic kiDney dIseAse (CADA DIA) trial and tested the hypothesis that fulacimstat—applied on top of standard of care medication—would reduce albuminuria in DKD patients.
MATERIALS AND METHODS
Study design and patients
CADA DIA was a multicentre, randomized, double-blinded Phase IIa trial in patients with T2DM and a clinical diagnosis of DKD. The trial was performed under the principles outlined in the declaration of Helsinki and supervised by an independent data monitoring committee. The study protocol was approved by the health authorities and institutional review boards in all participating countries. Written informed consent was obtained from all individual subjects before participation. Patients were enrolled in 27 study centres in Denmark, Sweden, Finland, Israel, Italy, Spain and Bulgaria (Supplementary data, Table S1). The study was registered at ClinicalTrials.gov (NCT 03412006) and was conducted between February 2018 and October 2019. Eligible patients (men and women without childbearing potential, 18–79 years of age) had to be treated with either an ACEi or an ARB—but not with both simultaneously—at the maximum tolerated dose for at least 3 months before the screening visit, whereby the maximum tolerated dose had to be at least as high as the minimal recommended target dose of an ACEi or ARB according to national or international guidelines. Creatinine-based eGFR (eGFRCrea) was ≥30 mL/min/1.73 m2 and <90 mL/min/1.73 m2 (Chronic Kidney Disease Epidemiology Collaboration formula) as determined by a central blinded core laboratory. For inclusion, UACR had to be >50 mg/g in two out of three consecutive morning void samples at the screening visit and at the baseline visit. Mean UACR at Visit 1 had to be >30 mg/g, otherwise the patient was withdrawn from the study according to protocol. Key exclusion criteria were systolic blood pressure >160 mmHg or diastolic blood pressure >100 mmHg; haemoglobin <9 g/dL, haemoglobin A1c level >12% (108 mmol/mol); non-DKD DM induced by immunosuppressant therapy; acute kidney injury, dialysis, stroke, acute coronary syndrome or hospitalization for heart failure within the last 3 months before the screening visit; previous kidney transplant; clinically relevant hepatic dysfunction; medication with strong CYP3A4 (cytochrom P450 3A4) inhibitors or inducers, and medication with sensitive CYP2C8 (cytochrom P450 2C8) substrates during the active treatment phase.
Visit scheme
The study consisted of a screening period, a treatment period and a washout phase. To ensure stable and persistent albuminuria despite optimal standard of care, a screening visit (within 3–6 weeks before Visit 1) and a confirmatory baseline visit (within 2 weeks before Visit 1) were performed. At Visit 1, eligible patients were randomized and assigned in a 2:1 ratio to receive either fulacimstat at a dose of 25 mg given twice daily (BID) as an immediate release tablet for 24 weeks or matching placebo. As UACR measurements were performed in a central core laboratory, the UACR values at Visit 1 were only known after randomization at Visit 1. Therefore, patients were pre-stratified according to high (∼75%) and very high albuminuria (∼25%) based on the UACR results obtained at the baseline visit. According to the protocol, patients with a mean UACR <30 mg/g at Visit 1 were withdrawn from the study. The final analysis was performed based on the stratification of UACR values obtained at Visit 1 [UACR high albuminuria (microalbuminuria) >30 mg/g and ≤300 mg/g; UACR very high albuminuria (macroalbuminuria) >300 mg/g and <3000 mg/g]. Visits 2, 3, 4, 5 and 6 were scheduled ∼2, 4, 8, 16 and 24 weeks after Visit 1. Following a washout phase of 14 ± 7 days after the last study drug intake at Visit 6, a follow-up visit was performed.
Safety monitoring
Vital signs, 12-lead electrocardiogram, haematology, serum chemistry, clotting status and urinalysis were monitored at all visits. Adverse events (AEs) mentioned during the patient interview performed by a member of the investigator team or spontaneously reported by the subject were documented. AEs starting after the first intake of study drug up to 3 days after the last intake of study drug were considered as treatment-emergent AEs (TEAEs).
Pharmacokinetics
Plasma concentrations of fulacimstat were determined within pre-defined time windows using a sparse sampling approach [120–240 min after study drug administration for Visit 1 (Day 0); towards end of the visit for Visits 4 (Day 55), 5 (Day 112) and 6 (Day 168); pre-administration, 30–90 min, 120–240 min after study drug administration, and towards the end of the visit for Visit 3 (Day 28)]. The plasma concentrations of fulacimstat were measured as described previously using a validated method [14, 16].
Dosing rationale
Preclinical pharmacokinetic (PK)/pharmacodynamic relationship revealed that therapeutically active doses should achieve exposures over the dosing interval that are at least as high as the in vitro half-maximal inhibitory concentration (IC50) of human chymase (52 μg/L, corrected for the fraction unbound) [12–14]. According to this analysis doses of 2 mg BID or 20 mg once daily would be the minimal doses expected to be therapeutically active. CADA DIA was planned to evaluate whether fulacimstat shows any effects on UACR in patients with DKD or not by applying one dose leading to almost complete chymase blockade. Therefore, 25 mg BID was selected leading to exposures higher than required for minimal therapeutic activity (covering the IC90 of human chymase over the dosing interval of 12 h) but still selective for chymase inhibition.
Study objectives and statistical methods
The primary objective of this explorative trial was the change in UACR (at Visit 1) to 24 weeks of treatment (Visit 6) with fulacimstat in comparison with placebo. The secondary objective was the assessment of safety and tolerability. Other objectives included the changes in eGFR from Visit 1 to Visits 2–6, as well as PK.
UACR was determined in the first morning void urines on three consecutive days before each study visit and a surrogate UACR (UACRc) was calculated from these three measurements as follows: UACRc was the median if the coefficient of variation (CV) was >25% and it was the geometric mean otherwise. The change in UACR from Visit 1 to each consecutive visit was expressed as ratio to baseline (obtained at Visit 1) using geometric means, standard deviation (SD) and geometric CVs. To demonstrate any effect of fulacimstat on UACR after 6 months of treatment, an analysis of covariance model was fitted to the log-transformed UACR at Visit 6 (167 ± 14 days after Visit 1) including a factor for treatment group and the log-transformed baseline UACR at Visit 1 as covariate. The UACR values and ratios were transformed because the primary variable was assumed to be log-normally distributed. Based on this analysis, a point estimate for the UACR ratio fulacimstat/placebo [least square (LS) mean ratio] and a 90% CI (confidence interval) were calculated by re-transformation of the results on the logarithmic scale. A sample size of at least 96 patients randomized 2:1 to fulacimstat (n = 64) and placebo (n = 32) would have 80% power to detect a difference to placebo in UACR change to baseline of at least 30%. Summary statistics were presented for quantitative variables. Qualitative data were described utilizing frequency tables. No adjustments for multiplicity were made in this exploratory trial.
RESULTS
Patient characteristics and disposition
A total of 361 patients were screened; 214 patients were screen failures and 147 patients were randomized in a 2:1 ratio to receive either fulacimstat (99 patients) or placebo (48 patients) (Figure 1). All randomized patients received at least one dose of study drug and were included in the safety analysis set and for the analysis of baseline characteristics (Table 1). All patients receiving fulacimstat were included in the PK analysis set. The reasons for discontinuation are depicted in Figure 1. In total, 139 patients completed the treatment phase, three of them had UACR values that were confounded either at Visit 1 or 6 by unusual physical activity, worsening glycaemic control or respiratory tract infection. Therefore, 136 patients having valid UACR values at Visits 1 and 6 were included in the intention to treat (ITT) analysis in accordance with the statistical analysis plan before unblinding (Figure 1). Fifteen patients were excluded from the per protocol set (PPS), which consisted of 38 patients treated with placebo and 83 treated with fulacimstat (Figure 1). Within the safety analysis set, the two treatment groups were well matched regarding their baseline characteristics, concomitant medication and medical history (Table 1). As required per protocol, all but one patient in the fulacimstat group received either an ARB or an ACEi. Patients were obese and almost all suffered from arterial hypertension (Table 1). Cardiovascular comorbidities were present in almost two-thirds of the overall patient population, encompassing stroke, transitory ischaemic attack, MI, heart failure, ischaemic heart disease, unstable angina, atrial fibrillation, LVD, left-ventricular hypertrophy, cardiac conduction deficits and peripheral artery disease. There was a small imbalance in baseline albuminuria, as slightly fewer patients in the placebo group than in the fulacimstat arm belonged to the high albuminuria stratum (Table 1).
