-
PDF
- Split View
-
Views
-
Cite
Cite
Charalampos Loutradis, Pantelis A Sarafidis, Charles J Ferro, Carmine Zoccali, Volume overload in hemodialysis: diagnosis, cardiovascular consequences, and management, Nephrology Dialysis Transplantation, Volume 36, Issue 12, December 2021, Pages 2182–2193, https://doi.org/10.1093/ndt/gfaa182
Close - Share Icon Share
Abstract
Volume overload in haemodialysis (HD) patients associates with hypertension and cardiac dysfunction and is a major risk factor for all-cause and cardiovascular mortality in this population. The diagnosis of volume excess and estimation of dry weight is based largely on clinical criteria and has a notoriously poor diagnostic accuracy. The search for accurate and objective methods to evaluate dry weight and to diagnose subclinical volume overload has been intensively pursued over the last 3 decades. Most methods have not been tested in appropriate clinical trials and their usefulness in clinical practice remains uncertain, except for bioimpedance spectroscopy and lung ultrasound (US). Bioimpedance spectroscopy is possibly the most widely used method to subjectively quantify fluid distributions over body compartments and produces reliable and reproducible results. Lung US provides reliable estimates of extravascular water in the lung, a critical parameter of the central circulation that in large part reflects the left ventricular end-diastolic pressure. To maximize cardiovascular tolerance, fluid removal in volume-expanded HD patients should be gradual and distributed over a sufficiently long time window. This review summarizes current knowledge about the diagnosis, prognosis and treatment of volume overload in HD patients.
INTRODUCTION
Over the past few years, mortality in patients with end-stage renal disease (ESRD) undergoing haemodialysis (HD) has only slightly decreased [1]. Cardiovascular disease is the leading cause of mortality, accounting for >50% of deaths with known causes, while cardiovascular mortality is 9-fold higher in these patients compared with age- and sex-matched individuals in the general population [2]. Cardiovascular events, such as sudden death and hospitalization for heart failure, show a specific weekly pattern in HD patients, being 25–40% higher during the first-weekly HD day (Monday or Tuesday) compared with any other weekday and concentrate within the last hours of the 3-day interval and the following session [3].
Several factors may be responsible for the increased cardiovascular mortality in dialysis patients, but sodium and volume overload are considered among the main mechanisms of this association [4]. In ESRD patients, the kidneys are unable to maintain sodium and water homeostasis [5]. Because of the intermittent nature of the thrice-weekly haemodialysis regimen, excessive interdialytic weight gain (IDWG) and the consequent excessive intradialytic weight loss constitute a cyclical cardiovascular stress [6]. Sodium and volume accumulation is inevitably larger during the 3-day interdialytic interval, which elevates pre-dialysis and ambulatory blood pressure (BP) levels as well as wave reflections [augmentation index (AIx) and augmentation pressure (AP)] from the periphery [7]. Hypervolaemia is also a frequent condition in peritoneal dialysis patients, and despite its more continuous nature, volume excess is no different compared with HD [8].
Defining the optimal hydration status in HD patients is challenging and the identification of an accurate and objective method to evaluate dry weight has been a focus of HD research for decades [9]. Most studies in this research area are observational in nature and there is a paucity of trials testing diagnostic methods that may guide treatment of volume overload and improve outcomes in these patients. Moreover, the Kidney Disease Outcomes Quality Initiative HD guidelines do not include recommendations for the diagnosis of fluid overload by objective methods [10]. The aim of this review is to summarize current knowledge regarding the associated factors, consequences, diagnosis and management of volume overload in HD.
Diagnosis of volume overload
An overview of the available methods for volume overload assessment is presented in Table 1. The lack of validated, easy to apply methods of measurement of body fluid volume status most likely contributes to the high prevalence of volume excess in HD patients. In most centres, volume status is usually estimated on the basis of clinical criteria, i.e. patient’s signs and symptoms, peridialytic BP measurements and intradialytic haemodynamic instability [5]. However, previous evidence suggests that clinical assessment of volume balance is a method with limited reliability. A bioimpedance spectroscopy–based study by Wabel et al. [11] including 500 HD patients indicated that pre-HD systolic BP (SBP) misclassifies the hydration status in ∼25% of patients. In addition, it is now well established that pre- and post-dialysis BP measurements are poorly reproducible, poorly associated with interdialytic BP assessed with ambulatory BP monitoring (ABPM) and have no association with outcomes [4]. A cross-sectional study in 146 HD patients examined the association of several volume-related parameters [inferior vena cava diameter (IVC) and collapsibility, blood volume monitoring markers, plasma renin, aldosterone, N-terminal pro B-type natriuretic peptide, C-reactive protein (CRP) and interleukin-6] with pedal oedema and results showed that only C-reactive protein levels were different in patients with and without oedema (1.22 ± 1.61 versus 0.90 ± 1.43 mg/dL; P = 0.03) while none of these measurements was associated with the presence of oedema, suggesting that pedal oedema may not accurately reflect volume [12]. A more recent study based on 1106 pre- and post-dialysis lung US scans in 79 HD patients showed that only 49% and 20% of the patients with severe congestion in this critical organ had lung crackles or peripheral oedema (Figure 1) [13].
![Correlation analysis between volume overload evaluated with lung US and (A) pulmonary crackles and (B) pedal oedema. Reprinted from Torino et al. [13], with permission.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ndt/36/12/10.1093_ndt_gfaa182/2/m_gfaa182fig1.jpeg?Expires=1748364594&Signature=WGD9N~-6LiOEUHbS~ZpHfZhzV8VQT5-Iy1UwWLURVtpjQZkO3XJdyYQKdXF~-JbGovPLer8HRpVJqbv9N905shCtLQK2tlREgOU4pcMCV1raQvKLJlBP206ULnwO4p1Z1yP-nZx~bDJeNVMxxLcBItLq1swMW4j-1Y8in~Wwwio9ZpW6abdzoscgLVW20psMXWJOuOC-bDNGvT7SA0mUkwW1QqOSFLc10p61upUWRjTyVl8mVcMxUMDtGKIUx-6-fUfXlJCzlxG3b5VvoXFcZo5vAZYuCZrhvze82duwoQ5GTB8hCEHQjOKwpngI~LgTpi4mqw37pbOUXNUzaQquAw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Correlation analysis between volume overload evaluated with lung US and (A) pulmonary crackles and (B) pedal oedema. Reprinted from Torino et al. [13], with permission.
