-
PDF
- Split View
-
Views
-
Cite
Cite
Xiu-Wu Pan, Da Xu, Wen-Jin Chen, Jia-Xin Chen, Jian-Qing Ye, Li Zuo, Xin-Gang Cui, Acute kidney injury during the COVID-19 outbreak, Nephrology Dialysis Transplantation, Volume 35, Issue 9, September 2020, Pages 1635–1641, https://doi.org/10.1093/ndt/gfaa218
Close - Share Icon Share
The novel coronavirus disease 2019 (COVID-19) is the first coronavirus pandemic in human history. Europe and the USA have become epicenters of the COVID-19 pandemic. Acute kidney injury (AKI) is one of the most common complications during COVID-19, which increases the risk of in-hospital death. In-depth analysis of the occurrence, underlying mechanisms and clinical evidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-induced AKI helps clinicians to be alert to kidney damage. We believe that people will triumph in this war and save as many lives as possible.
In December 2019, a frightening novel coronavirus (COVID-19) broke out in Wuhan, China and spread rapidly throughout the country. Currently >10 million cases of COVID-19 have been diagnosed by laboratory tests, including >490 000 deaths, especially in the USA, UK, Italy, Russia, Brazil, Spain and India. The number of occurrences of COVID-19 and associated deaths was far greater than those of SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV) infection. The genomic characterization of the novel coronavirus showed that it is closely related to SARS-like coronaviruses detected in bat-derived coronaviruses (sequence identity 96%) [1], therefore it was officially designated SARS-CoV-2 [2].
The common clinical symptoms of COVID-19 are fever, cough and diarrhea [3]. Although most patients have mild symptoms, nearly one-third of patients develop severe pneumonia with several complications, including septic shock, acute respiratory distress syndrome, AKI and even death [3, 4]. The kidney is one of the most vulnerable organs during the COVID-19 outbreak, which leads to AKI. According to previous studies, patients with SARS-CoV and MERS-CoV infection had a high incidence of AKI [5, 6].
INCIDENCE OF AKI DURING THE COVID-19 OUTBREAK
In this study we included 12 large-scale case studies with 3670 COVID-19 patients for meta-analysis (Supplementary data, Tables S1 and S2) [4, 7–17]. AKI occurred in 0.5–33.9% of cases and 2.5–78% of intensive care unit patients with COVID-19. There was no significant difference in COVID-19 patients with pre-existing renal diseases between non-severe and severe COVID-19 cases {odds ratio [OR] 0.83 [95% confidence interval (CI) 0.55–1.25], P = 0.37; Figure 1A}. Conversely, compared with non-severe COVID-19 patients, the AKI rate was significantly higher in severe COVID-19 patients [OR 12.57 (95% CI 9.15–17.27), P < 0.001; Figure 1B]. In addition, 50% (27/54) of COVID-19 patients with in-hospital death had AKI [9]. The incidence of AKI in non-survivors was significantly higher than that in survivors [OR 27.75 (95% CI 11.58–66.50), P < 0.001; Figure 1C]. Therefore SARS-CoV-2 infection is closely associated with the occurrence of AKI.
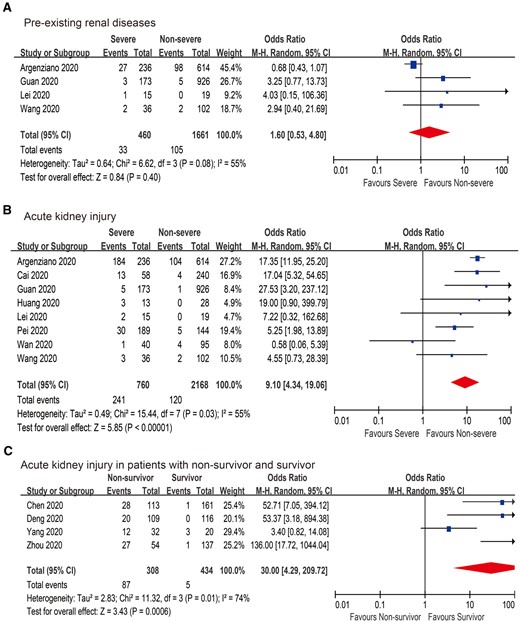
Pooled estimates of (A) pre-existing renal diseases, (B) AKI and (C) AKI in non-survivors and survivors.
POTENTIAL MECHANISM OF SARS-COV-2 AFFECTING THE KIDNEY
Angiotensin-converting enzyme 2 (ACE2) as a cell receptor and the transmembrane serine protease (TMPRSS) family (especially TMPRSS2) are key factors in assisting SARS-CoV-2 entry into host cells [18, 19]. After the spike (S) protein of SARS-CoV-2 binds to the host cell receptor ACE2, the S protein is cleaved by the cellular TMPRSSs, releasing the fusion peptides for virus-cell membrane fusion. In our study published in Intensive Care Medicine, co-expression of the ACE2 and TMPRSS genes was observably detected in podocytes and proximal straight tubule cells using single-cell transcriptome analysis, which was identified as potential host cells in the human kidney [20]. Meanwhile, the TMPRSS2 gene, as one of the most important cellular proteases, was co-expressed with ACE2 in podocytes [20]. The latest study reported that ACE2, TMPRSS2 and cathepsin L are enriched in multiple kidney cell types, regardless of whether they are faetal or adult, using single-cell RNA sequencing, which facilitates SARS-CoV-2-induced AKI [21]. Through in situ hybridization and indirect immunofluorescence with confocal microscopy, the SARS-CoV-2 viral load preferentially targeted glomerular cells [21], which is consistent with our previous research [20].