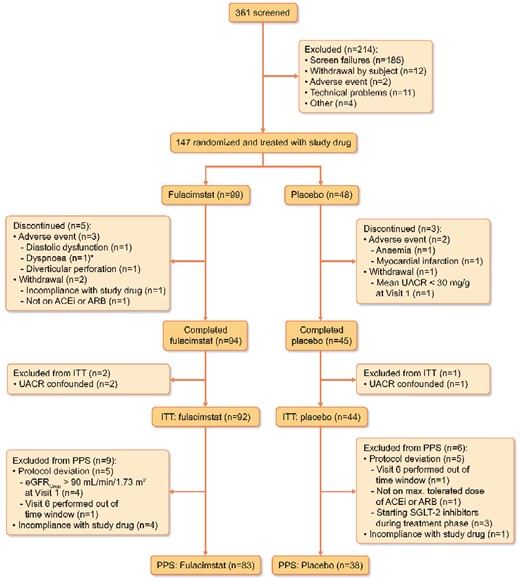
Patient flow. ITT, PPS. *The patient excluded due to dyspnoea also suffered from pollakiuria, dry mouth and peripheral oedema.
| Parameter . | Placebo, n = 48 . | Fulacimstat, n = 99 . | Total, n = 147 . |
|---|---|---|---|
| Male sex, % | 40 (83.3) | 84 (84.8) | 124 (84.4) |
| Age, years | 66.5 ± 7.8 | 69.1 ± 6.3 | 68.2 ± 6.9 |
| BMI, kg/m2 | 31.2 ± 4.7 | 31.4 ± 4.9 | 31.3 ± 4.8 |
| Systolic blood pressure, mmHg | 139 ± 16 | 135 ± 13 | 136 ± 14 |
| Diastolic blood pressure, mmHg | 77 ± 9 | 74 ± 10 | 75 ± 10 |
| High albuminuria, % | 32 (66.7) | 78 (78.8) | 110 (74.8) |
| UACR at Visit 1, mg/g | 140 (31–2429) | 129 (29–2165) | 131 (29–2429) |
| eGFR ≤60 mL/min/1.73 m2, % | 22 (45.8) | 49 (49.5) | 71 (48.3) |
| eGFR at Visit 1, mL/min/1.73 m2 | 61.2 ± 16.5 | 60.5 ± 17.2 | 60.8 ± 16.9 |
| Medical history,a % | |||
| Arterial hypertension | 47 (97.9) | 98 (99.0) | 145 (98.6) |
| Cardiovascular comorbidities | 33 (68.7) | 62 (62.6) | 95 (64.6) |
| Hyperlipidaemia/dyslipidaemia | 25 (52.1) | 51 (51.5) | 76 (51.7) |
| Obesity | 19 (39.6) | 41 (41.4) | 60 (40.8) |
| Diabetic neuropathy | 16 (33.3) | 23 (23.2) | 39 (26.5) |
| Diabetic retinopathy | 10 (20.8) | 25 (25.3) | 35 (23.8) |
| Hypercholesterinaemia | 8 (16.7) | 25 (25.3) | 33 (22.4) |
| Comedication,a % | |||
| ACEis | 15 (31.3) | 24 (24.2) | 39 (26.5)b |
| Ang II receptor blockers (%) | 33 (68.7) | 74 (74.7) | 107 (72.8)b |
| β-blockers | 31 (64.6) | 61 (61.6) | 92 (62.6) |
| Calcium channel blockers | 34 (70.8) | 64 (64.6) | 98 (66.7) |
| Diuretics | 26 (54.2) | 59 (59.6) | 85 (57.8) |
| Metformin | 40 (83.3) | 76 (76.8) | 116 (78.9) |
| Insulin and analogues | 20 (41.7) | 49 (49.5) | 69 (46.9) |
| DPP4 inhibitors | 17 (35.4) | 31 (31.3) | 48 (32.7) |
| SGLT2 inhibitors | 17 (35.4) | 32 (32.3) | 49 (33.3) |
| GLP-1 analogues | 14 (29.2) | 23 (23.2) | 37 (25.2) |
| Sulphonylureas | 14 (29.2) | 17 (17.2) | 31 (21.1) |
| Statins | 37 (77.1) | 78 (78.8) | 115 (78.2) |
| Acetylsalicylic acid | 21 (43.8) | 49 (49.5) | 70 (47.6) |
| Proton pump inhibitors | 9 (18.8) | 30 (30.3) | 39 (26.5) |
| Parameter . | Placebo, n = 48 . | Fulacimstat, n = 99 . | Total, n = 147 . |
|---|---|---|---|
| Male sex, % | 40 (83.3) | 84 (84.8) | 124 (84.4) |
| Age, years | 66.5 ± 7.8 | 69.1 ± 6.3 | 68.2 ± 6.9 |
| BMI, kg/m2 | 31.2 ± 4.7 | 31.4 ± 4.9 | 31.3 ± 4.8 |
| Systolic blood pressure, mmHg | 139 ± 16 | 135 ± 13 | 136 ± 14 |
| Diastolic blood pressure, mmHg | 77 ± 9 | 74 ± 10 | 75 ± 10 |
| High albuminuria, % | 32 (66.7) | 78 (78.8) | 110 (74.8) |
| UACR at Visit 1, mg/g | 140 (31–2429) | 129 (29–2165) | 131 (29–2429) |
| eGFR ≤60 mL/min/1.73 m2, % | 22 (45.8) | 49 (49.5) | 71 (48.3) |
| eGFR at Visit 1, mL/min/1.73 m2 | 61.2 ± 16.5 | 60.5 ± 17.2 | 60.8 ± 16.9 |
| Medical history,a % | |||
| Arterial hypertension | 47 (97.9) | 98 (99.0) | 145 (98.6) |
| Cardiovascular comorbidities | 33 (68.7) | 62 (62.6) | 95 (64.6) |
| Hyperlipidaemia/dyslipidaemia | 25 (52.1) | 51 (51.5) | 76 (51.7) |
| Obesity | 19 (39.6) | 41 (41.4) | 60 (40.8) |
| Diabetic neuropathy | 16 (33.3) | 23 (23.2) | 39 (26.5) |
| Diabetic retinopathy | 10 (20.8) | 25 (25.3) | 35 (23.8) |
| Hypercholesterinaemia | 8 (16.7) | 25 (25.3) | 33 (22.4) |
| Comedication,a % | |||
| ACEis | 15 (31.3) | 24 (24.2) | 39 (26.5)b |
| Ang II receptor blockers (%) | 33 (68.7) | 74 (74.7) | 107 (72.8)b |
| β-blockers | 31 (64.6) | 61 (61.6) | 92 (62.6) |
| Calcium channel blockers | 34 (70.8) | 64 (64.6) | 98 (66.7) |
| Diuretics | 26 (54.2) | 59 (59.6) | 85 (57.8) |
| Metformin | 40 (83.3) | 76 (76.8) | 116 (78.9) |
| Insulin and analogues | 20 (41.7) | 49 (49.5) | 69 (46.9) |
| DPP4 inhibitors | 17 (35.4) | 31 (31.3) | 48 (32.7) |
| SGLT2 inhibitors | 17 (35.4) | 32 (32.3) | 49 (33.3) |
| GLP-1 analogues | 14 (29.2) | 23 (23.2) | 37 (25.2) |
| Sulphonylureas | 14 (29.2) | 17 (17.2) | 31 (21.1) |
| Statins | 37 (77.1) | 78 (78.8) | 115 (78.2) |
| Acetylsalicylic acid | 21 (43.8) | 49 (49.5) | 70 (47.6) |
| Proton pump inhibitors | 9 (18.8) | 30 (30.3) | 39 (26.5) |
Continuous data are depicted as mean ± SD or as median (range) in case of UACR.
Cutoff >20% in total population.
One patient did neither receive an ACEi nor an ARB.
| Parameter . | Placebo, n = 48 . | Fulacimstat, n = 99 . | Total, n = 147 . |
|---|---|---|---|
| Male sex, % | 40 (83.3) | 84 (84.8) | 124 (84.4) |
| Age, years | 66.5 ± 7.8 | 69.1 ± 6.3 | 68.2 ± 6.9 |
| BMI, kg/m2 | 31.2 ± 4.7 | 31.4 ± 4.9 | 31.3 ± 4.8 |
| Systolic blood pressure, mmHg | 139 ± 16 | 135 ± 13 | 136 ± 14 |
| Diastolic blood pressure, mmHg | 77 ± 9 | 74 ± 10 | 75 ± 10 |
| High albuminuria, % | 32 (66.7) | 78 (78.8) | 110 (74.8) |
| UACR at Visit 1, mg/g | 140 (31–2429) | 129 (29–2165) | 131 (29–2429) |
| eGFR ≤60 mL/min/1.73 m2, % | 22 (45.8) | 49 (49.5) | 71 (48.3) |
| eGFR at Visit 1, mL/min/1.73 m2 | 61.2 ± 16.5 | 60.5 ± 17.2 | 60.8 ± 16.9 |
| Medical history,a % | |||
| Arterial hypertension | 47 (97.9) | 98 (99.0) | 145 (98.6) |
| Cardiovascular comorbidities | 33 (68.7) | 62 (62.6) | 95 (64.6) |
| Hyperlipidaemia/dyslipidaemia | 25 (52.1) | 51 (51.5) | 76 (51.7) |
| Obesity | 19 (39.6) | 41 (41.4) | 60 (40.8) |
| Diabetic neuropathy | 16 (33.3) | 23 (23.2) | 39 (26.5) |
| Diabetic retinopathy | 10 (20.8) | 25 (25.3) | 35 (23.8) |
| Hypercholesterinaemia | 8 (16.7) | 25 (25.3) | 33 (22.4) |
| Comedication,a % | |||
| ACEis | 15 (31.3) | 24 (24.2) | 39 (26.5)b |
| Ang II receptor blockers (%) | 33 (68.7) | 74 (74.7) | 107 (72.8)b |
| β-blockers | 31 (64.6) | 61 (61.6) | 92 (62.6) |
| Calcium channel blockers | 34 (70.8) | 64 (64.6) | 98 (66.7) |
| Diuretics | 26 (54.2) | 59 (59.6) | 85 (57.8) |
| Metformin | 40 (83.3) | 76 (76.8) | 116 (78.9) |
| Insulin and analogues | 20 (41.7) | 49 (49.5) | 69 (46.9) |
| DPP4 inhibitors | 17 (35.4) | 31 (31.3) | 48 (32.7) |
| SGLT2 inhibitors | 17 (35.4) | 32 (32.3) | 49 (33.3) |
| GLP-1 analogues | 14 (29.2) | 23 (23.2) | 37 (25.2) |
| Sulphonylureas | 14 (29.2) | 17 (17.2) | 31 (21.1) |
| Statins | 37 (77.1) | 78 (78.8) | 115 (78.2) |
| Acetylsalicylic acid | 21 (43.8) | 49 (49.5) | 70 (47.6) |
| Proton pump inhibitors | 9 (18.8) | 30 (30.3) | 39 (26.5) |
| Parameter . | Placebo, n = 48 . | Fulacimstat, n = 99 . | Total, n = 147 . |
|---|---|---|---|
| Male sex, % | 40 (83.3) | 84 (84.8) | 124 (84.4) |
| Age, years | 66.5 ± 7.8 | 69.1 ± 6.3 | 68.2 ± 6.9 |
| BMI, kg/m2 | 31.2 ± 4.7 | 31.4 ± 4.9 | 31.3 ± 4.8 |
| Systolic blood pressure, mmHg | 139 ± 16 | 135 ± 13 | 136 ± 14 |