| Method . | Principles of volume detection . | Body fluid compartment evaluated . | Advantages . | Disadvantages . | Evidence in HD patients . |
|---|---|---|---|---|---|
| Clinical criteria (peripheral oedema, lung auscultation, dyspnoea, blood pressure levels, jugular vein distension) | Clinical signs reflecting volume overload | TBW Extracellular volume Intravascular volume | Inexpensive Non-invasive Easy to apply Readily available | Non-standardized Observer dependent Associated with several clinical conditions | Yes (observational studies and randomized clinical trials) |
| Bioimpedance analysis (whole body or calf based) | Single- or multifrequency electric current flow to calculate body impedance and resistance | TBW Extracellular volume Intracellular volume | Non-invasive Easy to apply | Not widely available Interstitial and intravascular fluid compartment not estimated Highly variable results Poor association with outcomes | Yes (observational studies and randomized clinical trials) |
| Lung US | Ultrasonographic evaluation of interstitial lung water | Interstitial lung water | Inexpensive Non-invasive Easy to apply | Observer dependent Requires additional training Intravascular fluid compartment not estimated Results may be affected by the presence of heart failure or diffuse parenchymal lung disease | Yes (observational studies and randomized clinical trials) |
| Inferior vena cava diameter and collapsibility | Ultrasonographic evaluation of diameter and collapsibility during a respiratory cycle | Intravascular volume | Inexpensive Non-invasive Long-established method | Observer dependent Requires additional training Difficult to measure (obesity, poor image quality due to the intestinal air) No specific limits for diagnosis Extracellular volume not evaluated Results may be affected by the presence of diastolic dysfunction and intrathoracic or abdominal pressure changes | Yes (observational studies) |
| B-type natriuretic peptide | Hormone secreted by cardiomyocytes in response to stretching | Intravascular volume | Non-dialyzable Non-invasive Easy to measure | Expensive Not widely available Extracellular volume not evaluated Strongly associated with heart failure and diastolic dysfunction | Yes (observational studies) |
| Relative plasma volume (plethysmography, acoustic transmission) | Real-time measurement of haematocrit to estimate changes in plasma volume Changes in acoustic transmission due to changes in blood density | Intravascular volume | Non-invasive Real-time monitoring Reproducible | Only intradialytic assessment Relative changes in volume measured Extracellular volume not evaluated Increased vascular access related episodes | Yes (observational studies and randomized clinical trials) |
| Whole-body bioimpedance cardiography | Changes in electrical resistance of the arterial system, which are converted into changes in blood and stroke volume through a proprietary algorithm | TBW | Inexpensive Non-invasive Real-time monitoring Information on haemodynamic response to ultrafiltration | Not widely available Interstitial and intravascular fluid compartment not estimated Cardiac parameters may be difficult to interpret | Yes (observational studies) |
| Carotid artery corrected flow time | Ultrasonographic evaluation of carotid arteries’ flow time due to changes in blood’s density | Intravascular volume | Non-invasive Real-time monitoring Reproducible | Only intradialytic assessment Relative changes in volume measured Extracellular volume not evaluated | Yes (observational studies) |
| Artificial intelligence | Secondary analysis of the data obtained with another subjective method | Relevant to the method used | Non-invasive | Difficult to apply Includes of the disadvantages of the applied method Experimental Expensive Time-consuming | Yes (observational in paediatric HD patients) |
| Invasive techniques (pulmonary artery catheterization, transesophageal aortic flow, etc.) | Invasive estimation of the intravascular fluid compartment | Intravascular volume | More accurate | Invasive Expensive Time-consuming Difficult to apply on clinical routine Never tested in HD patients | No |
| Hydrodensitometry | Hydrostatic or underwater body weighing | TBW | More accurate Long-established method | Not widely available Expensive Time-consuming Difficult to apply on clinical routin Never tested in HD patients | No |
| Method . | Principles of volume detection . | Body fluid compartment evaluated . | Advantages . | Disadvantages . | Evidence in HD patients . |
|---|---|---|---|---|---|
| Clinical criteria (peripheral oedema, lung auscultation, dyspnoea, blood pressure levels, jugular vein distension) | Clinical signs reflecting volume overload | TBW Extracellular volume Intravascular volume | Inexpensive Non-invasive Easy to apply Readily available | Non-standardized Observer dependent Associated with several clinical conditions | Yes (observational studies and randomized clinical trials) |
| Bioimpedance analysis (whole body or calf based) | Single- or multifrequency electric current flow to calculate body impedance and resistance | TBW Extracellular volume Intracellular volume | Non-invasive Easy to apply | Not widely available Interstitial and intravascular fluid compartment not estimated Highly variable results Poor association with outcomes | Yes (observational studies and randomized clinical trials) |
| Lung US | Ultrasonographic evaluation of interstitial lung water | Interstitial lung water | Inexpensive Non-invasive Easy to apply | Observer dependent Requires additional training Intravascular fluid compartment not estimated Results may be affected by the presence of heart failure or diffuse parenchymal lung disease | Yes (observational studies and randomized clinical trials) |
| Inferior vena cava diameter and collapsibility | Ultrasonographic evaluation of diameter and collapsibility during a respiratory cycle | Intravascular volume | Inexpensive Non-invasive Long-established method | Observer dependent Requires additional training Difficult to measure (obesity, poor image quality due to the intestinal air) No specific limits for diagnosis Extracellular volume not evaluated Results may be affected by the presence of diastolic dysfunction and intrathoracic or abdominal pressure changes | Yes (observational studies) |
| B-type natriuretic peptide | Hormone secreted by cardiomyocytes in response to stretching | Intravascular volume | Non-dialyzable Non-invasive Easy to measure | Expensive Not widely available Extracellular volume not evaluated Strongly associated with heart failure and diastolic dysfunction | Yes (observational studies) |
| Relative plasma volume (plethysmography, acoustic transmission) | Real-time measurement of haematocrit to estimate changes in plasma volume Changes in acoustic transmission due to changes in blood density | Intravascular volume | Non-invasive Real-time monitoring Reproducible | Only intradialytic assessment Relative changes in volume measured Extracellular volume not evaluated Increased vascular access related episodes | Yes (observational studies and randomized clinical trials) |
| Whole-body bioimpedance cardiography | Changes in electrical resistance of the arterial system, which are converted into changes in blood and stroke volume through a proprietary algorithm | TBW | Inexpensive Non-invasive Real-time monitoring Information on haemodynamic response to ultrafiltration | Not widely available Interstitial and intravascular fluid compartment not estimated Cardiac parameters may be difficult to interpret | Yes (observational studies) |
| Carotid artery corrected flow time | Ultrasonographic evaluation of carotid arteries’ flow time due to changes in blood’s density | Intravascular volume | Non-invasive Real-time monitoring Reproducible | Only intradialytic assessment Relative changes in volume measured Extracellular volume not evaluated | Yes (observational studies) |
| Artificial intelligence | Secondary analysis of the data obtained with another subjective method | Relevant to the method used | Non-invasive | Difficult to apply Includes of the disadvantages of the applied method Experimental Expensive Time-consuming | Yes (observational in paediatric HD patients) |
| Invasive techniques (pulmonary artery catheterization, transesophageal aortic flow, etc.) | Invasive estimation of the intravascular fluid compartment | Intravascular volume | More accurate | Invasive Expensive Time-consuming Difficult to apply on clinical routine Never tested in HD patients | No |
| Hydrodensitometry | Hydrostatic or underwater body weighing | TBW | More accurate Long-established method | Not widely available Expensive Time-consuming Difficult to apply on clinical routin Never tested in HD patients | No |
| Method . | Principles of volume detection . | Body fluid compartment evaluated . | Advantages . | Disadvantages . | Evidence in HD patients . |
|---|---|---|---|---|---|
| Clinical criteria (peripheral oedema, lung auscultation, dyspnoea, blood pressure levels, jugular vein distension) | Clinical signs reflecting volume overload | TBW Extracellular volume Intravascular volume | Inexpensive Non-invasive Easy to apply Readily available | Non-standardized Observer dependent Associated with several clinical conditions | Yes (observational studies and randomized clinical trials) |
| Bioimpedance analysis (whole body or calf based) | Single- or multifrequency electric current flow to calculate body impedance and resistance | TBW Extracellular volume Intracellular volume | Non-invasive Easy to apply | Not widely available Interstitial and intravascular fluid compartment not estimated Highly variable results Poor association with outcomes | Yes (observational studies and randomized clinical trials) |
| Lung US | Ultrasonographic evaluation of interstitial lung water | Interstitial lung water | Inexpensive Non-invasive Easy to apply | Observer dependent Requires additional training Intravascular fluid compartment not estimated Results may be affected by the presence of heart failure or diffuse parenchymal lung disease | Yes (observational studies and randomized clinical trials) |
| Inferior vena cava diameter and collapsibility | Ultrasonographic evaluation of diameter and collapsibility during a respiratory cycle | Intravascular volume | Inexpensive Non-invasive Long-established method | Observer dependent Requires additional training Difficult to measure (obesity, poor image quality due to the intestinal air) No specific limits for diagnosis Extracellular volume not evaluated Results may be affected by the presence of diastolic dysfunction and intrathoracic or abdominal pressure changes | Yes (observational studies) |
| B-type natriuretic peptide | Hormone secreted by cardiomyocytes in response to stretching | Intravascular volume | Non-dialyzable Non-invasive Easy to measure | Expensive Not widely available Extracellular volume not evaluated Strongly associated with heart failure and diastolic dysfunction | Yes (observational studies) |