In addition, co-expression of ACE2 and TMPRSS genes in kidney cells was not less than that in the lung, esophagus, small intestine and colon, suggesting that the human kidney is an important organ targeted during SARS-CoV-2 infection [20]. The autopsies of COVID-19 patients showed that the kidney as one of the most common targets of SARS-CoV-2 is enriched with the SARS-CoV-2 viral load, the same as the respiratory tract, liver, heart and brain [21]. Interestingly, the expression of ACE2 in podocytes and proximal straight tubule cells was significantly higher in Occidental donors than in Asian donors, suggesting that Occidental populations might be at higher risk for developing AKI during COVID-19 [20]. According to the clinical characteristics of COVID-19 patients, the incidence of AKI (21.8–36.6%) from Occidental populations is significantly higher than that from Asian populations (0.5–7.3%) [14, 22, 23], which is consistent with our findings [20].
PROTEINURIA IS THE MAJOR SYMPTOM OF COVID-19 PATIENTS WITH AKI
It is well known that the major symptom of podocyte injury is heavy proteinuria. According to previous studies on SARS-CoV and MERS-CoV, proteinuria is also a major symptom in patients with AKI [5, 6]. A recent study reported that 43.9% of COVID-19 patients presented various degrees of proteinuria, especially patients with AKI, and 26.9% of COVID-19 patients had hematuria [24]. COVID-19 patients with proteinuria were closely associated with increased in-hospital death risk {proteinuria 1+: hazard ratio [HR] 2.47 (95% CI 1.15–5.33); proteinuria 2+~3+: HR 6.80 (95% CI 2.97–15.56); P < 0.001} [24]. Therefore it is highly likely that podocytes may be damaged by a SARS-CoV-2 attack.
OTHER CLINICAL EVIDENCE OF SARS-COV-2-MEDIATED AKI
Notably, the presence of SARS-CoV-2 in blood samples is a necessary condition for kidney damage. SARS-CoV-2 may spread to human kidney through the systemic circulation. According to clinical research data, 40% (6/15) of whole blood and 20% (3/15) of serum samples were SARS-CoV-2 nucleotide positive by a COVID-19 real-time quantitative polymerase chain reaction (RT-qPCR) test [25]. Several other studies showed that SARS-CoV-2 RNA viral load was detected in the serum of 41% of patients in general and up to 50% of critically ill patients [26, 27]. Moreover, detectable serum SARS-Cov-2 RNA (RNAaemia) is closely associated with elevated interleukin-6 level and poor prognosis, especially in critically ill COVID-19 patients [28], so decreasing RNAaemia might also help to blunt the excessive inflammatory response. Interestingly, although the oral swab test might be negative, blood samples can be detected as SARS-CoV-2 nucleotide positive, suggesting that adding blood tests can improve the positive rate of diagnosis for COVID-19 [25]. Last but not the least, the interval from symptom onset to the detection of SARS-CoV-2 in the blood is ∼1 week for patients with COVID-19 [4, 29, 30]. The length of time for blood SARS-CoV-2 positivity also coincides with the time (1 week) for developing AKI [3, 4].
Furthermore, the most direct evidence of coronavirus-induced kidney damage is that SARS-CoV-2 is found in the kidney tissue or urine of patients with COVID-19. A previous study demonstrated that SARS-CoV RNA was detected in kidney tissues from autopsy patients who died of SARS [31]. A recent study reported that the SARS-CoV-2 viral load was detected in kidney tissues from autopsies of COVID-19 patients, either with or without a history of chronic kidney disease, suggesting that SARS-CoV-2 directly infects the human kidney [21]. In addition, several studies found SARS-CoV-2 RNA viral load in the urine samples of patients with COVID-19 [7, 26].
CONCLUSIONS
In conclusion, this evidence indicates that SARS-CoV-2 may directly infect the human kidney, resulting in AKI, especially in severe COVID-19, either with or without a history of chronic kidney disease. According to single-cell transcriptome analysis combined with clinical characteristics analysis, multiple kidney compartments (especially podocytes and proximal straight tubule cells) in the human kidney may be potential host cells. Occidental populations may have a higher incidence of AKI, which requires more clinical case studies to verify. Given the current global outbreak of COVID-19, clinicians need to pay additional attention to SARS-CoV-2-mediated kidney damage. Notably, for severe cases with AKI, urinary excretion of these patients should be handled with caution to prevent accidental infection of medical workers.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
FUNDING
This work was sponsored by the National Natural Science Foundation of China (81772747, 81974391), the National Natural Science Foundation of China for Youths (81702515, 81702501), Shanghai Academic/Technology Research Leader Program (19XD1405100), Shanghai Sailing Program (17YF1425400), Shanghai ‘Rising Stars of Medical Talent’ Youth Development Program [Outstanding Youth Medical Talents (X.C.) and Youth Medical Talents – Specialist Program (X.P.)].
CONFLICT OF INTEREST STATEMENT
All authors declare no conflicts of interest.
REFERENCES
Gorbalenya AE, Baker SC, Baric RS et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 2020;
,
,
,
,
Author notes
Xiu-Wu Pan, Da Xu and Wen-Jin Chen contributed equally to this work as joint first authors.





Comments