| Diastolic blood pressure, mmHg | 77 ± 9 | 74 ± 10 | 75 ± 10 |
| High albuminuria, % | 32 (66.7) | 78 (78.8) | 110 (74.8) |
| UACR at Visit 1, mg/g | 140 (31–2429) | 129 (29–2165) | 131 (29–2429) |
| eGFR ≤60 mL/min/1.73 m2, % | 22 (45.8) | 49 (49.5) | 71 (48.3) |
| eGFR at Visit 1, mL/min/1.73 m2 | 61.2 ± 16.5 | 60.5 ± 17.2 | 60.8 ± 16.9 |
| Medical history,a % | |||
| Arterial hypertension | 47 (97.9) | 98 (99.0) | 145 (98.6) |
| Cardiovascular comorbidities | 33 (68.7) | 62 (62.6) | 95 (64.6) |
| Hyperlipidaemia/dyslipidaemia | 25 (52.1) | 51 (51.5) | 76 (51.7) |
| Obesity | 19 (39.6) | 41 (41.4) | 60 (40.8) |
| Diabetic neuropathy | 16 (33.3) | 23 (23.2) | 39 (26.5) |
| Diabetic retinopathy | 10 (20.8) | 25 (25.3) | 35 (23.8) |
| Hypercholesterinaemia | 8 (16.7) | 25 (25.3) | 33 (22.4) |
| Comedication,a % | |||
| ACEis | 15 (31.3) | 24 (24.2) | 39 (26.5)b |
| Ang II receptor blockers (%) | 33 (68.7) | 74 (74.7) | 107 (72.8)b |
| β-blockers | 31 (64.6) | 61 (61.6) | 92 (62.6) |
| Calcium channel blockers | 34 (70.8) | 64 (64.6) | 98 (66.7) |
| Diuretics | 26 (54.2) | 59 (59.6) | 85 (57.8) |
| Metformin | 40 (83.3) | 76 (76.8) | 116 (78.9) |
| Insulin and analogues | 20 (41.7) | 49 (49.5) | 69 (46.9) |
| DPP4 inhibitors | 17 (35.4) | 31 (31.3) | 48 (32.7) |
| SGLT2 inhibitors | 17 (35.4) | 32 (32.3) | 49 (33.3) |
| GLP-1 analogues | 14 (29.2) | 23 (23.2) | 37 (25.2) |
| Sulphonylureas | 14 (29.2) | 17 (17.2) | 31 (21.1) |
| Statins | 37 (77.1) | 78 (78.8) | 115 (78.2) |
| Acetylsalicylic acid | 21 (43.8) | 49 (49.5) | 70 (47.6) |
| Proton pump inhibitors | 9 (18.8) | 30 (30.3) | 39 (26.5) |
Continuous data are depicted as mean ± SD or as median (range) in case of UACR.
Cutoff >20% in total population.
One patient did neither receive an ACEi nor an ARB.
UACR analysis
The primary readout parameter, the UACR ratio to baseline, was reduced by fulacimstat in a statistically non-significant manner by 19.6% within the overall study population in the PPS after 24 weeks of treatment when compared with placebo [LS mean UACR ratio (fulacimstat/placebo) = 0.804, 90% CI 0.627–1.030, P = 0.1477; Table 2]. Throughout treatment, the UACR ratio to baseline remained quite constant in the fulacimstat arm (+3% increase at Visit 6, Table 2 and Figure 2A) and increased by 27.4% in the placebo group starting after 2–4 months of treatment in the PPS (Figure 2A) and by 13% in the ITT population (Figure 2B). Despite the overall neutral study outcome, we performed an exploratory analysis of the primary readout parameter within pre-specified subgroups in the PPS. To facilitate oversight, UACR analysis in Table 2 is restricted to the overall group and a subset of the pre-specified subgroups. The other pre-specified subgroup analyses using stratification based on extent of albuminuria, gender, ACEi or ARB use, use of sodium–glucose-linked transporter 2 (SGLT-2) inhibitors and body mass index at Visit 1 are depicted in Supplementary data, Table S2 and did not provide evidence for any treatment effects. Apparently, fulacimstat reduced the LS mean UACR ratio in patients with an eGFRCrea ≤60 mL/min/1.73 m2 and in patients belonging to the high-risk group (characterized by the presence of cardiovascular comorbidities and an eGFRCrea ≤60 mL/min/1.73 m2) by 45.5 and 43.9%, respectively (Table 2). There were no differences between the two treatments in patients with an eGFRCrea >60 mL/min/1.73 m2 within the PPS (Figure 2E) or the ITT population (Figure 2F). Within the low eGFRCrea subgroup, placebo-treated patients showed an even more pronounced UACR increase of 80.6% (Table 2) than in the overall PPS [starting after 2–4 months of treatment in the PPS (Figure 2C)] and of 69% in the ITT population (Figure 2D), whereas there was only a slight UACR increase of 3.2% (Figure 2C and Table 2, PPS) or 2.8% (Figure 2D, ITT) in the fulacimstat arm. There seemed to be only one country showing a treatment effect [LS mean UACR ratio (fulacimstat/placebo) = 0.453, 90% CI 0.266–0.772, P = 0.0178] that was based on 16.6% albuminuria reduction after fulacimstat treatment and on 84.9% albuminuria increase after placebo treatment (Table 2). To assess whether the potential ‘treatment effects’ seen in these three subgroups mentioned above were chance findings, we performed a sensitivity analysis within the PPS (Table 3). About 15 subjects with implausible UACR and/or eGFR changes during the pre-treatment phase (from screening to the confirmatory baseline visit to Visit 1) or up to 2 weeks of treatment were excluded from the PPS. We also omitted the only country that seemed to show an albuminuria reduction after fulacimstat treatment. Since the elderly, diabetic patient population in this study was obese (Table 1), the use of eGFRCrea for stratification might have caused an overestimation of kidney function due to reduced creatinine levels in the relative absence of muscle mass [17]. Therefore, in the sensitivity analysis, patients were stratified to high and low eGFR subgroups using cystatin C-based eGFR (eGFRCysC) (Table 3). Following these three approaches, we could not demonstrate that fulacimstat reduced albuminuria compared with placebo treatment (Table 3). Similar results were obtained in the ITT population (data not shown).
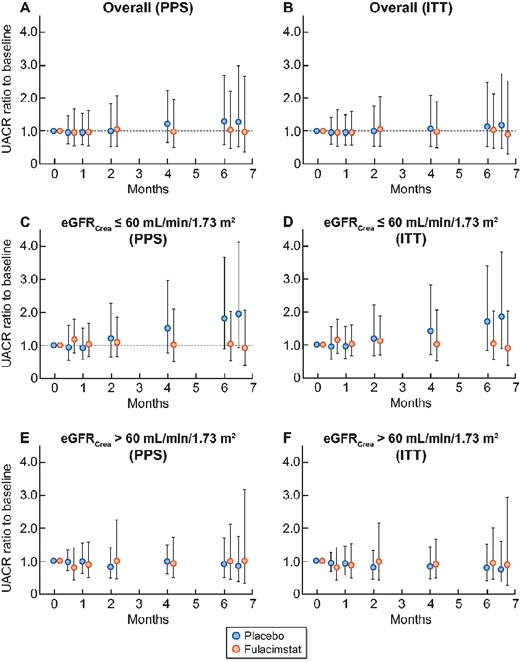
Time course of UACR ratio to baseline (PPS and ITT). Data are depicted as geometric mean (SD) from baseline at Visit 1 (0 months) to Visit 2 (0.5 months), Visit 3 (1 month), Visit 4 (2 months), Visit 5 (4 months), Visit 6 (6 months of treatment with study drug) and to the follow-up visit (6.5 months). Placebo (blue symbols), fulacimstat (orange symbols). (A) Overall PPS population (fulacimstat = 83, placebo = 38 patients), (B) overall ITT population (fulacimstat = 92, placebo = 44 patients), (C) patients with eGFRCrea ≤60 mL/min/1.73 m2 at Visit 1 in the PPS (fulacimstat = 40, placebo = 19 patients), (D) patients with eGFRCrea ≤60 mL/min/1.73 m2 at Visit 1 in the ITT (fulacimstat = 44, placebo = 21 patients), (E) patients with eGFRCrea >60 mL/min/1.73 m2 at Visit 1 in the PPS (fulacimstat = 42, placebo = 19 patients), (F) patients with eGFRCrea >60 mL/min/1.73 m2 at Visit 1 in the ITT (fulacimstat = 47, placebo = 23 patients). Please note that the sum of patients treated with fulacimstat in the two eGFR subgroups is 82 and not 83 (PPS) or 91 and not 92 (ITT), as for one patient treated with fulacimstat the eGFRCrea value at Visit 1 is lacking.