| Relative plasma volume (plethysmography, acoustic transmission) | Real-time measurement of haematocrit to estimate changes in plasma volume Changes in acoustic transmission due to changes in blood density | Intravascular volume | Non-invasive Real-time monitoring Reproducible | Only intradialytic assessment Relative changes in volume measured Extracellular volume not evaluated Increased vascular access related episodes | Yes (observational studies and randomized clinical trials) |
| Whole-body bioimpedance cardiography | Changes in electrical resistance of the arterial system, which are converted into changes in blood and stroke volume through a proprietary algorithm | TBW | Inexpensive Non-invasive Real-time monitoring Information on haemodynamic response to ultrafiltration | Not widely available Interstitial and intravascular fluid compartment not estimated Cardiac parameters may be difficult to interpret | Yes (observational studies) |
| Carotid artery corrected flow time | Ultrasonographic evaluation of carotid arteries’ flow time due to changes in blood’s density | Intravascular volume | Non-invasive Real-time monitoring Reproducible | Only intradialytic assessment Relative changes in volume measured Extracellular volume not evaluated | Yes (observational studies) |
| Artificial intelligence | Secondary analysis of the data obtained with another subjective method | Relevant to the method used | Non-invasive | Difficult to apply Includes of the disadvantages of the applied method Experimental Expensive Time-consuming | Yes (observational in paediatric HD patients) |
| Invasive techniques (pulmonary artery catheterization, transesophageal aortic flow, etc.) | Invasive estimation of the intravascular fluid compartment | Intravascular volume | More accurate | Invasive Expensive Time-consuming Difficult to apply on clinical routine Never tested in HD patients | No |
| Hydrodensitometry | Hydrostatic or underwater body weighing | TBW | More accurate Long-established method | Not widely available Expensive Time-consuming Difficult to apply on clinical routin Never tested in HD patients | No |
| Method . | Principles of volume detection . | Body fluid compartment evaluated . | Advantages . | Disadvantages . | Evidence in HD patients . |
|---|---|---|---|---|---|
| Clinical criteria (peripheral oedema, lung auscultation, dyspnoea, blood pressure levels, jugular vein distension) | Clinical signs reflecting volume overload | TBW Extracellular volume Intravascular volume | Inexpensive Non-invasive Easy to apply Readily available | Non-standardized Observer dependent Associated with several clinical conditions | Yes (observational studies and randomized clinical trials) |
| Bioimpedance analysis (whole body or calf based) | Single- or multifrequency electric current flow to calculate body impedance and resistance | TBW Extracellular volume Intracellular volume | Non-invasive Easy to apply | Not widely available Interstitial and intravascular fluid compartment not estimated Highly variable results Poor association with outcomes | Yes (observational studies and randomized clinical trials) |
| Lung US | Ultrasonographic evaluation of interstitial lung water | Interstitial lung water | Inexpensive Non-invasive Easy to apply | Observer dependent Requires additional training Intravascular fluid compartment not estimated Results may be affected by the presence of heart failure or diffuse parenchymal lung disease | Yes (observational studies and randomized clinical trials) |
| Inferior vena cava diameter and collapsibility | Ultrasonographic evaluation of diameter and collapsibility during a respiratory cycle | Intravascular volume | Inexpensive Non-invasive Long-established method | Observer dependent Requires additional training Difficult to measure (obesity, poor image quality due to the intestinal air) No specific limits for diagnosis Extracellular volume not evaluated Results may be affected by the presence of diastolic dysfunction and intrathoracic or abdominal pressure changes | Yes (observational studies) |
| B-type natriuretic peptide | Hormone secreted by cardiomyocytes in response to stretching | Intravascular volume | Non-dialyzable Non-invasive Easy to measure | Expensive Not widely available Extracellular volume not evaluated Strongly associated with heart failure and diastolic dysfunction | Yes (observational studies) |
| Relative plasma volume (plethysmography, acoustic transmission) | Real-time measurement of haematocrit to estimate changes in plasma volume Changes in acoustic transmission due to changes in blood density | Intravascular volume | Non-invasive Real-time monitoring Reproducible | Only intradialytic assessment Relative changes in volume measured Extracellular volume not evaluated Increased vascular access related episodes | Yes (observational studies and randomized clinical trials) |
| Whole-body bioimpedance cardiography | Changes in electrical resistance of the arterial system, which are converted into changes in blood and stroke volume through a proprietary algorithm | TBW | Inexpensive Non-invasive Real-time monitoring Information on haemodynamic response to ultrafiltration | Not widely available Interstitial and intravascular fluid compartment not estimated Cardiac parameters may be difficult to interpret | Yes (observational studies) |
| Carotid artery corrected flow time | Ultrasonographic evaluation of carotid arteries’ flow time due to changes in blood’s density | Intravascular volume | Non-invasive Real-time monitoring Reproducible | Only intradialytic assessment Relative changes in volume measured Extracellular volume not evaluated | Yes (observational studies) |
| Artificial intelligence | Secondary analysis of the data obtained with another subjective method | Relevant to the method used | Non-invasive | Difficult to apply Includes of the disadvantages of the applied method Experimental Expensive Time-consuming | Yes (observational in paediatric HD patients) |
| Invasive techniques (pulmonary artery catheterization, transesophageal aortic flow, etc.) | Invasive estimation of the intravascular fluid compartment | Intravascular volume | More accurate | Invasive Expensive Time-consuming Difficult to apply on clinical routine Never tested in HD patients | No |
| Hydrodensitometry | Hydrostatic or underwater body weighing | TBW | More accurate Long-established method | Not widely available Expensive Time-consuming Difficult to apply on clinical routin Never tested in HD patients | No |
The most widely used method to quantify fluid distributions over body compartments is bioimpedance spectroscopy, which performs an analysis of the body’s resistance and reactance by measuring the electric current applied on distant electrodes on the body surface [14]. A comprehensive, multicentre validation study including 152 subjects (120 healthy individuals and 32 HD patients) showed good agreement (shared variance 91%, R2 = 0.91) between total body water (TBW) measured by bioimpedance spectroscopy and the golden standard methods (deuterium or tritium dilution) [15]. In the Bland–Altman plot of these variables, only 5 subjects among 152 had values >2 standard deviations of the average difference between the gold standard measurements of TBW and the corresponding bioimpedance measurement, while there was no bias in the bioimpedance TBW measurement versus the gold standard methods. Apart from the agreement with the golden standard, it is important to note that bioimpedance spectroscopy is a reproducible measurement with an interobserver and intra-observer coefficient of variation of ∼2–5% [16, 17]. In another study by Moissl et al. [18], bioimpedance was proved to be useful to prospectively guide fluid volume optimization in 55 dialysis patients. In another study, in which 156 HD patients were randomized to bioimpedance-assisted fluid management or standard-of-care treatment, results showed that bioimpedance use was associated with a clear-cut improvement in left ventricular (LV) mass index {mean between groups difference −10.2 g/m2 [95% confidence interval (CI) −19.2 to −1.17]} and arterial stiffness (two surrogate endpoints) [19]. In a population of 39 566 HD patients from 26 countries, it was found that chronic fluid overload as assessed by bioimpedance spectroscopy (body composition monitor, Fresenius, Bad Homburg vor der Höhe, Germany) doubles the death risk independent of other risk factors [20]. By the same token, another cohort study in 8883 HD patients fully confirmed a dose–response relationship between the degree of fluid overload and mortality [21].
During the last decade, a novel and easy to apply ultrasound (US) technique to quantify water excess in the lungs in these patients has been developed [22]. Lung US takes advantage of basic principles of ultrasonography, i.e. excessive lung water in the thickened subpleural interlobular septa completely reflect the US beam, forming highly echogenic structures, the US-B lines [22], which are hyperechoic reverberation artefacts between the subpleural interlobular septa and the overlying pleura (Figure 2) [23]. In a cross-sectional analysis of baseline data from the Lung water by UltraSound guided Treatment to prevent death and cardiovascular complications in high risk end-stage renal disease patients with cardiomyopathy (LUST) study, lung US was superior to clinical criteria in detecting and monitoring volume excess in haemodialysis patients [13]. The feasibility and the validation of this technique in HD patients were examined in a study including 75 HD patients [24]. In this study, the number of US-B lines was reproducible and had small interoperator variability. Of note, lung water as estimated by this technique modestly correlated with LV mass (r = 0.28, P = 0.01), diastolic dysfunction and LV filling pressures [early transmitral diastolic velocity (E): r = 0.31, P = 0.008; early transmitral diastolic velocities ratio (E:e‵): r = 0.48, P < 0.001], but was only weakly associated with the hydration status as measured by bioimpedance analysis [24]. Previous ultrasonographic techniques include measurement of the IVC diameter and collapsibility [25]. Measurement of IVC diameter, relative plasma volume and bioimpedance analysis during a dry weight reduction protocol guided by clinical criteria has been examined in a pilot study in 16 incident HD patients [26]. In this study, all methods, except bioimpedance, were overtly inadequate for capturing meaningful volume changes induced by a dry weight reduction intervention [26]. Recently an automated method to evaluate IVC diameter has been developed, but this device has not yet been tested in dialysis patients [27]. Although the inferior vena cava reflects central venous pressure, it is a poor marker of hydration status because it may be affected by respiration, right heart function and by intrathoracic or abdominal pressure changes [28].
