-
PDF
- Split View
-
Views
-
Cite
Cite
Martina Tedesco, Isabella Pisani, Marco Allinovi, Giovanni Casazza, Lucia Del Vecchio, Marisa Santostefano, Francesca Ferrario, Ciro Esposito, Pasquale Esposito, Domenico Santoro, Roberta Lazzarin, Elisa Delbarba, Angelo Ferrantelli, Renato A Sinico, Mario Gennaro Cozzolino, Maurizio Gallieni, Francesco Scolari, Calogero Cirami, Augusto Vaglio, Federico Alberici, P0372
RITUXIMAB IN IDIOPATHIC FOCAL SEGMENTAL GLOMERULOSCLEROSIS OF THE ADULT: A MULTICENTRE RETROSPECTIVE SURVEY OF 31 PATIENTS, Nephrology Dialysis Transplantation, Volume 35, Issue Supplement_3, June 2020, gfaa142.P0372, https://doi.org/10.1093/ndt/gfaa142.P0372Close - Share Icon Share
Abstract
Idiopathic Focal Segmental Glomerulosclerosis (FSGS) is a rare glomerulonephritis often complicated by a chronic relapsing course frequently characterized by dependency or resistance to immunosuppressive treatment. Moreover, about half of the patients with active disease would develop end-stage renal disease within 10 years from the diagnosis, highlighting the need of novel therapeutic approaches. Rituximab (RTX), a chimeric monoclonal antibody against CD20, showed promising results in pediatric steroid-dependent/frequently relapsing FSGS and in post-transplantation recurrence. However, evidence about its role in the FSGS of the adult is still lacking with small case series suggesting conflicting results. In this study we assess the efficacy of RTX in the largest cohort of adults with FSGS currently available in literature.
Adults with biopsy proven idiopathic FSGS treated with RTX were retrospectively identified among several Italian nephrology units. Response to RTX was evaluated at 3, 6, 12 months and, when available, during the long-term follow-up. A positive response (POR) was defined as: (1) proteinuria <3.5 g/die with a decrease >50% compared to baseline, (2) stable renal function (3) decreased or stable dose of glucocorticoids and other immunosuppressants. Severe Adverse Events (SAEs) have been recorded.
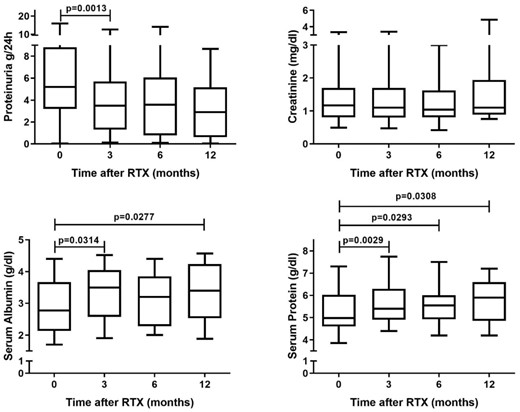
Figure: Changes in serum albumin, serum protein, serum creatinine and 24/h proteinuria over the first year of follow-up after RTX in the overall cohort of 31 patients affected by idiopathic FSGS
31 patients have been identified: 18 steroid-dependent, 11 steroid-resistant, and 2 patients with major contraindication to steroid therapy. RTX has been administered at a median of 87 months (IQR 54–96) from the diagnoses using heterogeneous schedules of administration. Overall, the POR rate at 6 months was 52% (steroid-dependent=69%; steroid-resistant=22%). At univariate analyses, POR to RTX at 6 months was associated to the steroid-dependent status (p=0.0347) and a proteinuria at RTX <5 g/die (p=0.0173); a trend towards better response was observed in patients with IgG at RTX <500 mg/dl (p=0.0774). Over the first year of follow-up, the proteinuria and serum albumin significantly improved (respectively, p=0.0021 and p=0.0277 at 12 months), while serum creatinine remained stable (figure). Among treated patients, the median dose of prednisone decreased from 15 mg/die (IQR 12.5–25) at baseline to 10 mg/die (IQR 5–15) at 12 months, while the proportion of patients free from glucocorticoids respectively increased from 42% to 54%. Six patients have been retreated within a year since the first RTX: of these only the 2 patients who have experienced a POR to the first administration obtained a further POR after retreatment. After the 12th month, 11 patients have been followed for a median time of 17 months (IQR 15–33.5): of the 5/11 with a POR at the 12th month, 2/5 maintained a POR without needing further immunosuppression, 2/5 maintained a POR with a pre-emptive RTX based maintenance treatment, 1/5 experienced a relapse successfully managed with RTX needing then a pre-emptive RTX based maintenance therapy able to allow a persistent POR. Overall, 9 SAEs have been recorded with requirement of hospital admission for clinical deterioration being the most frequent.
RTX may be an option in the FSGS of the adult, especially in the steroid-dependent patients and the ones with less severe nephrotic syndrome. In the responders, a RTX based maintenance therapy may be required.
- nephrotic syndrome
- proteinuria
- prednisone
- glomerulonephritis
- immunosuppressive agents
- renal function
- kidney failure, chronic
- biopsy
- glucocorticoids
- heterogeneity
- adult
- monoclonal antibodies
- cd20 antigens
- chimera organism
- death
- follow-up
- focal glomerulosclerosis
- nephrology
- pediatrics
- retreatments
- steroids
- immunoglobulin g
- therapeutic immunosuppression
- natural immunosuppression
- diagnosis
- serum albumin
- transplantation
- rituximab
- persistence
- steroid dependency
- creatinine tests, serum
- steroid therapy
- hospital admission
- adverse event
- univariate analysis
- clinical deterioration





Comments