UACR analysis in overall study population and a subset of pre-specified subgroups (PPS)
| Analysis group . | Placebo UACRc . | Fulacimstat UACRc . | LS mean ratio (90% CI) . | P-value . | ||||
|---|---|---|---|---|---|---|---|---|
| Visit 1 (mg/g)a . | Visit 6 (mg/g)a . | Rel. changea . | Visit 1 (mg/g)a . | Visit 6 (mg/g)a . | Rel. changea . | . | . | |
| Overall study population (fulacimstat = 83, placebo = 38) | 196.7 (149.6) | 250.7 (182.1) | 1.274 (86.0) | 159.0 (126.9) | 163.8 (197.3) | 1.030 (88.9) | 0.804 (0.627–1.030) | 0.1477 |
| eGFRCrea ≤60 mL/min/1.73 m2 (fulacimstat = 40, placebo = 19)b | 226.6 (186.0) | 409.2 (200.1) | 1.806 (80.4) | 142.9 (114.5) | 147.5 (147.6) | 1.032 (76.0) | 0.545 (0.393–0.754) | 0.0029 |
| eGFRCrea >60 mL/min/1.73 m2 (fulacimstat = 42, placebo = 19)b | 170.9 (118.7) | 153.6 (119.7) | 0.899 (68.4) | 177.4 (141.3) | 172.9 (248.4) | 0.975 (91.5) | 1.083 (0.769–1.524) | 0.6978 |
| High-risk group (fulacimstat = 25, placebo = 10) | 221.8 (164.6) | 413.8 (242.7) | 1.866 (73.8) | 167.9 (101.9) | 177.6 (143.0) | 1.058 (86.1) | 0.561 (0.351–0.897) | 0.0452 |
| No high-risk group (fulacimstat = 58, placebo = 28) | 188.6 (148.7) | 209.6 (156.1) | 1.112 (84.7) | 155.4 (139.3) | 158.2 (226.2) | 1.018 (91.1) | 0.910 (0.677–1.223) | 0.5984 |
| Israel (fula (fulacimstat = 24, placebo = 10) | 199.7 (151.7) | 228.9 (206.5) | 1.146 (44.3) | 145.5 (105.0) | 196.1 (223.3) | 1.348 (117.1) | 1.224 (0.717– 2.082) | 0.5274 |
| Bulgaria (fulacimstat = 17, palcebo = 11) | 142.3 (114.5) | 263.2 (255.6) | 1.849 (111.9) | 174.2 (226.4) | 145.3 (248.3) | 0.834 (80.7) | 0.453 (0.266–0.772) | 0.0178 |
| Finland (fulacimstat = 13, placebo = 6) | 339.7 (246.9) | 295.8 (231.3) | 0.871 (63.5) | 138.6 (90.7) | 116.6 (187.8) | 0.841 (70.0) | 1.070 (0.594–1.929) | 0.8422 |
| Spain (fulacimstat = 12, placebo = 5) | 207.1 (271.9) | 268.8 (158.3) | 1.298 (117.1) | 198.4 (145.4) | 214.7 (187) | 1.082 (58.8) | 0.828 (0.442–1.551) | 0.6062 |
| Analysis group . | Placebo UACRc . | Fulacimstat UACRc . | LS mean ratio (90% CI) . | P-value . | ||||
|---|---|---|---|---|---|---|---|---|
| Visit 1 (mg/g)a . | Visit 6 (mg/g)a . | Rel. changea . | Visit 1 (mg/g)a . | Visit 6 (mg/g)a . | Rel. changea . | . | . | |
| Overall study population (fulacimstat = 83, placebo = 38) | 196.7 (149.6) | 250.7 (182.1) | 1.274 (86.0) | 159.0 (126.9) | 163.8 (197.3) | 1.030 (88.9) | 0.804 (0.627–1.030) | 0.1477 |
| eGFRCrea ≤60 mL/min/1.73 m2 (fulacimstat = 40, placebo = 19)b | 226.6 (186.0) | 409.2 (200.1) | 1.806 (80.4) | 142.9 (114.5) | 147.5 (147.6) | 1.032 (76.0) | 0.545 (0.393–0.754) | 0.0029 |
| eGFRCrea >60 mL/min/1.73 m2 (fulacimstat = 42, placebo = 19)b | 170.9 (118.7) | 153.6 (119.7) | 0.899 (68.4) | 177.4 (141.3) | 172.9 (248.4) | 0.975 (91.5) | 1.083 (0.769–1.524) | 0.6978 |
| High-risk group (fulacimstat = 25, placebo = 10) | 221.8 (164.6) | 413.8 (242.7) | 1.866 (73.8) | 167.9 (101.9) | 177.6 (143.0) | 1.058 (86.1) | 0.561 (0.351–0.897) | 0.0452 |
| No high-risk group (fulacimstat = 58, placebo = 28) | 188.6 (148.7) | 209.6 (156.1) | 1.112 (84.7) | 155.4 (139.3) | 158.2 (226.2) | 1.018 (91.1) | 0.910 (0.677–1.223) | 0.5984 |
| Israel (fula (fulacimstat = 24, placebo = 10) | 199.7 (151.7) | 228.9 (206.5) | 1.146 (44.3) | 145.5 (105.0) | 196.1 (223.3) | 1.348 (117.1) | 1.224 (0.717– 2.082) | 0.5274 |
| Bulgaria (fulacimstat = 17, palcebo = 11) | 142.3 (114.5) | 263.2 (255.6) | 1.849 (111.9) | 174.2 (226.4) | 145.3 (248.3) | 0.834 (80.7) | 0.453 (0.266–0.772) | 0.0178 |
| Finland (fulacimstat = 13, placebo = 6) | 339.7 (246.9) | 295.8 (231.3) | 0.871 (63.5) | 138.6 (90.7) | 116.6 (187.8) | 0.841 (70.0) | 1.070 (0.594–1.929) | 0.8422 |
| Spain (fulacimstat = 12, placebo = 5) | 207.1 (271.9) | 268.8 (158.3) | 1.298 (117.1) | 198.4 (145.4) | 214.7 (187) | 1.082 (58.8) | 0.828 (0.442–1.551) | 0.6062 |
UACRc values are given as geometric means (geometric CV%).
One subject in the fulacimstat group did not have eGFR data at Visit 1. Subgroups for Sweden, Denmark and Italy were too small for analysis of covariance analysis and are not depicted. P-values are exploratory and not adjusted for multiple testing.
UACR analysis in overall study population and a subset of pre-specified subgroups (PPS)
| Analysis group . | Placebo UACRc . | Fulacimstat UACRc . | LS mean ratio (90% CI) . | P-value . | ||||
|---|---|---|---|---|---|---|---|---|
| Visit 1 (mg/g)a . | Visit 6 (mg/g)a . | Rel. changea . | Visit 1 (mg/g)a . | Visit 6 (mg/g)a . | Rel. changea . | . | . | |
| Overall study population (fulacimstat = 83, placebo = 38) | 196.7 (149.6) | 250.7 (182.1) | 1.274 (86.0) | 159.0 (126.9) | 163.8 (197.3) | 1.030 (88.9) | 0.804 (0.627–1.030) | 0.1477 |
| eGFRCrea ≤60 mL/min/1.73 m2 (fulacimstat = 40, placebo = 19)b | 226.6 (186.0) | 409.2 (200.1) | 1.806 (80.4) | 142.9 (114.5) | 147.5 (147.6) | 1.032 (76.0) | 0.545 (0.393–0.754) | 0.0029 |
| eGFRCrea >60 mL/min/1.73 m2 (fulacimstat = 42, placebo = 19)b | 170.9 (118.7) | 153.6 (119.7) | 0.899 (68.4) | 177.4 (141.3) | 172.9 (248.4) | 0.975 (91.5) | 1.083 (0.769–1.524) | 0.6978 |
| High-risk group (fulacimstat = 25, placebo = 10) | 221.8 (164.6) | 413.8 (242.7) | 1.866 (73.8) | 167.9 (101.9) | 177.6 (143.0) | 1.058 (86.1) | 0.561 (0.351–0.897) | 0.0452 |
| No high-risk group (fulacimstat = 58, placebo = 28) | 188.6 (148.7) | 209.6 (156.1) | 1.112 (84.7) | 155.4 (139.3) | 158.2 (226.2) | 1.018 (91.1) | 0.910 (0.677–1.223) | 0.5984 |
| Israel (fula (fulacimstat = 24, placebo = 10) | 199.7 (151.7) | 228.9 (206.5) | 1.146 (44.3) | 145.5 (105.0) | 196.1 (223.3) | 1.348 (117.1) | 1.224 (0.717– 2.082) | 0.5274 |
| Bulgaria (fulacimstat = 17, palcebo = 11) | 142.3 (114.5) | 263.2 (255.6) | 1.849 (111.9) | 174.2 (226.4) | 145.3 (248.3) | 0.834 (80.7) | 0.453 (0.266–0.772) | 0.0178 |
| Finland (fulacimstat = 13, placebo = 6) | 339.7 (246.9) | 295.8 (231.3) | 0.871 (63.5) | 138.6 (90.7) | 116.6 (187.8) | 0.841 (70.0) | 1.070 (0.594–1.929) | 0.8422 |
| Spain (fulacimstat = 12, placebo = 5) | 207.1 (271.9) | 268.8 (158.3) | 1.298 (117.1) | 198.4 (145.4) | 214.7 (187) | 1.082 (58.8) | 0.828 (0.442–1.551) | 0.6062 |
| Analysis group . | Placebo UACRc . | Fulacimstat UACRc . | LS mean ratio (90% CI) . | P-value . | ||||
|---|---|---|---|---|---|---|---|---|
| Visit 1 (mg/g)a . | Visit 6 (mg/g)a . | Rel. changea . | Visit 1 (mg/g)a . | Visit 6 (mg/g)a . | Rel. changea . | . | . | |
| Overall study population (fulacimstat = 83, placebo = 38) | 196.7 (149.6) | 250.7 (182.1) | 1.274 (86.0) | 159.0 (126.9) | 163.8 (197.3) | 1.030 (88.9) | 0.804 (0.627–1.030) | 0.1477 |
| eGFRCrea ≤60 mL/min/1.73 m2 (fulacimstat = 40, placebo = 19)b | 226.6 (186.0) | 409.2 (200.1) | 1.806 (80.4) | 142.9 (114.5) | 147.5 (147.6) | 1.032 (76.0) | 0.545 (0.393–0.754) | 0.0029 |
| eGFRCrea >60 mL/min/1.73 m2 (fulacimstat = 42, placebo = 19)b | 170.9 (118.7) | 153.6 (119.7) | 0.899 (68.4) | 177.4 (141.3) | 172.9 (248.4) | 0.975 (91.5) | 1.083 (0.769–1.524) | 0.6978 |
| High-risk group (fulacimstat = 25, placebo = 10) | 221.8 (164.6) | 413.8 (242.7) | 1.866 (73.8) | 167.9 (101.9) | 177.6 (143.0) | 1.058 (86.1) | 0.561 (0.351–0.897) | 0.0452 |
| No high-risk group (fulacimstat = 58, placebo = 28) | 188.6 (148.7) | 209.6 (156.1) | 1.112 (84.7) | 155.4 (139.3) | 158.2 (226.2) | 1.018 (91.1) | 0.910 (0.677–1.223) | 0.5984 |
| Israel (fula (fulacimstat = 24, placebo = 10) | 199.7 (151.7) | 228.9 (206.5) | 1.146 (44.3) | 145.5 (105.0) | 196.1 (223.3) | 1.348 (117.1) | 1.224 (0.717– 2.082) | 0.5274 |
| Bulgaria (fulacimstat = 17, palcebo = 11) | 142.3 (114.5) | 263.2 (255.6) | 1.849 (111.9) | 174.2 (226.4) | 145.3 (248.3) | 0.834 (80.7) | 0.453 (0.266–0.772) | 0.0178 |
| Finland (fulacimstat = 13, placebo = 6) | 339.7 (246.9) | 295.8 (231.3) | 0.871 (63.5) | 138.6 (90.7) | 116.6 (187.8) | 0.841 (70.0) | 1.070 (0.594–1.929) | 0.8422 |
| Spain (fulacimstat = 12, placebo = 5) | 207.1 (271.9) | 268.8 (158.3) | 1.298 (117.1) | 198.4 (145.4) | 214.7 (187) | 1.082 (58.8) | 0.828 (0.442–1.551) | 0.6062 |
UACRc values are given as geometric means (geometric CV%).