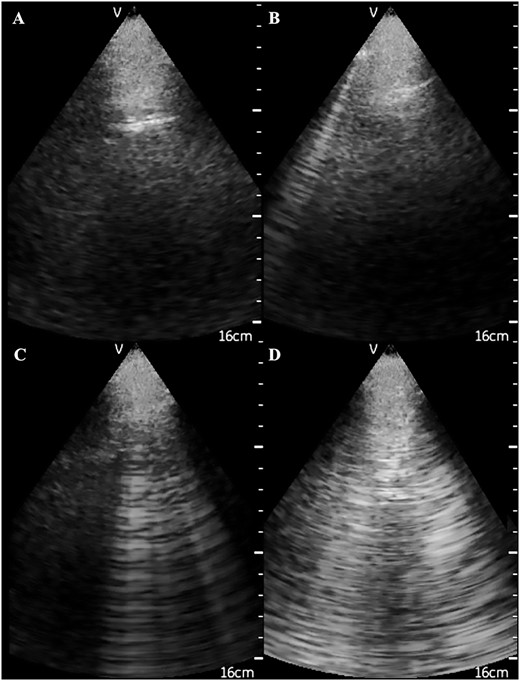
Ultrasonographic appearance of (A) normal lungs and the presence of (B) 1, (C) 4 and (D) 10 US B-lines.
Additional methods for the evaluation of hydration status in dialysis patients include the use of circulating biomarkers. However, biomarkers are costly and are variably removed during HD, while their levels are highly dependent on cardiac function [29]. Results from observational studies suggest a significant association of cardiac natriuretic peptides with volume status assessed with bioimpedance analysis and mortality [30, 31], but their simultaneous association with LV hypertrophy (LVH) and systolic dysfunction [32] substantially weakens their diagnostic potential for volume overload. The Crit-Line (Hema Metrics, Kaysville, UT, USA), which uses a transmissive photometric technique to provide intradialytic real-time assessment of hematocrit levels and to estimate changes in relative plasma volume, is of dubious value for assessing volume status and for guiding fluid removal in HD patients [28]. The use of whole-body bioimpedance cardiography, which provides multidimensional insight into intradialytic haemodynamic changes (peripheral vascular resistance, cardiac output), was tested in a single study including 27 HD patients, but results indicated no significant differences in the recorded parameters between patients with and without intradialytic hypotension [33]. Flow time (FT), or LV ejection time, reflects the duration of systole and is measured from the beginning of the upstroke to the trough of the incisura notch on pulse waveform analysis. FT is corrected for the heart rate (FTc) and the change in this measurement may reflect variations in stroke volume and circulating volume [34]. FTc in HD patients decreases during standard dialysis, but the value of the technique for the diagnosis of volume status and for monitoring this parameter in dialysis are unknown [35]. Invasive techniques such as pulmonary artery catheterization and transesophageal Doppler evaluation of aortic FT changes have never been tested in the HD population [28]. A small study in 14 paediatric HD patients compared optimal dry weight estimation between an algorithmic analysis through neuronal networks of data obtained with bioimpedance spectroscopy and intradialytic plasma volume monitoring with clinical evaluation by treating physicians and showed that standard-of-care treatment was associated with non-significantly higher dry weight [mean difference 0.497 kg (95% CI −1.33–1.29), P = 0.99] [36]. Perhaps not surprisingly, no study so far has ever compared any of the above methods with the best available technique for measuring lean body mass, that of hydrodensitometry (hydrostatic or underwater weighing), in HD patients.
Direct consequences of volume overload on BP and cardiovascular regulation in HD patients
During interdialytic intervals, HD patients are subjected to fluid accumulation, a parameter defined by the oral fluid and food–water intake minus residual urine output (when present), stool water output, sweat and respiratory water loss. Chronic fluid overload develops as the patient’s fluid gains exceed the prescribed ultrafiltration rate needed to achieve or maintain dry weight. Higher IDWG is closely associated with higher pre-dialysis BP [37] and results in a gradual upward shift in ambulatory BP levels, which is worse during the third day and night of the long interdialytic interval [38, 39]. Thus volume overload is currently considered the main pathogenic mechanism of hypertension in the ESRD population [5]. As a matter of fact, the worldwide prevalence of hypertension in this population is as high as 85% [40]. The increase in volume during the interdialytic intervals likely contributes to short-term BP variability from Day 1 to Day 2 of the regular interdialytic interval [41], which has been implicated in the risk for cardiovascular events independent of BP levels [42]. Further, chronic volume overload and increased IDWG are considered major factors for the BP increase that occurs in some patients during the HD session [43]. On the other hand, higher ultrafiltration volume is also associated with intradialytic hypotensive episodes [44]. This can result in interruption of sessions, shorter dialysis time, inappropriate adjustment of target dry weight to higher levels or infusion of saline solutions, all factors that further contribute to volume expansion [45]. High ultrafiltration volumes (>2.5 kg) go along with higher pre- and lower post-dialysis BP as compared with ultrafiltration volumes ≤2.5 kg and this association may be triggered by poor compliance with fluid restriction and non-achievement of the true dry weight, a hypothesis supported by a large survey [46]. Results from another study examining target weight achievement patterns in 152 196 HD patients suggested that higher ultrafiltration volume (2.9 ± 1.5 versus 2.4 ± 1.2 L) and rate (10.2 ± 5.2 versus 8.6 ± 4.5 mL/h/kg) were significantly higher in patients not achieving dry weight (post-dialysis weight ≥1 kg from the prescribed dry weight) [47].
The number of antihypertensive drugs prescribed to HD patients paradoxically associates with higher BP levels [48]. This may depend on the fact that antihypertensive drugs may prompt haemodynamic fragility during dialysis, eventually hindering the achievement of dry weight, thus causing volume expansion and apparent resistance to drug treatment. Moreover, a cross-sectional study compared two different approaches for the treatment of hypertension in 423 HD patients and showed that antihypertensive drug–based therapy was associated with more intradialytic hypotensive episodes compared with salt restriction and dry weight reduction (27 versus 11 per 100 patient sessions; P < 0.01) [49]. It is likely that this association is confounded by the fact that too much medication may actually limit the opportunity to probe dry weight and leads to BP resistance through expanded volume [48].
Beyond BP, volume accumulation during the interdialytic interval and the subsequent volume removal during HD associate with intermittent changes in other important cardiovascular parameters. Arterial stiffness is probably the major vascular abnormality in ESRD patients and represents the main underlying factors for isolated systolic hypertension and LVH in this population [50]. The AP, AIx and central pulse pressure (PP), all alterations resulting from arterial stiffness, gradually increase during both the 3-day and 2-day interdialytic interval, whereas pulse wave velocity (PWV) remains unchanged during these short time frames. Thus intermittent volume accumulation may trigger cardiovascular damage via arterial stiffness–related mechanisms, leading to the hypothesis that premature arrival of the reflected waves is generated by intermittent volume accumulation causing alterations in peripheral vascular tone and, consequently, changes in the morphology of peripheral reflection sites [51]. Subsequent ABPM studies registering BP and arterial stiffness–related measurements (AIx and central BP) during the 2-day interval confirm this interpretation and an additive increase during the third day of the 3-day interval with minimal increase in PWV [38, 39].
In the same vein, interdialytic volume accumulation during the long interval goes along with dilatation of the left and right cardiac chambers, pulmonary circulation overload and diastolic dysfunction in these patients. Cardiac chambers dilatation and IDWG are closely associated, indicating that the recurrent stretching of cardiac chambers between sessions results in long-term cardiac chambers remodelling [52]. The long (3-day) interdialytic interval associates with more pronounced left and right atrial enlargement and right ventricular systolic pressure (RVSP) increase, and the IDWG is the strongest factor driving the increase in RVSP, suggesting that continuous volume accumulation underlies not only left but also right atrium enlargement and pulmonary overload [53]. In clinical practice, higher IDWG prompts the prescription of higher ultrafiltration volumes, which in turn leads to diastolic dysfunction and myocardial stunning, possibly due to a rapid decrease of the intravascular fluid compartment [54]. The increase in central BP accompanying interdialytic volume accumulation directly increases cardiac afterload and raises myocardial oxygen demand, thereby favouring the occurrence of acute ischaemic cardiac events [6].
