-
PDF
- Split View
-
Views
-
Cite
Cite
Desmond Yap, Colin Siu On Tang, Ho Yin Chung, Julia Chan, Joseph M W Lo, Benjamin Y F So, Daniel Tak Mao Chan, SO077
A PROSPECTIVE RANDOMIZED STUDY ON PRE-EMPTIVE IMMUNOSUPPRESSIVE TREATMENT IN LUPUS NEPHRITIS PATIENTS WITH ASYMPTOMATIC SEROLOGICAL REACTIVATION, Nephrology Dialysis Transplantation, Volume 35, Issue Supplement_3, June 2020, gfaa139.SO077, https://doi.org/10.1093/ndt/gfaa139.SO077Close - Share Icon Share
Abstract
The optimal management for asymptomatic serological reactivation (ASR) in lupus nephritis (LN) patients remains undefined. Our previous retrospective study suggested that pre-emptive treatment with moderate increase of immunosuppression may reduce subsequent clinical flares.
This prospective study randomized LN patients with ASR (defined as >2-fold increase of anti-dsDNA to >100 IU/mL, with or without change in complement level, and absence of clinical lupus exacerbation) to receive pre-emptive treatment (‘Pre-emptive’ group) or unchanged management (‘Control’ group). Pre-emptive treatment included increasing prednisolone dose to 0.5 mg/kg/D, and the dose of mycophenolate to 1g/D or azathioprine to 75 mg/D, then tapered over 12-16 weeks back to the original dosages.
Thirty-four patients were randomized (17 in each group). Pre-emptive group showed lower anti-dsDNA and higher C3 levels after 12 weeks compared with Controls (90.3±58.3 IU/mL vs. 153.8±92.9 IU/mL, and 98.7±33.4 mg/dL vs. 81.8±26.2 mg/dL, respectively; p<0.001 for both) (Figure 1). Pre-emptive group showed significantly lower incidence rates of all clinical flares and renal flares during subsequent 9 months of follow-up compared with Controls (11.7% vs 47.1%, p=0.01; and 0% vs 17.7%, p=0.03; respectively). Mean time-to-flare was 6.4±4.2 months and 5.2±3.4 months in Pre-emptive and Control groups respectively (p=0.77). There was no clinically significant adverse event.
Our results suggest that pre-emptive moderate increase of immunosuppressive treatment reduces the risk of disease flare in LN patients with ASR and is well tolerated.
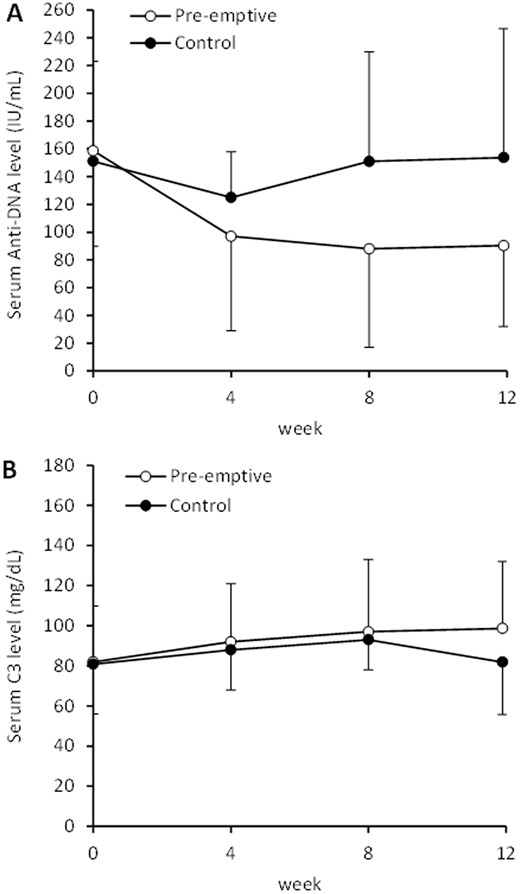
Figure 1. Serial changes in (A) anti-dsDNA level and (B) C3 level in lupus nephritis patients with asymptomatic serological reactivation who have or have not received pre-emptive immunosuppressive treatment.





Comments