-
PDF
- Split View
-
Views
-
Cite
Cite
Juliet Schurder, David Buob, Peggy Perrin, Eric Thervet, Alexandre Karras, Alexandre Hertig, Acute interstitial nephritis: aetiology and management, Nephrology Dialysis Transplantation, Volume 36, Issue 10, October 2021, Pages 1799–1802, https://doi.org/10.1093/ndt/gfz262
Close - Share Icon Share
INTRODUCTION
Acute interstitial nephritis (AIN) complicates a heterogeneous spectrum of diseases (see Table 1). Its characteristic histological lesion is the infiltration of the interstitial renal tissue by inflammatory cells (see Figure 1). AIN per se is a cause of acute kidney injury (AKI) and increasingly so: from 10 to 35% of the biopsy samples taken in AKI patients show AIN [1]. Renal impairment is occasionally severe and can be isolated, i.e. without gross proteinuria or haematuria. However, aseptic leucocyturia is found in one of the two cases [2]. While we are still short of non-invasive specific markers, urinary tumour necrosis factor α (TNF-α) and interleukin-9 were recently shown to perform well [3]. Renal biopsy ensures diagnosis of AIN, points to possible cause and informs prognosis.
Historically reported during infectious disease such as scarlet fever or diphtheria, AIN is nowadays mainly due to drugs, including anti-infectious ones. In the 1960s, the first cases of methicillin- and phenindione-related AIN showed symptoms suggestive of hypersensitivity (rash, fever and eosinophilia) [4, 5], but today these symptoms are generally absent. Instead, symptoms such as asthenia, nausea or lumbar pain (due to renal oedema) are frequent but not specific.
DRUGS
Drug-induced AIN remains one of exclusion, but an exhaustive list of patient medications (including self-medications) must be established in a patient with AIN. Antibiotics, non-steroidal anti-inflammatory drugs and proton pump inhibitors are the main culprits [1]. Theoretically, however, any drug can lead to AIN through an immune-allergic reaction. It has been suggested that humoral immunity is involved in this reaction because of the (exceptional) detection of antibodies directed against tubular basement membrane antigens [6], but experimental data strongly support the implication of cellular immunity [7]. The recent description of cases caused by immune checkpoint inhibitors [8] or TNF-α inhibitors [9] suggests a loss of tolerance against a native antigen expressed by renal cells (tubular or other). This hypothesis shares pathophysiological mechanisms with the alloimmune response observed in renal grafts during cell-mediated rejection, where lymphocytes infiltrate the tubular epithelium, inducing ‘tubulitis’, a lesion that can also be observed in native kidneys with AIN. How an antibiotic, for instance, can generate such a reaction is debated, but among other hypotheses, drugs may act as haptens, and cross-reactivity between native and drug epitopes—molecular mimicry—has been suggested [10]. Alternatively, drugs can also display a direct toxicity or crystallize within the tubular lumen, where they will initiate an inflammatory reaction, occasionally granulomatous, as has been described for atazanavir, ciprofloxacin and others [11, 12].
OXALATE CRYSTALS
Enteric, food-derived oxalate ions are poorly available for intestinal absorption because they complex with calcium ions. In the case of fat malabsorption (after intestinal resection or gastric bypass, or in patients with a deficiency in exocrine pancreatic functions, or in patients exposed to orlistat, e.g. Ferraz et al. [13]), calcium is saponified with fat, which leaves oxalate ions unbound and free for absorption in the colon. Hyperoxalaemia ensues and hence hyperoxaluria. Such patients, if fed with oxalate-rich vegetables or fruits, may present with AKI. This is because of tubular obstruction, but also because precipitation of intratubular oxalate crystals, visible under a microscope using a polarized light, is frequently associated with renal inflammation [14]. Calcium oxalate crystals may indeed translocate into the renal interstitium and be picked up by endothelial cells or macrophages, where they will produce a danger signal and elicit inflammation through a mechanism involving the NLRP3 inflammasome [15–17]. Of note, intoxication with ethylene glycol (an oxalate precursor used as antifreeze) or ascorbic acid (another precursor) shows similar pathogenesis and association with metabolic acidosis [18].
Patients with active inflammatory bowel disease may similarly develop hyperoxaluria, but the differential diagnosis is occasionally complicated by the intake of 5-aminosalicylic acid, which is another cause of drug-induced AIN (typically observed within 3 years following the beginning of treatment) [19]. In this category of patients, renal biopsy is mandatory to distinguish isolated AIN from oxalate crystal nephropathy.
CORTINAR MUSHROOMS
Ingestion of orellanine-containing cortinar mushrooms can lead to AIN, sometimes granulomatous. Gastrointestinal symptoms will appear first (Day 3 post-intoxication), followed by renal damage (Day 8) [20]. There is no antidote and patients often progress to chronic renal failure.