Long-term consequences of volume overload in haemodialysis
Volume overload is considered a major underlying risk factor for all-cause and cardiovascular death in ESRD patients [55]. Large studies focusing on IDWG, a proxy of volume overload and the simplest biomarker of fluid overload, coherently exposed the potential deleterious effects of volume expansion on survival and cardiovascular disease [56–58]. Both high dietary sodium intake and high sodium concentration in the dialysate induce a positive sodium balance, activate the thirst mechanism and increase fluid intake [59]. Total body sodium is distributed in different compartments, namely the osmotically active sodium of the extracellular fluid, which directly affects haemodynamic response and fluid tonicity, and the osmotically inactive exchangeable sodium stored in the bones, skin, muscle and artery interstitium, which replenishes extracellular sodium but also affects local hypertonicity, immune cell activity and BP [60]. As expected, salt restriction associates with lower IDWG in this population and such an association is independent of pre-haemodialysis BP levels [49]. Similarly, high dialysate sodium to induce a positive sodium gradient results in an increase in serum sodium across dialysis, which in turn triggers thirst and increases the IDWG [61].
Several studies have associated objectively measured fluid overload with adverse clinical outcomes (Table 2). As analysed above, a study including ∼40 000 incident HD patients showed that cumulative 1-year fluid overload by whole-body bioimpedance spectroscopy associates with all-cause mortality, regardless of BP levels [20]. The hypothesis that bioimpedance-guided fluid removal might translate into better clinical outcomes is supported by a small trial (131 HD patients) with a very limited number of events (one death in the intervention and nine deaths in the control arm) [73]. Another randomized trial in 298 haemodialysis patients in Taiwan testing the same intervention showed that bioimpedance-guided ultrafiltration did not influence the risk of hospitalization but reduced the incidence of acute fluid overload and cardiovascular events, as well as the incidence of hypertension and intradialysis hypotension, while mortality did not differ in the two study arms, but the number of deaths was again very small again (three in the intervention arm and three in the control arm) [74]. Even though these trials showed signals of benefit of bioimpedance-guided treatment strategies, no conclusion can be drawn from the data gathered until now.
Observational studies examining the association of volume overload and hard outcomes in patients undergoing HD; adjusted HRs or β-coefficients are reported
| Study . | . | N . | Study design . | Follow-up . | Volume parameter . | Primary outcome and results . | Secondary outcomes and results . |
|---|---|---|---|---|---|---|---|
| Foley et al. [62] | 11 142 | Prospective | 3.8 years | IDWG >4.8% of body weight | ACM: HR 1.12 (95% CI 1.02–1.23) | – | |
| Saran et al. [63] | Euro-DOPPS: 2337 Japan: 1980 USA: 3359 | Prospective | Euro-DOPPS: 1.8 years Japan: 2.0 years United States: 2.9 years | IDWG >5.7% of body weight | ACM: HR 1.12 (95% CI 1.00–1.26) | Hospitalization: 1.09 (95% CI 0.99–1.19) | |
| Kalantar-Zadeh et al. [56] | 34 107 | Retrospective | 2.0 years | IDWG ≥4.0 kg | ACM: HR 1.28 (95% CI 1.15–1.39) | CVM: HR 1.25 (95% CI 1.12–1.39) | |
| Wizemann et al. [64] | 269 | Prospective | 3.5 years | FO/extracellular water ≥15% with BIA | ACM: HR 2.102 (95% CI 1.389–3.179) | – | |
| Chazot et al. [65] | 208 | Retrospective | 6.5 years | HS: 3.5±1.2 L with BIA FO/extracellular water ≥15% with BIA | ACM: HR 3.41 (95% CI 1.62–7.17) | – | |
| Zoccali et al. [66] | 392 | Retrospective | 2.1 (IQR 1.8–2.4) years | US B-lines >60 with LUS | ACM: HR 4.20, (95% CI 2.45–7.23) | CVE: HR 3.20 (95% CI 1.75–5.88) | |
| Siriopol et al. [67] | 96 | Prospective | 405.5 (IQR 234.8–518.0) days | Pre-HD US B-lines >30 with LUS Pre-HD FO/extracellular water >15% with BIA | ACM: HR 3.63 (95% CI 1.03–12.74) for LUS HR 1.22 (95% CI 0.26–5.74) for BIA | – | |
| Cabrera et al. [57] | 39 256 | Retrospective | 1–3 years | IDWG >3.5% of post-dialysis weight over 3 months | ACM: HR 1.26 (95% CI 1.20–1.33) | MI: HR 1.18 (95% CI 1.08–1.28) CVM: HR 1.23 (95% CI 1.14–1.34) | |
| Kim et al. [68] | 344 | Prospective | 24.0 months | FO/extracellular water ≥15% with BIA | ACM: HR 2.582 (95% CI 1.159–5.750) | – | |
| Onofriescu et al. [69] | 157 | Prospective | 66.2 (IQR 42.4–70.2) months | BIA FO/extracellular water >15% and >17.4% with BIA | ACM: HR 1.87 (95% CI 1.12–3.13 for RFO >15%) HR 2.72 (95% CI 1.60–4.63) for RFO >17.4% | CVE: HR 2.31 (95% CI 1.42–3.77) for RFO >15% HR 4.17 (95% CI 2.48–7.02) for RFO >17.4% Hospitalization: HR 0.84 (95% CI 0.71–1.01) HR 0.78 (95% CI 0.64–0.95) DHF hospitalization: HR 0.53 (95% CI 0.24–1.19) HR 0.52 (95% CI 0.22–1.36) | |
| Siriopol et al. [70] | 173 | Prospective | 21.3 (IQR 19.9–30.3) months | US B-lines >22 with LUS FO/extracellular water ≥6.88% with BIA | ACM: HR 2.72 (95% CI 1.19–6.16) for LUS HR 2.93 (95% CI 1.30–6.58) for BIA | – | |
| Wong et al. [58] | 21 919 | Prospective | 2.0 (IQR 1.0–2.6) years | IDWG ≥5.7% of post-dialysis weight | ACM: HR 1.23 (95% CI 1.08–1.49) | Fluid overload-related hospitalization: HR 1.64 (95% CI 1.27–2.13) | |
| Zoccali et al.[20] | 39 566 | Prospective | 497 ± 358 days | FO/extracellular water ≥15% for male and ≥13% for female over 1 year with BIA | ACM: HR 1.94 (95% CI 1.68–2.23) for pre-HD SBP <130 mmHg HR 1.62 (95% CI 1.39–1.90) for pre-HD SBP >160 mmHg | – | |
| Hecking et al. [71] | 38 614 | Retrospective | 12 months | IDWG 7.5 (IQR 2.3) Pre-HD FO/extracellular water: 21.8% (IQR 5.6) Post-HD FO/extracellular water: 11.3% (IQR 6.2) with BIA | ACM: HR 0.89 (95% CI 0.80–0.98) for IDWG HR 1.75 (95% CI 1.59–1.92) for pre-HD HR 1.74 (95% CI 1.60–1.90) for post-HD bioimpedance analysis | – | |
| Saad et al. [72] | 71 | Prospective | 1.19 years | US B-lines ≥60 with LUS | CVM: HR 7.98 (P = 0.013) | – |
| Study . | . | N . | Study design . | Follow-up . | Volume parameter . | Primary outcome and results . | Secondary outcomes and results . |
|---|---|---|---|---|---|---|---|
| Foley et al. [62] | 11 142 | Prospective | 3.8 years | IDWG >4.8% of body weight | ACM: HR 1.12 (95% CI 1.02–1.23) | – | |
| Saran et al. [63] | Euro-DOPPS: 2337 Japan: 1980 USA: 3359 | Prospective | Euro-DOPPS: 1.8 years Japan: 2.0 years United States: 2.9 years | IDWG >5.7% of body weight | ACM: HR 1.12 (95% CI 1.00–1.26) | Hospitalization: 1.09 (95% CI 0.99–1.19) | |
| Kalantar-Zadeh et al. [56] | 34 107 | Retrospective | 2.0 years | IDWG ≥4.0 kg | ACM: HR 1.28 (95% CI 1.15–1.39) | CVM: HR 1.25 (95% CI 1.12–1.39) | |