One subject in the fulacimstat group did not have eGFR data at Visit 1. Subgroups for Sweden, Denmark and Italy were too small for analysis of covariance analysis and are not depicted. P-values are exploratory and not adjusted for multiple testing.
Sensitivity analysis performed with the primary efficacy parameter UACR (PPS)
| Procedure . | Analysis group . | Placebo UACRc . | Fulacimstat UACRc . | LS mean ratio (90% CI) . | P-value . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Visit 1 (mg/g)a . | Visit 6 (mg/g)a . | Rel. changea . | Visit 1 (mg/g)a . | Visit 6 (mg/g)a . | Rel. changea . | . | . | ||
| Omission of 15 subjects | Overall study population (fulacimstat = 73, placebo = 33) | 206.3 (155.4) | 220.4 (167.4) | 1.068 (62.3) | 158.6 (117.0) | 184.3 (181.2) | 1.161 (79.7) | 1.087 (0.860–1.374) | 0.5541 |
| eGFRCrea ≤60 mL/min/1.73 m2 (fulacimstat = 37, placebo = 15)b | 269.0 (194.4) | 390.9 (198.2) | 1.453 (52.2) | 147.8 (117.6) | 161.4 (145.6) | 1.091 (74.3) | 0.714 (0.513–0.992) | 0.093 | |
| eGFRCrea >60 mL/min/1.73 m2 (fulacimstat = 35, placebo = 18)b | 165.3 (121.7) | 136.7 (97.6) | 0.826 (55.2) | 172.3 (120.1) | 201.3 (218.2) | 1.168 (74.5) | 1.410 (1.041–1.908) | 0.0633 | |
| Omission of Bulgaria | Overall study population (fulacimstat = 66, placebo = 27) | 224.5 (162.0) | 245.7 (163.7) | 1.094 (69.4) | 155.3 (125.6) (106.9) | 168.9 (188.1) | 1.087 (90.4) | 0.994 (0.747–1.321) | 0.9716 |
| eGFRCrea ≤60 mL/min/1.73 m2 (fulacimstat = 32, placebo = 12)b | 289.1 (201.0) | 459.4 (171.6) | 1.588 (53.5) | 148.9 (125.4) (96.7) | 163.0 (144.1) | 1.094 (79.4) | 0.653 (0.440–0.966) | 0.074 | |
| eGFRCrea >60 mL/min/1.73 m2 (fulacimstat = 33, placebo = 15)b | 183.4 (133.4) | 149.0 (104.4) | 0.812 (61.2) | 163.2 (120.9) | 165.1 (234.1) | 1.011 (89.2) | 1.252 (0.859–1.822) | 0.320 | |
| Stratification using eGFRCysC | eGFRCysC ≤60 mL/min/1.73 m2 (fulacimstat = 61, placebo = 27)c | 185.3 (156.1) | 264.1 (189.3) | 1.425 (77.1) | 167.7 (119.6) | 172.9 (186.6) | 1.031 (87.6) | 0.723 (0.543–0.960) | 0.0606 |
| eGFRCysC >60 mL/min/1.73 m2 (fulacimstat = 19, placebo = 11)c | 227.9 (142.1) | 224.9 (196.2) | 1.079 (95.6) | 136.6 (169.7) | 117.6 (230.7) | 0.861 (71.1) | 0.796 (0.490–1.289) | 0.427 | |
| Procedure . | Analysis group . | Placebo UACRc . | Fulacimstat UACRc . | LS mean ratio (90% CI) . | P-value . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Visit 1 (mg/g)a . | Visit 6 (mg/g)a . | Rel. changea . | Visit 1 (mg/g)a . | Visit 6 (mg/g)a . | Rel. changea . | . | . | ||
| Omission of 15 subjects | Overall study population (fulacimstat = 73, placebo = 33) | 206.3 (155.4) | 220.4 (167.4) | 1.068 (62.3) | 158.6 (117.0) | 184.3 (181.2) | 1.161 (79.7) | 1.087 (0.860–1.374) | 0.5541 |
| eGFRCrea ≤60 mL/min/1.73 m2 (fulacimstat = 37, placebo = 15)b | 269.0 (194.4) | 390.9 (198.2) | 1.453 (52.2) | 147.8 (117.6) | 161.4 (145.6) | 1.091 (74.3) | 0.714 (0.513–0.992) | 0.093 | |
| eGFRCrea >60 mL/min/1.73 m2 (fulacimstat = 35, placebo = 18)b | 165.3 (121.7) | 136.7 (97.6) | 0.826 (55.2) | 172.3 (120.1) | 201.3 (218.2) | 1.168 (74.5) | 1.410 (1.041–1.908) | 0.0633 | |
| Omission of Bulgaria | Overall study population (fulacimstat = 66, placebo = 27) | 224.5 (162.0) | 245.7 (163.7) | 1.094 (69.4) | 155.3 (125.6) (106.9) | 168.9 (188.1) | 1.087 (90.4) | 0.994 (0.747–1.321) | 0.9716 |
| eGFRCrea ≤60 mL/min/1.73 m2 (fulacimstat = 32, placebo = 12)b | 289.1 (201.0) | 459.4 (171.6) | 1.588 (53.5) | 148.9 (125.4) (96.7) | 163.0 (144.1) | 1.094 (79.4) | 0.653 (0.440–0.966) | 0.074 | |
| eGFRCrea >60 mL/min/1.73 m2 (fulacimstat = 33, placebo = 15)b | 183.4 (133.4) | 149.0 (104.4) | 0.812 (61.2) | 163.2 (120.9) | 165.1 (234.1) | 1.011 (89.2) | 1.252 (0.859–1.822) | 0.320 | |
| Stratification using eGFRCysC | eGFRCysC ≤60 mL/min/1.73 m2 (fulacimstat = 61, placebo = 27)c | 185.3 (156.1) | 264.1 (189.3) | 1.425 (77.1) | 167.7 (119.6) | 172.9 (186.6) | 1.031 (87.6) | 0.723 (0.543–0.960) | 0.0606 |
| eGFRCysC >60 mL/min/1.73 m2 (fulacimstat = 19, placebo = 11)c | 227.9 (142.1) | 224.9 (196.2) | 1.079 (95.6) | 136.6 (169.7) | 117.6 (230.7) | 0.861 (71.1) | 0.796 (0.490–1.289) | 0.427 | |
UACRc values are given as geometric means (geometric CV%).
One subject in the fulacimstat group had no creatinine data at Visit 1.
Three subjects in the fulacimstat group had no cystatin C data at Visit 1 . P-values are exploratory and not adjusted for multiple testing.
