-
PDF
- Split View
-
Views
-
Cite
Cite
Eleni Petra, Tianlin He, Agnieszka Latosinska, Rafael Stroggilos, Harald Mischak, Antonia Vlahou, P0650
URINE PEPTIDOME ANALYSIS IN HEART FAILURE, CHRONIC KIDNEY DISEASE AND CARDIORENAL SYNDROME TOWARDS THE DEFINITION OF DISEASE-SPECIFIC MARKERS, Nephrology Dialysis Transplantation, Volume 35, Issue Supplement_3, June 2020, gfaa143.P0650, https://doi.org/10.1093/ndt/gfaa143.P0650Close - Share Icon Share
Abstract
The cardiorenal syndrome (CRS) reflects the complex interplay between kidney and heart diseases, but its molecular basis remains poorly understood. Multiple studies have demonstrated the association of urinary biomarkers with both heart and kidney diseases. However, their relevance and involvement in CRS have not been investigated yet. To address this gap, a study was designed with the aim to compare urinary biomarkers specific for heart failure (HF) and chronic kidney disease (CKD) with peptides representing CRS, with the ultimate target to connect these findings towards a better understanding of CRS pathophysiology.
A total of 3.463 urinary peptidomic datasets from patients with HF, CKD, or with both HF and CKD (CRS) as well as patients with no apparent diseases (controls) were retrieved and analyzed from the urinary peptidomics database (Latosinska A et al., Electrophoresis 2019; 40: 2294-2308). Following the matching for age, gender, heart and kidney function, differences in the abundance of urinary peptides were investigated in a cohort comprised of 390 patients with HF, 257 patients with CKD, 392 patients with CRS and 356 controls. The non-parametric Mann-Whitney U test was applied, followed by correction for multiple testing using the Benjamini-Hochberg method. To map the peptides to the protein precursor, the alignment tool Geneious (www. geneious.com) was applied, while the PeptideRanker (http://distilldeep.ucd.ie/PeptideRanker/) was used to predict probability of peptide being bioactive.
The multiple pair-wise comparisons resulted in the identification of numerous differentially abundant peptides (p<0.05) between the studied conditions, including among others 176 HF-specific, 146 CKD-specific and 35 CRS-specific peptides. Among the HF-specific peptides, the majority (n=94, 53.4%) originated from collagen type I, II and III. In the case of CKD-specific peptides, 24 (16.43%) originated from alpha-1-antitrypsin, 19 (13.0%) from b2-microglobulin and 15 (10.27%) from collagen type I. For the CRS specific peptides, fragments of Ig lambda-2 chain C regions (n=4, 11.42%), collagen type III (n=4, 11.42%), secreted and transmembrane protein 1 (n=3, 8.57%) and gelsolin (n=1, 2.85%) were identified (figure: 1). Of the 176 HF-specific peptides, 94 (53.40%) were predicted as bioactive, including, among others, fragments of collagen types I (n=43, 45.74%) and III (n=21, 22.34%). In the former, peptides with the higher bioactivity scores were aligned close to the N terminus of the precursor protein, whereas in the latter, peptides were in close proximity to both N and C termini. Along the same lines, 32 (21.91%) of the 146 CKD-specific peptides were predicted as bioactive, including peptides from collagen types I and III with the highest score, as well as fragments from collagen type V and the C terminus of the b2-microglobulin and alpha-1-antitrypsin proteins. No CRS-specific peptides could be predicted as bioactive.
Specific urinary peptides significantly associated with CRS, but not with HF or CKD, could be identified. These data indicate that on a molecular level, CRS is not merely the result of a combination of HF and CKD, but may represent a distinct pathology, defined via specific proteomic changes. It is expected that interpretation of these findings in the context of existing literature as well as in vitro activity assays will help to understand their biological relevance in CRS.
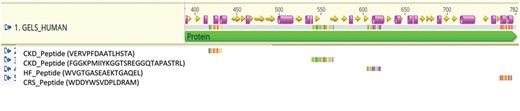
Figure: Specific peptides are associated with distinct pathologies. As example, peptides significantly associated with CKD, HR, or CRS derived from Gelsolin are depicted. The data indicate potential activation of distinct proteases specific for the different pathologies.
- kidney diseases
- renal function
- heart diseases
- alpha 1-antitrypsin
- kidney failure, chronic
- heart failure
- biological markers
- collagen
- collagen type i
- collagen type iii
- collagen type v
- electrophoresis
- endopeptidases
- gelsolin
- peptides
- urinary tract
- immunoglobulins
- heart
- kidney
- pathology
- gender
- urine
- peptide hydrolases
- transmembrane protein 1
- mann-whitney u test
- cardiorenal syndrome
- datasets
- user-centered design





Comments