| Wizemann et al. [64] | 269 | Prospective | 3.5 years | FO/extracellular water ≥15% with BIA | ACM: HR 2.102 (95% CI 1.389–3.179) | – | |
| Chazot et al. [65] | 208 | Retrospective | 6.5 years | HS: 3.5±1.2 L with BIA FO/extracellular water ≥15% with BIA | ACM: HR 3.41 (95% CI 1.62–7.17) | – | |
| Zoccali et al. [66] | 392 | Retrospective | 2.1 (IQR 1.8–2.4) years | US B-lines >60 with LUS | ACM: HR 4.20, (95% CI 2.45–7.23) | CVE: HR 3.20 (95% CI 1.75–5.88) | |
| Siriopol et al. [67] | 96 | Prospective | 405.5 (IQR 234.8–518.0) days | Pre-HD US B-lines >30 with LUS Pre-HD FO/extracellular water >15% with BIA | ACM: HR 3.63 (95% CI 1.03–12.74) for LUS HR 1.22 (95% CI 0.26–5.74) for BIA | – | |
| Cabrera et al. [57] | 39 256 | Retrospective | 1–3 years | IDWG >3.5% of post-dialysis weight over 3 months | ACM: HR 1.26 (95% CI 1.20–1.33) | MI: HR 1.18 (95% CI 1.08–1.28) CVM: HR 1.23 (95% CI 1.14–1.34) | |
| Kim et al. [68] | 344 | Prospective | 24.0 months | FO/extracellular water ≥15% with BIA | ACM: HR 2.582 (95% CI 1.159–5.750) | – | |
| Onofriescu et al. [69] | 157 | Prospective | 66.2 (IQR 42.4–70.2) months | BIA FO/extracellular water >15% and >17.4% with BIA | ACM: HR 1.87 (95% CI 1.12–3.13 for RFO >15%) HR 2.72 (95% CI 1.60–4.63) for RFO >17.4% | CVE: HR 2.31 (95% CI 1.42–3.77) for RFO >15% HR 4.17 (95% CI 2.48–7.02) for RFO >17.4% Hospitalization: HR 0.84 (95% CI 0.71–1.01) HR 0.78 (95% CI 0.64–0.95) DHF hospitalization: HR 0.53 (95% CI 0.24–1.19) HR 0.52 (95% CI 0.22–1.36) | |
| Siriopol et al. [70] | 173 | Prospective | 21.3 (IQR 19.9–30.3) months | US B-lines >22 with LUS FO/extracellular water ≥6.88% with BIA | ACM: HR 2.72 (95% CI 1.19–6.16) for LUS HR 2.93 (95% CI 1.30–6.58) for BIA | – | |
| Wong et al. [58] | 21 919 | Prospective | 2.0 (IQR 1.0–2.6) years | IDWG ≥5.7% of post-dialysis weight | ACM: HR 1.23 (95% CI 1.08–1.49) | Fluid overload-related hospitalization: HR 1.64 (95% CI 1.27–2.13) | |
| Zoccali et al.[20] | 39 566 | Prospective | 497 ± 358 days | FO/extracellular water ≥15% for male and ≥13% for female over 1 year with BIA | ACM: HR 1.94 (95% CI 1.68–2.23) for pre-HD SBP <130 mmHg HR 1.62 (95% CI 1.39–1.90) for pre-HD SBP >160 mmHg | – | |
| Hecking et al. [71] | 38 614 | Retrospective | 12 months | IDWG 7.5 (IQR 2.3) Pre-HD FO/extracellular water: 21.8% (IQR 5.6) Post-HD FO/extracellular water: 11.3% (IQR 6.2) with BIA | ACM: HR 0.89 (95% CI 0.80–0.98) for IDWG HR 1.75 (95% CI 1.59–1.92) for pre-HD HR 1.74 (95% CI 1.60–1.90) for post-HD bioimpedance analysis | – | |
| Saad et al. [72] | 71 | Prospective | 1.19 years | US B-lines ≥60 with LUS | CVM: HR 7.98 (P = 0.013) | – |
ACM, all-cause mortality; BIA, bioimpedance analysis; CVM, cardiovascular mortality; CVE, cardiovascular events; DHF; decompensated heart failure; FO, fluid overload; HS, hydration status; IQR, interquartile range; LUS, lung ultrasound; MI, myocardial infarction; RFO, relative fluid overload.
Observational studies examining the association of volume overload and hard outcomes in patients undergoing HD; adjusted HRs or β-coefficients are reported
| Study . | . | N . | Study design . | Follow-up . | Volume parameter . | Primary outcome and results . | Secondary outcomes and results . |
|---|---|---|---|---|---|---|---|
| Foley et al. [62] | 11 142 | Prospective | 3.8 years | IDWG >4.8% of body weight | ACM: HR 1.12 (95% CI 1.02–1.23) | – | |
| Saran et al. [63] | Euro-DOPPS: 2337 Japan: 1980 USA: 3359 | Prospective | Euro-DOPPS: 1.8 years Japan: 2.0 years United States: 2.9 years | IDWG >5.7% of body weight | ACM: HR 1.12 (95% CI 1.00–1.26) | Hospitalization: 1.09 (95% CI 0.99–1.19) | |
| Kalantar-Zadeh et al. [56] | 34 107 | Retrospective | 2.0 years | IDWG ≥4.0 kg | ACM: HR 1.28 (95% CI 1.15–1.39) | CVM: HR 1.25 (95% CI 1.12–1.39) | |
| Wizemann et al. [64] | 269 | Prospective | 3.5 years | FO/extracellular water ≥15% with BIA | ACM: HR 2.102 (95% CI 1.389–3.179) | – | |
| Chazot et al. [65] | 208 | Retrospective | 6.5 years | HS: 3.5±1.2 L with BIA FO/extracellular water ≥15% with BIA | ACM: HR 3.41 (95% CI 1.62–7.17) | – | |
| Zoccali et al. [66] | 392 | Retrospective | 2.1 (IQR 1.8–2.4) years | US B-lines >60 with LUS | ACM: HR 4.20, (95% CI 2.45–7.23) | CVE: HR 3.20 (95% CI 1.75–5.88) | |
| Siriopol et al. [67] | 96 | Prospective | 405.5 (IQR 234.8–518.0) days | Pre-HD US B-lines >30 with LUS Pre-HD FO/extracellular water >15% with BIA | ACM: HR 3.63 (95% CI 1.03–12.74) for LUS HR 1.22 (95% CI 0.26–5.74) for BIA | – | |
| Cabrera et al. [57] | 39 256 | Retrospective | 1–3 years | IDWG >3.5% of post-dialysis weight over 3 months | ACM: HR 1.26 (95% CI 1.20–1.33) | MI: HR 1.18 (95% CI 1.08–1.28) CVM: HR 1.23 (95% CI 1.14–1.34) | |
| Kim et al. [68] | 344 | Prospective | 24.0 months | FO/extracellular water ≥15% with BIA | ACM: HR 2.582 (95% CI 1.159–5.750) | – | |
| Onofriescu et al. [69] | 157 | Prospective | 66.2 (IQR 42.4–70.2) months | BIA FO/extracellular water >15% and >17.4% with BIA | ACM: HR 1.87 (95% CI 1.12–3.13 for RFO >15%) HR 2.72 (95% CI 1.60–4.63) for RFO >17.4% | CVE: HR 2.31 (95% CI 1.42–3.77) for RFO >15% HR 4.17 (95% CI 2.48–7.02) for RFO >17.4% Hospitalization: HR 0.84 (95% CI 0.71–1.01) HR 0.78 (95% CI 0.64–0.95) DHF hospitalization: HR 0.53 (95% CI 0.24–1.19) HR 0.52 (95% CI 0.22–1.36) | |
| Siriopol et al. [70] | 173 | Prospective | 21.3 (IQR 19.9–30.3) months | US B-lines >22 with LUS FO/extracellular water ≥6.88% with BIA | ACM: HR 2.72 (95% CI 1.19–6.16) for LUS HR 2.93 (95% CI 1.30–6.58) for BIA | – | |
| Wong et al. [58] | 21 919 | Prospective | 2.0 (IQR 1.0–2.6) years | IDWG ≥5.7% of post-dialysis weight | ACM: HR 1.23 (95% CI 1.08–1.49) | Fluid overload-related hospitalization: HR 1.64 (95% CI 1.27–2.13) | |
| Zoccali et al.[20] | 39 566 | Prospective | 497 ± 358 days | FO/extracellular water ≥15% for male and ≥13% for female over 1 year with BIA | ACM: HR 1.94 (95% CI 1.68–2.23) for pre-HD SBP <130 mmHg HR 1.62 (95% CI 1.39–1.90) for pre-HD SBP >160 mmHg | – | |
| Hecking et al. [71] | 38 614 | Retrospective | 12 months | IDWG 7.5 (IQR 2.3) Pre-HD FO/extracellular water: 21.8% (IQR 5.6) Post-HD FO/extracellular water: 11.3% (IQR 6.2) with BIA | ACM: HR 0.89 (95% CI 0.80–0.98) for IDWG HR 1.75 (95% CI 1.59–1.92) for pre-HD HR 1.74 (95% CI 1.60–1.90) for post-HD bioimpedance analysis | – | |
| Saad et al. [72] | 71 | Prospective | 1.19 years | US B-lines ≥60 with LUS | CVM: HR 7.98 (P = 0.013) | – |
| Study . | . | N . | Study design . | Follow-up . | Volume parameter . | Primary outcome and results . | Secondary outcomes and results . |
|---|---|---|---|---|---|---|---|
| Foley et al. [62] | 11 142 | Prospective | 3.8 years | IDWG >4.8% of body weight | ACM: HR 1.12 (95% CI 1.02–1.23) | – | |