Sensitivity analysis performed with the primary efficacy parameter UACR (PPS)
| Procedure . | Analysis group . | Placebo UACRc . | Fulacimstat UACRc . | LS mean ratio (90% CI) . | P-value . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Visit 1 (mg/g)a . | Visit 6 (mg/g)a . | Rel. changea . | Visit 1 (mg/g)a . | Visit 6 (mg/g)a . | Rel. changea . | . | . | ||
| Omission of 15 subjects | Overall study population (fulacimstat = 73, placebo = 33) | 206.3 (155.4) | 220.4 (167.4) | 1.068 (62.3) | 158.6 (117.0) | 184.3 (181.2) | 1.161 (79.7) | 1.087 (0.860–1.374) | 0.5541 |
| eGFRCrea ≤60 mL/min/1.73 m2 (fulacimstat = 37, placebo = 15)b | 269.0 (194.4) | 390.9 (198.2) | 1.453 (52.2) | 147.8 (117.6) | 161.4 (145.6) | 1.091 (74.3) | 0.714 (0.513–0.992) | 0.093 | |
| eGFRCrea >60 mL/min/1.73 m2 (fulacimstat = 35, placebo = 18)b | 165.3 (121.7) | 136.7 (97.6) | 0.826 (55.2) | 172.3 (120.1) | 201.3 (218.2) | 1.168 (74.5) | 1.410 (1.041–1.908) | 0.0633 | |
| Omission of Bulgaria | Overall study population (fulacimstat = 66, placebo = 27) | 224.5 (162.0) | 245.7 (163.7) | 1.094 (69.4) | 155.3 (125.6) (106.9) | 168.9 (188.1) | 1.087 (90.4) | 0.994 (0.747–1.321) | 0.9716 |
| eGFRCrea ≤60 mL/min/1.73 m2 (fulacimstat = 32, placebo = 12)b | 289.1 (201.0) | 459.4 (171.6) | 1.588 (53.5) | 148.9 (125.4) (96.7) | 163.0 (144.1) | 1.094 (79.4) | 0.653 (0.440–0.966) | 0.074 | |
| eGFRCrea >60 mL/min/1.73 m2 (fulacimstat = 33, placebo = 15)b | 183.4 (133.4) | 149.0 (104.4) | 0.812 (61.2) | 163.2 (120.9) | 165.1 (234.1) | 1.011 (89.2) | 1.252 (0.859–1.822) | 0.320 | |
| Stratification using eGFRCysC | eGFRCysC ≤60 mL/min/1.73 m2 (fulacimstat = 61, placebo = 27)c | 185.3 (156.1) | 264.1 (189.3) | 1.425 (77.1) | 167.7 (119.6) | 172.9 (186.6) | 1.031 (87.6) | 0.723 (0.543–0.960) | 0.0606 |
| eGFRCysC >60 mL/min/1.73 m2 (fulacimstat = 19, placebo = 11)c | 227.9 (142.1) | 224.9 (196.2) | 1.079 (95.6) | 136.6 (169.7) | 117.6 (230.7) | 0.861 (71.1) | 0.796 (0.490–1.289) | 0.427 | |
| Procedure . | Analysis group . | Placebo UACRc . | Fulacimstat UACRc . | LS mean ratio (90% CI) . | P-value . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Visit 1 (mg/g)a . | Visit 6 (mg/g)a . | Rel. changea . | Visit 1 (mg/g)a . | Visit 6 (mg/g)a . | Rel. changea . | . | . | ||
| Omission of 15 subjects | Overall study population (fulacimstat = 73, placebo = 33) | 206.3 (155.4) | 220.4 (167.4) | 1.068 (62.3) | 158.6 (117.0) | 184.3 (181.2) | 1.161 (79.7) | 1.087 (0.860–1.374) | 0.5541 |
| eGFRCrea ≤60 mL/min/1.73 m2 (fulacimstat = 37, placebo = 15)b | 269.0 (194.4) | 390.9 (198.2) | 1.453 (52.2) | 147.8 (117.6) | 161.4 (145.6) | 1.091 (74.3) | 0.714 (0.513–0.992) | 0.093 | |
| eGFRCrea >60 mL/min/1.73 m2 (fulacimstat = 35, placebo = 18)b | 165.3 (121.7) | 136.7 (97.6) | 0.826 (55.2) | 172.3 (120.1) | 201.3 (218.2) | 1.168 (74.5) | 1.410 (1.041–1.908) | 0.0633 | |
| Omission of Bulgaria | Overall study population (fulacimstat = 66, placebo = 27) | 224.5 (162.0) | 245.7 (163.7) | 1.094 (69.4) | 155.3 (125.6) (106.9) | 168.9 (188.1) | 1.087 (90.4) | 0.994 (0.747–1.321) | 0.9716 |
| eGFRCrea ≤60 mL/min/1.73 m2 (fulacimstat = 32, placebo = 12)b | 289.1 (201.0) | 459.4 (171.6) | 1.588 (53.5) | 148.9 (125.4) (96.7) | 163.0 (144.1) | 1.094 (79.4) | 0.653 (0.440–0.966) | 0.074 | |
| eGFRCrea >60 mL/min/1.73 m2 (fulacimstat = 33, placebo = 15)b | 183.4 (133.4) | 149.0 (104.4) | 0.812 (61.2) | 163.2 (120.9) | 165.1 (234.1) | 1.011 (89.2) | 1.252 (0.859–1.822) | 0.320 | |
| Stratification using eGFRCysC | eGFRCysC ≤60 mL/min/1.73 m2 (fulacimstat = 61, placebo = 27)c | 185.3 (156.1) | 264.1 (189.3) | 1.425 (77.1) | 167.7 (119.6) | 172.9 (186.6) | 1.031 (87.6) | 0.723 (0.543–0.960) | 0.0606 |
| eGFRCysC >60 mL/min/1.73 m2 (fulacimstat = 19, placebo = 11)c | 227.9 (142.1) | 224.9 (196.2) | 1.079 (95.6) | 136.6 (169.7) | 117.6 (230.7) | 0.861 (71.1) | 0.796 (0.490–1.289) | 0.427 | |
UACRc values are given as geometric means (geometric CV%).
One subject in the fulacimstat group had no creatinine data at Visit 1.
Three subjects in the fulacimstat group had no cystatin C data at Visit 1 . P-values are exploratory and not adjusted for multiple testing.
eGFR analysis
There were no obvious differences in eGFRCrea, eGFRCycC or the respective eGFR ratios to baseline between the two treatment arms (Figure 3, Supplementary data, Table S3). Fulacimstat did not cause an early transient drop in eGFR (Figure 3).
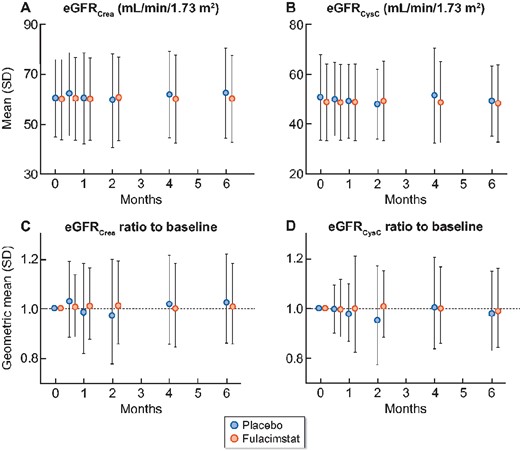
Time course of eGFR and eGFR ratio to baseline (PPS). (A and B) eGFR values from Visit 1 (0 months), to Visit 2 (0.5 months), Visit 3 (1 months), Visit 4 (2 months), Visit 5 (4 months) and to Visit 6 (6 months) are depicted as arithmetic means (SD) using (A) the creatinine-based eGFR (eGFRCrea) and (B) the cystatin C-based eGFR (eGFRCysC). (C and D) The ratios of eGFR to baseline are shown as geometric means (geometric SD) for (C) eGFRCrea and (D) eGFRCysC. Placebo (blue symbols), fulacimstat (orange symbols).
Fulacimstat plasma concentrations
Geometric mean plasma concentrations at trough (i.e. before dosing at Visit 3) were 475 µg/L and thus ∼9-fold higher than predicted to be required for minimal therapeutic activity (see dosing rationale) [12–14] (Table 4). The concentrations determined at the end of visit were similar for Visits 3–6, whereas the time interval of sampling was quite large, i.e. 20 min to 10.75 h post-dose.
| Visit . | Planned time point . | N . | Fulacimstat (µg/L)a . | Minimum . | Maximum . |
|---|---|---|---|---|---|
| Visit 1 | 2–4 h after dosing | 83 | 941 (87.3) | 17.8 | 2530 |
| Visit 3 | Before dosing | 81 | 475 (95.7) | 31.9 | 1990 |
| 0.5–1.5 h after dosing | 92 | 877 (109.0) | 86.1 | 5370 | |
| 2–4 h after dosing | 92 | 1380 (65.0) | 170 | 3810 | |
| End of visit | 91 | 1390 (63.9) | 173 | 3790 | |
| Visit 4 | End of visit | 92 | 1350 (69.0) | 162 | 5490 |
| Visit 5 | End of visit | 88 | 1280 (84.8) | 107 | 4230 |
| Visit 6 | End of visit | 87 | 1510 (56.6) | 310 | 4940 |
| Visit . | Planned time point . | N . | Fulacimstat (µg/L)a . | Minimum . | Maximum . |
|---|---|---|---|---|---|
| Visit 1 | 2–4 h after dosing | 83 | 941 (87.3) | 17.8 | 2530 |
| Visit 3 | Before dosing | 81 | 475 (95.7) | 31.9 | 1990 |
| 0.5–1.5 h after dosing | 92 | 877 (109.0) | 86.1 | 5370 | |
| 2–4 h after dosing | 92 | 1380 (65.0) | 170 | 3810 | |
| End of visit | 91 | 1390 (63.9) | 173 | 3790 | |
| Visit 4 | End of visit | 92 | 1350 (69.0) | 162 | 5490 |
| Visit 5 | End of visit | 88 | 1280 (84.8) | 107 | 4230 |
| Visit 6 | End of visit | 87 | 1510 (56.6) | 310 | 4940 |
Total plasma concentrations of fulacimstat are given as geometric mean (geometric CV%).