AUTOIMMUNE DISEASES
Sjögren’s syndrome, inflammatory bowel diseases, primary biliary cholangitis and sarcoidosis are known to cause AIN [1]. During lupus and antineutrophil cytoplasmic antibody-associated vasculitis, interstitial inflammation is one possible feature [1], but it is associated with glomerular lesions. All these syndromes are associated with extrarenal signs, generally at the origin of presentation. Tubulointerstitial nephritis and uveitis are a rare syndrome with isolated uveitis, but the causes are still obscure. Immunoglobulin G4 (IgG4)-related syndrome is systemic (and in many organs may induce pseudo-tumoral manifestations) and of more recent description. The histological pattern of the renal parenchyma is a key to aid diagnosis: the infiltration is characterized by a high density of IgG4+ plasmocytes (which is not specific, however) and by a storiform fibrosis (i.e. with a woven pattern or like a straw).
INFECTIONS
Stricto sensu, bacterial pyelonephritis is by far the leading cause of AIN, but it is unilateral and typically localized to a renal pyramid. In patients with malnutrition and, often, chronic alcohol consumption, or in kidney recipients, the pyelonephritis can be diffuse and cause AKI [21]. A positive uroculture, or (in most cases) blood cultures, should therefore alert the physician to this hypothesis, especially when AKI occurs in the absence of septic shock. A biopsy sample may be considered to guide the therapeutic strategy (duration of antibiotherapy would be extended for weeks).
Apart from conventional bacteria, Leptospira interrogans is a cause of severe AIN some geographical areas of the world [22]. Paradoxically, workers exposed to rodents (the vehicle of leptospirosis) are not at risk because vaccination is mandatory. Leptospirosis has other characteristic features such as jaundice and confusion, which accelerates the correct diagnosis.
Patients infected with human immunodeficiency virus (HIV) may develop AIN because of the virus itself—HIV has a tropism for renal epithelial cells—and an exaggerated CD8 response to viraemia may cause a systemic disease with a sicca syndrome, interstitial pneumonitis and AIN (the syndrome is called diffuse infiltrative lymphocytosis syndrome) [23]. Likewise, the BK virus, also highly tropic for the tubular epithelium, may cause AIN in kidney recipients [24] (and anecdotally in other contexts [25]). Because BK-related AIN is usually not observed in other categories of immunodeficient patients (including recipients of an organ other than a kidney, or after a bone marrow transplantation, where immunosuppression is heavy), it is thought that another hit is required for AIN to develop (probably a local inflammation of the kidney). During HIV infection, though, indirect (HIV-independent) AIN may occur after the immune reconstitution that follows the introduction of drugs aimed at repressing HIV replication [23]. In the latter situation, the presence of a urinary infection by Mycobacterium tuberculosis (not a cause of diffuse AIN in immunocompetent patients) may elicit a spectacular pyelonephritis (just as a chronic but indolent Cryptococcus neoformans infection may lead to fatal meningitis and encephalitis after immune wake-up).
The Hantaan virus is endemic worldwide (it is inhaled from wood soiled with vole faeces) and—although pathological interstitial changes are usually slight—is another direct cause of AIN and, like leptospirosis, induces an infectious course that facilitates diagnosis [26]. The Hantaan virus also causes microvascular inflammation [27].
Other germs anecdotally cause AIN: brucella, herpes virus, adenovirus, measles, rubella, toxoplasmosis, leishmaniasis and even fungi if disseminated (Candida albicans, Histoplasma gondii and C. neoformans).
TREATMENT
Except for short-lasting and idiosyncratic infections by Hantaan virus or L. interrogans, there is a high risk of developing interstitial fibrosis after AIN, which is why the cause must be identified as soon as possible to give the appropriate treatment. This treatment is well codified concerning autoimmune disease, consisting of steroids: from 0.5 to 1 mg prednisone/kg/day for several weeks and slowly tapered over months. During drug-induced AIN, there should be a rapid withdrawal of all suspected drugs. Steroids are frequently used, in a shorter course (from 0.5 to 1 mg prednisone/kg/day for at least 2 weeks and then slowly tapered during several weeks). Although evidence is lacking to strongly recommend the use of steroids (to date, no randomized prospective trials have included a large and homogeneous population of patients), a pragmatic approach is usually followed, whereby a number of factors are integrated (severity of AKI, presence of fibrosis, type of drugs, etc.) and a relatively high dose (1 mg/kg/day of prednisolone) is given for >2 weeks.
CONCLUSIONS
Eventually AIN is not a disease per se but a syndrome potentially due to a number of causes eliciting renal injury via divert mechanisms. It is our opinion that the time has come to undertake a randomized precision trial. Such a trial would test the efficacy of steroids in one category of patients expected to respond: (i) a recent history of drug exposure, (ii) renal inflammation without crystals on the biopsy sample (intuitively, AIN with crystals will not respond to steroids) and (iii) a severe phenotype defined both biologically (AKI) and histologically (the Banff classification for allograft histological lesions offers semi-quantitation of tubular and interstitial injury). Modern tools are now routinely available that would help us to exclude patients from other categories, who may have polluted previous trials: to name a few, infrared microscopy of biopsy samples, infectious and immune tests, eye fundus, etc.
CONFLICT OF INTEREST STATEMENT
None declared.