| Saran et al. [63] | Euro-DOPPS: 2337 Japan: 1980 USA: 3359 | Prospective | Euro-DOPPS: 1.8 years Japan: 2.0 years United States: 2.9 years | IDWG >5.7% of body weight | ACM: HR 1.12 (95% CI 1.00–1.26) | Hospitalization: 1.09 (95% CI 0.99–1.19) | |
| Kalantar-Zadeh et al. [56] | 34 107 | Retrospective | 2.0 years | IDWG ≥4.0 kg | ACM: HR 1.28 (95% CI 1.15–1.39) | CVM: HR 1.25 (95% CI 1.12–1.39) | |
| Wizemann et al. [64] | 269 | Prospective | 3.5 years | FO/extracellular water ≥15% with BIA | ACM: HR 2.102 (95% CI 1.389–3.179) | – | |
| Chazot et al. [65] | 208 | Retrospective | 6.5 years | HS: 3.5±1.2 L with BIA FO/extracellular water ≥15% with BIA | ACM: HR 3.41 (95% CI 1.62–7.17) | – | |
| Zoccali et al. [66] | 392 | Retrospective | 2.1 (IQR 1.8–2.4) years | US B-lines >60 with LUS | ACM: HR 4.20, (95% CI 2.45–7.23) | CVE: HR 3.20 (95% CI 1.75–5.88) | |
| Siriopol et al. [67] | 96 | Prospective | 405.5 (IQR 234.8–518.0) days | Pre-HD US B-lines >30 with LUS Pre-HD FO/extracellular water >15% with BIA | ACM: HR 3.63 (95% CI 1.03–12.74) for LUS HR 1.22 (95% CI 0.26–5.74) for BIA | – | |
| Cabrera et al. [57] | 39 256 | Retrospective | 1–3 years | IDWG >3.5% of post-dialysis weight over 3 months | ACM: HR 1.26 (95% CI 1.20–1.33) | MI: HR 1.18 (95% CI 1.08–1.28) CVM: HR 1.23 (95% CI 1.14–1.34) | |
| Kim et al. [68] | 344 | Prospective | 24.0 months | FO/extracellular water ≥15% with BIA | ACM: HR 2.582 (95% CI 1.159–5.750) | – | |
| Onofriescu et al. [69] | 157 | Prospective | 66.2 (IQR 42.4–70.2) months | BIA FO/extracellular water >15% and >17.4% with BIA | ACM: HR 1.87 (95% CI 1.12–3.13 for RFO >15%) HR 2.72 (95% CI 1.60–4.63) for RFO >17.4% | CVE: HR 2.31 (95% CI 1.42–3.77) for RFO >15% HR 4.17 (95% CI 2.48–7.02) for RFO >17.4% Hospitalization: HR 0.84 (95% CI 0.71–1.01) HR 0.78 (95% CI 0.64–0.95) DHF hospitalization: HR 0.53 (95% CI 0.24–1.19) HR 0.52 (95% CI 0.22–1.36) | |
| Siriopol et al. [70] | 173 | Prospective | 21.3 (IQR 19.9–30.3) months | US B-lines >22 with LUS FO/extracellular water ≥6.88% with BIA | ACM: HR 2.72 (95% CI 1.19–6.16) for LUS HR 2.93 (95% CI 1.30–6.58) for BIA | – | |
| Wong et al. [58] | 21 919 | Prospective | 2.0 (IQR 1.0–2.6) years | IDWG ≥5.7% of post-dialysis weight | ACM: HR 1.23 (95% CI 1.08–1.49) | Fluid overload-related hospitalization: HR 1.64 (95% CI 1.27–2.13) | |
| Zoccali et al.[20] | 39 566 | Prospective | 497 ± 358 days | FO/extracellular water ≥15% for male and ≥13% for female over 1 year with BIA | ACM: HR 1.94 (95% CI 1.68–2.23) for pre-HD SBP <130 mmHg HR 1.62 (95% CI 1.39–1.90) for pre-HD SBP >160 mmHg | – | |
| Hecking et al. [71] | 38 614 | Retrospective | 12 months | IDWG 7.5 (IQR 2.3) Pre-HD FO/extracellular water: 21.8% (IQR 5.6) Post-HD FO/extracellular water: 11.3% (IQR 6.2) with BIA | ACM: HR 0.89 (95% CI 0.80–0.98) for IDWG HR 1.75 (95% CI 1.59–1.92) for pre-HD HR 1.74 (95% CI 1.60–1.90) for post-HD bioimpedance analysis | – | |
| Saad et al. [72] | 71 | Prospective | 1.19 years | US B-lines ≥60 with LUS | CVM: HR 7.98 (P = 0.013) | – |
ACM, all-cause mortality; BIA, bioimpedance analysis; CVM, cardiovascular mortality; CVE, cardiovascular events; DHF; decompensated heart failure; FO, fluid overload; HS, hydration status; IQR, interquartile range; LUS, lung ultrasound; MI, myocardial infarction; RFO, relative fluid overload.
Three observational studies using lung US showed a significant association between the number of US-B lines (an estimate of lung water) and clinical outcomes in HD patients. In the first, among 392 HD patients, those with severe lung congestion (>30 US-B lines) had higher all-cause [hazard ratio (HR) 4.20 (95% CI 2.45–7.23)] and cardiovascular mortality [HR 3.20 (95% CI 1.75–5.88)] in analyses adjusted for New York Heart Association class and other risk factors [66]. In the second, which performed a head-to-head comparison between bioimpedance analysis and lung US in a series of 96 patients, only the pre-HD number of US-B lines predicted all-cause mortality, and these results were echoed in a third study that enrolled 71 HD patients [67, 75].
LVH is common in the HD population and predisposes to systolic and diastolic dysfunction, arrhythmias and sudden death [76]. The coexistence of hypertension and LVH likely reflects the causal role of hypertension in LVH and suggests that chronic volume expansion is the triggering event in the pathway leading to LVH [77]. However, volume overload in HD patients may also cause adverse events by mechanisms independent of hypertension. Indeed, chronic fluid overload predicts mortality across all BP strata, from hypotension to normotension and frank hypertension [20]. Volume overload per se associates with interstitial lung oedema, airway obstruction, pleural effusions and pulmonary hypertension [78]. Moreover, due to hypopharyngeal oedema, fluid overload is a trigger of sleep apnoea [79], which is per se a strong risk factor for cardiovascular events in the HD population [80]. Subclinical lung congestion, a consequence of chronic volume expansion, associates with poor physical functioning and quality of life independent of heart failure in dialysis patients [81].
Inflammation and volume overload in HD patients
Over the past few years, the use of 23Na magnetic resonance imaging (MRI) has made the measurement of exchangeable body sodium feasible [82]. Recent evidence from studies using this technique showed that tissue sodium concentration is higher in patients undergoing dialysis compared with healthy controls or CKD patients [83]. Non-osmotically active tissue sodium is a critical determinant of extracellular fluid volume and arterial pressure in the HD population [60]. Apart from expanding extracellular volume, sodium in HD patients is stored non-osmotically in the connective tissue, in the skin and muscles [84]. Non-osmotic accumulation of sodium critically depends on tissue inflammation and on the secretion of vascular endothelial growth factor-C (VEGF-C) by macrophages. Low VEGF-C levels, which typically occur in the elderly, associate with a higher accumulation of sodium in the skin after HD. Non-osmotic sodium accumulation in the skin and in muscles likely explains the long time lag needed in some HD patients for the normalization of BP after fluid overload has been corrected [85]. Of note, the link between inflammation and fluid accumulation extends to the lung. Chronic, systemic inflammation as measured by serum CRP amplifies lung congestion in HD patients. Indeed, in this study, serum CRP was a consistent effect modifier of the relationship between left atrial volume and lung water [86]. In other words, at a similar degree of fluid overload (as estimated by the left atrial volume), lung congestion is greater in patients with inflammation than in those without, a phenomenon likely attributable to inflammation-driven capillary leakage and albumin extravasation [87].