| Visit . | Planned time point . | N . | Fulacimstat (µg/L)a . | Minimum . | Maximum . |
|---|---|---|---|---|---|
| Visit 1 | 2–4 h after dosing | 83 | 941 (87.3) | 17.8 | 2530 |
| Visit 3 | Before dosing | 81 | 475 (95.7) | 31.9 | 1990 |
| 0.5–1.5 h after dosing | 92 | 877 (109.0) | 86.1 | 5370 | |
| 2–4 h after dosing | 92 | 1380 (65.0) | 170 | 3810 | |
| End of visit | 91 | 1390 (63.9) | 173 | 3790 | |
| Visit 4 | End of visit | 92 | 1350 (69.0) | 162 | 5490 |
| Visit 5 | End of visit | 88 | 1280 (84.8) | 107 | 4230 |
| Visit 6 | End of visit | 87 | 1510 (56.6) | 310 | 4940 |
| Visit . | Planned time point . | N . | Fulacimstat (µg/L)a . | Minimum . | Maximum . |
|---|---|---|---|---|---|
| Visit 1 | 2–4 h after dosing | 83 | 941 (87.3) | 17.8 | 2530 |
| Visit 3 | Before dosing | 81 | 475 (95.7) | 31.9 | 1990 |
| 0.5–1.5 h after dosing | 92 | 877 (109.0) | 86.1 | 5370 | |
| 2–4 h after dosing | 92 | 1380 (65.0) | 170 | 3810 | |
| End of visit | 91 | 1390 (63.9) | 173 | 3790 | |
| Visit 4 | End of visit | 92 | 1350 (69.0) | 162 | 5490 |
| Visit 5 | End of visit | 88 | 1280 (84.8) | 107 | 4230 |
| Visit 6 | End of visit | 87 | 1510 (56.6) | 310 | 4940 |
Total plasma concentrations of fulacimstat are given as geometric mean (geometric CV%).
Safety and tolerability
About 58.3% of patients treated with placebo and 64.6% of patients receiving fulacimstat experienced at least one TEAE; most of them were of mild to moderate intensity and resolved. In general, TEAEs (Table 5) were quite well balanced between the treatment arms. Slight imbalances to the disadvantage of fulacimstat seemed to exist for fatigue, back pain, upper respiratory tract infection and headache (Table 5). About 5.1% of subjects in the fulacimstat arm and 10.4% of subjects in the placebo arm reported a treatment-emergent serious AE (TESAE). None of those TESAEs were considered related to treatment with study drug (Table 5).
| Number of patients (%) with at least one such AE . | Placebo, n = 48 (100%) . | Fulacimstat, n = 99 (100%) . | Total, n = 147 (100%) . |
|---|---|---|---|
| Any TEAE, % | 28 (58.3) | 64 (64.6) | 92 (62.6) |
| Study drug-related TEAE, % | 2 (4.2) | 12 (12.1) | 14 (9.5) |
| Any SAE, % | 5 (10.4) | 5 (5.1) | 10 (6.8) |
| Study drug-related SAE | 0 | 0 | 0 |
| TEAE with outcome death | 0 | 0 | 0 |
| Most frequent TEAEs,a % | |||
| Nasopharyngitis | 5 (10.4) | 4 (4.0) | 9 (6.1) |
| Constipation | 3 (6.3) | 4 (4.0) | 7 (4.8) |
| Hypoglycaemia | 3 (6.3) | 4 (4.0) | 7 (4.8) |
| Urinary tract infection | 1 (2.1) | 5 (5.1) | 6 (4.1) |
| Back pain | 0 | 5 (5.1) | 5 (3.4) |
| Abdominal pain | 2 (4.2) | 2 (2.0) | 4 (2.7) |
| Anaemia | 1 (2.1) | 3 (3.0) | 4 (2.7) |
| Arthralgia | 2 (4.2) | 2 (2.0) | 4 (2.7) |
| Blood pressure increased | 2 (4.2) | 2 (2.0) | 4 (2.7) |
| Diarrhoea | 1 (2.1) | 3 (3.0) | 4 (2.7) |
| Fatigue | 0 | 4 (4.0) | 4 (2.7) |
| Headache | 0 | 4 (4.0) | 4 (2.7) |
| Hyperglycaemia | 1 (2.1) | 3 (3.0) | 4 (2.7) |
| Upper respiratory tract infection | 0 | 4 (4.0) | 4 (2.7) |
| Number of patients (%) with at least one such AE . | Placebo, n = 48 (100%) . | Fulacimstat, n = 99 (100%) . | Total, n = 147 (100%) . |
|---|---|---|---|
| Any TEAE, % | 28 (58.3) | 64 (64.6) | 92 (62.6) |
| Study drug-related TEAE, % | 2 (4.2) | 12 (12.1) | 14 (9.5) |
| Any SAE, % | 5 (10.4) | 5 (5.1) | 10 (6.8) |
| Study drug-related SAE | 0 | 0 | 0 |
| TEAE with outcome death | 0 | 0 | 0 |
| Most frequent TEAEs,a % | |||
| Nasopharyngitis | 5 (10.4) | 4 (4.0) | 9 (6.1) |
| Constipation | 3 (6.3) | 4 (4.0) | 7 (4.8) |
| Hypoglycaemia | 3 (6.3) | 4 (4.0) | 7 (4.8) |
| Urinary tract infection | 1 (2.1) | 5 (5.1) | 6 (4.1) |
| Back pain | 0 | 5 (5.1) | 5 (3.4) |
| Abdominal pain | 2 (4.2) | 2 (2.0) | 4 (2.7) |
| Anaemia | 1 (2.1) | 3 (3.0) | 4 (2.7) |
| Arthralgia | 2 (4.2) | 2 (2.0) | 4 (2.7) |
| Blood pressure increased | 2 (4.2) | 2 (2.0) | 4 (2.7) |
| Diarrhoea | 1 (2.1) | 3 (3.0) | 4 (2.7) |
| Fatigue | 0 | 4 (4.0) | 4 (2.7) |
| Headache | 0 | 4 (4.0) | 4 (2.7) |
| Hyperglycaemia | 1 (2.1) | 3 (3.0) | 4 (2.7) |
| Upper respiratory tract infection | 0 | 4 (4.0) | 4 (2.7) |
Cutoff four or more subjects in total population.
| Number of patients (%) with at least one such AE . | Placebo, n = 48 (100%) . | Fulacimstat, n = 99 (100%) . | Total, n = 147 (100%) . |
|---|---|---|---|
| Any TEAE, % | 28 (58.3) | 64 (64.6) | 92 (62.6) |
| Study drug-related TEAE, % | 2 (4.2) | 12 (12.1) | 14 (9.5) |
| Any SAE, % | 5 (10.4) | 5 (5.1) | 10 (6.8) |
| Study drug-related SAE | 0 | 0 | 0 |
| TEAE with outcome death | 0 | 0 | 0 |
| Most frequent TEAEs,a % | |||
| Nasopharyngitis | 5 (10.4) | 4 (4.0) | 9 (6.1) |
| Constipation | 3 (6.3) | 4 (4.0) | 7 (4.8) |
| Hypoglycaemia | 3 (6.3) | 4 (4.0) | 7 (4.8) |
| Urinary tract infection | 1 (2.1) | 5 (5.1) | 6 (4.1) |
| Back pain | 0 | 5 (5.1) | 5 (3.4) |
| Abdominal pain | 2 (4.2) | 2 (2.0) | 4 (2.7) |
| Anaemia | 1 (2.1) | 3 (3.0) | 4 (2.7) |
| Arthralgia | 2 (4.2) | 2 (2.0) | 4 (2.7) |
| Blood pressure increased | 2 (4.2) | 2 (2.0) | 4 (2.7) |
| Diarrhoea | 1 (2.1) | 3 (3.0) | 4 (2.7) |
| Fatigue | 0 | 4 (4.0) | 4 (2.7) |
| Headache | 0 | 4 (4.0) | 4 (2.7) |
| Hyperglycaemia | 1 (2.1) | 3 (3.0) | 4 (2.7) |
| Upper respiratory tract infection | 0 | 4 (4.0) | 4 (2.7) |
| Number of patients (%) with at least one such AE . | Placebo, n = 48 (100%) . | Fulacimstat, n = 99 (100%) . | Total, n = 147 (100%) . |
|---|---|---|---|
| Any TEAE, % | 28 (58.3) | 64 (64.6) | 92 (62.6) |
| Study drug-related TEAE, % | 2 (4.2) | 12 (12.1) | 14 (9.5) |
| Any SAE, % | 5 (10.4) | 5 (5.1) | 10 (6.8) |
| Study drug-related SAE | 0 | 0 | 0 |
| TEAE with outcome death | 0 | 0 | 0 |
| Most frequent TEAEs,a % | |||
| Nasopharyngitis | 5 (10.4) | 4 (4.0) | 9 (6.1) |
| Constipation | 3 (6.3) | 4 (4.0) | 7 (4.8) |
| Hypoglycaemia | 3 (6.3) | 4 (4.0) | 7 (4.8) |
| Urinary tract infection | 1 (2.1) | 5 (5.1) | 6 (4.1) |
| Back pain | 0 | 5 (5.1) | 5 (3.4) |
| Abdominal pain | 2 (4.2) | 2 (2.0) | 4 (2.7) |
| Anaemia | 1 (2.1) | 3 (3.0) | 4 (2.7) |
| Arthralgia | 2 (4.2) | 2 (2.0) | 4 (2.7) |
| Blood pressure increased | 2 (4.2) | 2 (2.0) | 4 (2.7) |
| Diarrhoea | 1 (2.1) | 3 (3.0) | 4 (2.7) |
| Fatigue | 0 | 4 (4.0) | 4 (2.7) |
| Headache | 0 | 4 (4.0) | 4 (2.7) |
| Hyperglycaemia | 1 (2.1) | 3 (3.0) | 4 (2.7) |
| Upper respiratory tract infection | 0 | 4 (4.0) | 4 (2.7) |
Cutoff four or more subjects in total population.