Main causes of AIN, respective mechanisms, suggested interrogation about food and drug intake and minimum biological or radiological workup
| Cause . | Mechanism . | Workup . |
|---|---|---|
| Drugs |
|
|
| Sarcoidosis | Unknown |
|
| TINU | Autoimmune | Eye fundus |
| Sjögren’s syndrome | Autoimmune | Serum protein electrophoresis |
| Lupus | Autoimmune | Anti-nuclear antibody, anti-DNA, C3 and C4 complement proteins |
| IgG4 disease | Autoimmune | IgG4 concentration |
| Primary biliary cholangitis | Autoimmune | Liver enzymes |
| Inflammatory bowel disease | Autoinflammatory | Crystalluria/pharmacovigilance |
| Bacteria | Infectious | Cytobacteriological urine examination and specific cultures |
| Virus | Infectious/immune | Specific serologies |
| Fungi/protozoa | Infectious | Urine-specific cultures |
| Oxalosis | Crystallization |
|
| Cortinar intoxication | Acute tubular necrosis | Food inquiry |
| Cause . | Mechanism . | Workup . |
|---|---|---|
| Drugs |
|
|
| Sarcoidosis | Unknown |
|
| TINU | Autoimmune | Eye fundus |
| Sjögren’s syndrome | Autoimmune | Serum protein electrophoresis |
| Lupus | Autoimmune | Anti-nuclear antibody, anti-DNA, C3 and C4 complement proteins |
| IgG4 disease | Autoimmune | IgG4 concentration |
| Primary biliary cholangitis | Autoimmune | Liver enzymes |
| Inflammatory bowel disease | Autoinflammatory | Crystalluria/pharmacovigilance |
| Bacteria | Infectious | Cytobacteriological urine examination and specific cultures |
| Virus | Infectious/immune | Specific serologies |
| Fungi/protozoa | Infectious | Urine-specific cultures |
| Oxalosis | Crystallization |
|
| Cortinar intoxication | Acute tubular necrosis | Food inquiry |
TINU, tubulointerstitial nephritis and uveitis.
Main causes of AIN, respective mechanisms, suggested interrogation about food and drug intake and minimum biological or radiological workup
| Cause . | Mechanism . | Workup . |
|---|---|---|
| Drugs |
|
|
| Sarcoidosis | Unknown |
|
| TINU | Autoimmune | Eye fundus |
| Sjögren’s syndrome | Autoimmune | Serum protein electrophoresis |
| Lupus | Autoimmune | Anti-nuclear antibody, anti-DNA, C3 and C4 complement proteins |
| IgG4 disease | Autoimmune | IgG4 concentration |
| Primary biliary cholangitis | Autoimmune | Liver enzymes |
| Inflammatory bowel disease | Autoinflammatory | Crystalluria/pharmacovigilance |
| Bacteria | Infectious | Cytobacteriological urine examination and specific cultures |
| Virus | Infectious/immune | Specific serologies |
| Fungi/protozoa | Infectious | Urine-specific cultures |
| Oxalosis | Crystallization |
|
| Cortinar intoxication | Acute tubular necrosis | Food inquiry |
| Cause . | Mechanism . | Workup . |
|---|---|---|
| Drugs |
|
|
| Sarcoidosis | Unknown |
|
| TINU | Autoimmune | Eye fundus |
| Sjögren’s syndrome | Autoimmune | Serum protein electrophoresis |
| Lupus | Autoimmune | Anti-nuclear antibody, anti-DNA, C3 and C4 complement proteins |
| IgG4 disease | Autoimmune | IgG4 concentration |
| Primary biliary cholangitis | Autoimmune | Liver enzymes |
| Inflammatory bowel disease | Autoinflammatory | Crystalluria/pharmacovigilance |
| Bacteria | Infectious | Cytobacteriological urine examination and specific cultures |
| Virus | Infectious/immune | Specific serologies |
| Fungi/protozoa | Infectious | Urine-specific cultures |
| Oxalosis | Crystallization |
|
| Cortinar intoxication | Acute tubular necrosis | Food inquiry |
TINU, tubulointerstitial nephritis and uveitis.
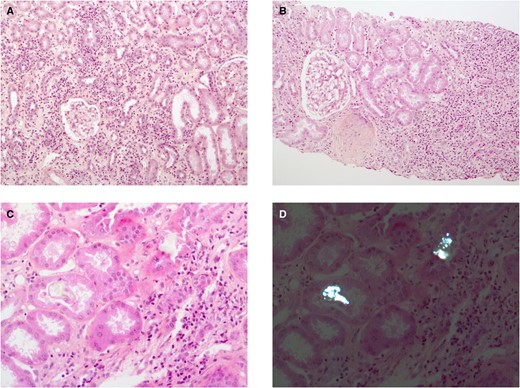
Histological features of AIN. (A) A case of interstitial inflammatory infiltrate, tubulitis and interstitial oedema (haematoxylin and eosin and saffron staining, original magnification ×100). (B) A case with severe interstitial fibrosis containing dense inflammatory infiltrate (Masson’s trichrome staining, original magnification ×100). (C and D) The usefulness of polarized light microscopy (D) to detect intratubular, unstained crystals (C, haematoxylin and eosin and saffron, original magnification ×200) with slight interstitial inflammation.





Comments