Management of volume overload
Controlling the fluid overload in HD patients is an unmet clinical need [88]. Restraint due to patients' symptoms (intradialytic hypotension, muscle cramps, nausea and vomiting) and the use of ‘fast and easy’ solutions to treat intradialytic hypotension (i.e. cessation of ultrafiltration, hypertonic sodium infusions, increasing dialysate sodium concentration, premature termination of dialysis) are all recognized barriers towards achievement of dry weight in HD patients [4].
In theory, low salt intake and low dialysate sodium may be useful for the control of fluid overload and hypertension in HD patients. Results from an observational pilot study in 20 HD patients suggest that 23Na-MRI can reliably quantify the tissue sodium stores and can prove a useful diagnostic tool to assess total body sodium removal in these patients [89]. In a study in eight hypertensive patients, a salt diet of 6 g/day (about 100 mmol of sodium) in association with a reduction in dialysate sodium to 135 mmol/L caused a meaningful hypotensive effect in HD patients [90]. However, it is almost a generalized experience that maintaining a low salt intake is very difficult in these patients. Lowering dialysate sodium to levels close to pre-dialysis serum sodium levels resulted in reduced pre-dialysis BP levels and IDWG and interdialytic thirst in two small studies [91, 92], but a recent meta-analysis collating 266 patients in 12 studies found that besides causing a modest decrease in BP, this intervention lowers serum sodium and increases hypotensive episodes and cramps during HD [93].
On the other hand, it is well demonstrated that longer and more frequent HD schedules have a favourable impact on BP control and may improve clinical outcomes. The effects of more frequent dialysis over thrice-weekly HD on clinical outcomes were tested in the Frequent Haemodialysis Network (FHN) Daily Trial, which compared the standard thrice-weekly HD schedule versus six HD sessions per week [94]. In this trial, SBP at 1 year was lower by 10 mmHg in the frequent-dialysis arm than in the control arm and the incidence of the composite primary endpoint (mortality or reduction in LV mass index measured by nuclear magnetic resonance) was by 39% lower in the same arm [94]. However, these beneficial effects were counterbalanced in part by a higher risk of arteriovenous fistula occlusion. Of note, observation of patients in this trial extended to an average period of 3.6 years, which documented that patients in the active arm of the trial had a 46% reduction in the risk of death [95]. This was a legacy effect, because patients in this trial all returned to conventional dialysis at the end of the trial, which lasted for 1 year. Another pilot study, which randomized 18 patients to a thrice-weekly or every-other-day HD regimen, showed that dry weight (69.7 ± 9.1 versus 67.4 ± 9.1 kg; P < 0.05) was lower at study-end and pre-HD mean BP decreased by 7 mmHg with less antihypertensive medications (2.6 ± 1 versus 2.0 ± 0.9; P < 0.05) in the frequent HD group but were similar in the control group [96].
Identification of the optimal dry weight remains a challenge. The effects of dry weight reduction on BP were evaluated in the Dry Weight Reduction in Hypertensive Haemodialysis Patients (DRIP) study, a randomized clinical trial in which ultrafiltration was intensified until patients became symptomatic [97]. This intervention resulted in a 7.6/3.4 mmHg reduction in 44-h BP favouring the active arm [97]. However, patients in the active arm of the trial had a higher risk of intradialytic hypotensive episodes. Similarly, observations in an uncontrolled study with dry weight reduction guided by clinical criteria suggested that ultrafiltration intensification may increase arteriovenous fistula problems and the risk of cardiovascular events [98].
Implementation of non-invasive methods to guide dry weight reduction interventions may result in favourable outcomes. Results from the previously discussed study by Onofriescu et al. [73] show that a bioimpedance-guided intervention does not modify SBP but reduces PWV, a robust surrogate endpoint reflecting arterial stiffness. As previously remarked, survival in the active arm of the trial was higher than in the control arm, but the number of deaths (one in the active arm and eight in the control arm) was too low to allow sensible conclusions. Similarly, in another trial, a bioimpedance-guided dry weight reduction strategy reduced the incidence of hypertension and of intradialytic complications but did not affect survival (three deaths in each arm) [74].
The usefulness of the intradialytic blood volume monitoring technique was evaluated in the Crit-Line Intradialytic Monitoring Benefit (CLIMB) study, which randomized 443 patients to Crit-Line or conventional ultrafiltration monitoring, and results indicated an increased risk for non-access [HR 1.61 (95% CI 1.15–2.25); P < 0.01] and access-related [HR 1.52 (95% CI 1.02–2.28); P < 0.04] hospitalizations and higher mortality (8.7 versus 3.3%; P = 0.021) in the active group [99]. Similarly, a randomized crossover study in 32 patients with intradialytic hypotension failed to show a benefit of a blood volume–guided biofeedback technology for the prevention of symptomatic hypotensive episodes during HD [100].
Over the past few years, biofeedback control sensors on dialysis machines have been used to automatically regulate ultrafiltration and dialysate conductivity (UCR) or ultrafiltration and temperature (UTR) to offer individualization in these parameters [101]. The use of these techniques was compared with conventional dialysis in a randomized controlled trial including 244 HD patients, 15% of whom were fluid overloaded, and results suggested that dry weight reduction was higher in the UTR compared with the UCR group (5.0 ± 3.4 versus 2.0 ± 2.7%, P = 0.013) but not compared with standard-of-care treatment (versus 3.9 ± 2.1% body weight; P = 0.31) [102].
There is emerging evidence that the lung US-guided treatment policies may be useful to improve hypertension and to reduce LVH in HD patients. A randomized controlled trial corollary to LUST showed that the gradual dry weight reduction with a lung US-guided strategy reduced 48-h SBP (−6.61 ± 9.57 versus −0.67 ± 13.07 mmHg, P = 0.033) and DBP (−3.85 ± 6.34 versus −0.55 ± 8.28 mmHg, P = 0.031) as compared with standard treatment with no excess of dialysis hypotension episodes [103]. Furthermore, the lung US-guided treatment reduced cardiac chamber dimensions and LV filling pressure as estimated by the E/e‵ ratio (E/e’: −0.38 ± 3.14 versus 1.36 ± 3.54; P = 0.034) [104] and 48-h PWV (−0.23 ± 0.59 versus 0.05 ± 0.45 m/s; P = 0.030) [105], but not in ambulatory BP variability parameters during follow-up [106].
CONCLUSION
Fluid overload is a major risk factor for all-cause and cardiovascular mortality in the HD population. Volume overload underlies hypertension, LVH and dysfunction, as well as pulmonary circulation overload in these patients, and is a major contributor to the dire prognosis of ESRD. Dry weight assessment in clinical practice still rests on subjective evaluation of patient signs and symptoms rather than on objective measurements. However, clinical criteria to evaluate fluid balance are notoriously unreliable and for this reason volume overload goes undetected in HD patients. Inaccurate evaluation of fluid volume status may lead to incorrect antihypertensive drugs and ultrafiltration prescriptions.
The diagnosis of subclinical volume overload in HD patients has been a matter of research for decades. Bioimpedance spectroscopy is a widely used technique that provides reliable estimates of fluid overload in these patients, but adequately powered clinical trials based on clinical endpoints for recommending this technique as a useful instrument to guide ultrafiltration prescription in clinical practice are still lacking. Lung US subjectively quantifies interstitial fluid accumulation in a critical area of the central circulation, like the lung. Recent evidence suggests that application of this method may be useful to safely reduce ambulatory BP in hypertensive HD patients. Whether a lung US-guided treatment strategy can decrease major outcomes in high-risk HD patients remains to be answered by the ongoing LUST study. Apart from optimal dry weight estimation, management of volume overload should also include gradual fluid removal to avoid hypotensive episodes, provision of sufficient HD time to achieve ultrafiltration and sodium removal and a significant reduction of sodium intake to decrease thirst and IDGW. Proper implementation of these strategies would help to reduce volume overload for the benefit of our patients.
FUNDING
This article was not supported by any source and represents an original effort by the authors.
CONFLICT OF INTEREST STATEMENT
All authors disclose that they do not have any financial or other relationships, which might lead to a conflict of interest regarding this paper.





Comments