DISCUSSION
CADA DIA investigated the effects of the chymase inhibitor fulacimstat on albuminuria in patients with T2DM and a clinical diagnosis of DKD receiving stable standard of care. Fulacimstat was safe and well tolerated, and achieved mean total trough concentrations that were ∼9-fold higher than those predicted to be required for minimal therapeutic efficacy [12–14]. Contrary to the established standard of care for DKD, such as ARBs [18, 19], ACEis [20] and SGLT-2 inhibitors [21], fulacimstat did not reduce albuminuria after 24 weeks of treatment.
While the established standard of care for DKD is haemodynamically active, which may partly contribute to UACR decrease [18–21], fulacimstat and other chymase inhibitors are devoid of any blood pressure-lowering effects [11, 14, 15]. At first sight it was therefore not surprising that fulacimstat exhibiting anti-remodelling activity did not reduce but rather stopped further albuminuria increase when compared with placebo. However, this observation was most likely biased due to an unusual high UACR increase within the placebo group, i.e. 27% increase in the overall PPS and 80% increase within the low eGFRCrea subgroup. In contrast, placebo-treated patients showed increases in albuminuria of ±8–10% at maximum in other DKD trials after 6 months of treatment [18, 19, 21] and the annual increase in albuminuria in the absence of any treatment was ∼20% (see ref. [22] for a review). The reason for the unexpected high albuminuria increase starting after 2–4 months of placebo treatment remains enigmatic. We could not identify any statistically significant differences in any baseline characteristics between the two treatment arms neither in the PPS nor in any of the subgroups. Notably, albuminuria at Visit 1 tended to be slightly higher in many (but not all) placebo groups when compared with the corresponding fulacimstat arms. It has been described previously that baseline albuminuria predicts disease progression [23]. Hence, we cannot rule out the possibility that even a statistically nonsignificant difference in baseline albuminuria might have caused the strong deterioration in albuminuria seen within several subgroups of placebo treatment. However, the subgroup analyses performed in the very high and the high albuminuria subgroup as well as in Bulgaria do not support this hypothesis: despite lower starting UACR values in the placebo compared with the fulacimstat group, there was a stronger UACR increase in the placebo arms compared with fulacimstat (high and very high albuminuria subgroup) or even a decrease in UACR after fulacimstat treatment (Bulgaria).
Further analysis revealed that within the low eGFRCrea subgroup, TEAEs and changes in concomitant medication during active treatment with study drug were reported with reduced frequency in the placebo arm (changes in concomitant medication: 21.1% of placebo—versus 52.5% of fulacimstat-treated patients; TEAE reporting: 42.1% of placebo—versus 65% of fulacimstat-treated patients; PPS). As the majority of comedication changes was transient (for the treatment of TEAEs) and did not affect ARB or ACEi medication, this imbalance during the treatment phase could not explain the unusual UACR increase within the placebo group. It is therefore reasonable to assume that the different response of albuminuria to treatment within this low eGFRCrea subgroup reflected a chance finding based on unusual placebo group behaviour. The sensitivity analysis supported this notion. Remarkably, any treatment effect vanished when a more adequate eGFR stratification based on cystatin C was performed. In line with previous results [17], we observed that kidney function was overestimated using eGFRCrea in our obese, diabetic patient population with low muscle mass: at Visit 1, the mean eGFRCysC was ∼10 mL/min/1.73 m2 smaller than the respective mean eGFRCrea (Figure 3).
Following CHIARA MIA 2 (chymase inhibitor in adverse remodelling after myocardial infarction 2) [16], CADA DIA was the second clinical trial that failed to translate findings obtained with fulacimstat in preclinical animal models to the human situation. There are two most likely reasons why fulacimstat did not reduce albuminuria in patients with DKD as expected from animal experiments [9–11]. First, the therapeutic effects of fulacimstat might have been overestimated in animal models. The animals were not treated with standard of care therapy for DKD, which may have limited the applicability of the obtained findings to humans. Secondly, as chymase does not belong to the strongly conserved genes during evolution, it could also be that chymase function is not conserved across species. Notably, mice and rats express different chymase isoenzymes that form and degrade Ang II, whereas hamsters and dogs as humans express only one isoenzyme and therefore resemble the most appropriate model for studying chymase inhibitors [24].
By choosing a treatment duration of 24 weeks that might be sufficiently long for the analysis of changes in albuminuria, we may have failed to detect any beneficial effect of fulacimstat on kidney function (resembling an exploratory readout variable in this study). During the active treatment phase, kidney function remained largely unchanged in the fulacimstat arm. As expected for a haemodynamically neutral principle [11, 14, 15], fulacimstat did not trigger an initial transient eGFR decline as it was observed with ARBs, ACEIs or SGLT-2 inhibitors [18–21].
Retrospectively, the chosen 2:1 randomization was not optimal in this small trial. As the strong increase in UACR in placebo-treated patients was unexpected, a larger placebo group would have increased the confidence in the obtained results.
Taken together, major limitations of this small exploratory trial were as follows: a relatively short treatment duration of 6 months (not allowing for reliable eGFR analysis); the 2:1 randomization leading to a relatively small placebo group; unexpectedly strong deterioration of the UACR in placebo-treated patients with reduced eGFR.
In conclusion, despite sufficiently high plasma exposures, fulacimstat did not reduce albuminuria in patients with a clinical diagnosis of DKD. Although failure to reduce albuminuria does not preclude beneficial clinical effects, we conclude that chymase inhibition does not suffice as a novel treatment option for DKD. The perception that fulacimstat could stop albuminuria progression was most likely biased by an unusual high albuminuria increase within the placebo group.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
ACKNOWLEDGEMENTS
The authors are grateful to the following clinical investigators for reviewing the study protocol, enrolment of patients and collection of data: Thomas Almdal, Mads Hornum, Lise Tarnow, Peter Kristensen, Yoram Yagil, Julio Wainstein, Ofri Mosenzon, Keren Doenyas-Barak, Zaher Amaly, Zhulieta Prakova, Rosen Rashkov, Neli Klyuchkova, Francisco Martinez Deben, Isabel Fernandez Martinez, Francesca Calero, Ilkka Kantola, Jyrki Taurio, Sakari Nieminen, Salvatore de Cosmo, Domenico Russo, Gaetano La Manna, Ken Eliasson, Ingemar Torstensson, Ake Olsson and Bo Liu. The authors wish to thank Mitra Brenner, Thilo Krueger, Iouri Bachmakov, Robert Kraemer and Thomas Bernhardt for critical discussion; Sari Soisalon-Soininen, Isabel Caballero (Linical) and Carlos Hortelano (Linical) for ongoing medical review; Mark Enzler (Swiss BioQuant AG) and Richard Abbott for performing bioanalysis of plasma PK samples; and Achim Fritsch for PK evaluation. We are grateful to all patients who participated in this study.
FUNDING
The study was funded by its sponsor Bayer AG.
AUTHORS’ CONTRIBUTIONS
P.R.: trial concept and design, trial conduct and oversight, data analysis and interpretation, writing, review, editing and approval of the manuscript, J.S.: trial concept and design, trial conduct and oversight, data analysis and interpretation, writing-review, editing and approval of the manuscript, A.A.: trial concept and design, trial conduct and oversight, data analysis and interpretation, writing, review, editing and approval of the manuscript, M.B.: trial concept and design, data curation, formal analysis, methodology, project administration, software, supervision, data validation and visualization, writing, review, editing and approval of the manuscript, F.K.: trial concept and design, data curation, formal analysis, methodology, project administration, supervision, data validation and visualization, writing, review, editing and approval of the manuscript, C.O. trial concept and design, feasibility, monitoring, data curation, formal analysis, data interpretation, project administration, supervision, data validation and visualization, writing (original draft), writing, review, editing and approval of the manuscript.
CONFLICT OF INTEREST STATEMENT
P.R. reports fees to his institution from Bayer during the conduct of the study and fees to his institution from Novo Nordisk, Gilead, Bayer, Sanofi Aventis, Eli Lilly, Boehringer Ingelheim, Astellas, Mundipharma, Merck and Astra Zeneca outside the submitted work. A.A. reports fees to his institution from Bayer during conduct of the study, grants from Astra Zeneca and Mundipharma outside the submitted work, as well as personal fees from Astra Zeneca, Boehringer, Sanofi Aventis, Novartis, Mundipharma, Servier, Eli Lilly, Novo Nordisk and Merck Sharp & Dohme outside the submitted work. J.S. reports fees to his institution from Bayer during the conduct of the study. F.K., M.B. and C.O. are employees of Bayer AG. C.O. is stockholder at Bayer AG. The results presented in this article have not been published previously, either in whole or in part.
DATA AVAILABILITY STATEMENT
The data underlying this article cannot be shared publicly to ensure patients’ privacy rights.





Comments