-
PDF
- Split View
-
Views
-
Cite
Cite
Sune Moeller Skov-Jeppesen, Knud Bonnet Yderstraede, Claus Bistrup, Boyle L Jensen, Niels Marcussen, Milad Hanna, Lars Lund, Low-intensity shockwave therapy in the treatment of diabetic nephropathy: a prospective Phase 1 study, Nephrology Dialysis Transplantation, Volume 35, Issue 8, August 2020, Pages 1385–1392, https://doi.org/10.1093/ndt/gfy375
Close - Share Icon Share
Abstract
Low-intensity shockwave therapy (LI-SWT) is suggested as a therapy for promoting tissue regeneration. In pigs, it was recently found that LI-SWT improved renal function after ischaemic injury. Our objectives were to study glomerular filtration rate (GFR) and albuminuria in diabetic nephropathy (DN) after treatment with LI-SWT. The present pilot study reports on the clinical safety of LI-SWT in DN.
A total of 14 patients with diabetes mellitus and Stage 3 chronic kidney disease were recruited for this prospective, one-arm Phase 1 study. The patients were treated with six sessions of LI-SWT during a 3-week period. At each session, 3000 shockwaves were applied to each kidney with 0.265 mJ/mm2, extended focal size and 4 Hz. Follow-up visits were performed at 1, 3 and 6 months.
In general, the treatment was well tolerated. Transient macroscopic haematuria was observed in three patients immediately after LI-SWT. The majority of patients experienced lower back tenderness lasting up to 2 days after treatment. There was no need for analgesic treatment. LI-SWT showed no negative effect on GFR and albuminuria. At baseline, median (interquartile range) GFR was 33.5 mL/min/1.73 m2 (27.8–43.8) compared with 36.0 mL/min/1.73 m2 (27.5–52.0) at 6 months follow-up. In parallel, median albuminuria was 256 mg/24 h (79–619) at baseline and tended to decrease to 137 mg/24 h (41–404) 6 months after LI-SWT. There was no statistical difference between baseline and follow-up results.
LI-SWT is a safe treatment for DN. Inclusion of more patients is needed to determine whether LI-SWT can improve renal functional outcomes.
INTRODUCTION
The prevalence of diabetes mellitus (DM) is increasing worldwide. Accordingly, the burden of diabetic complications is expected to expand [1–3]. Diabetic nephropathy (DN) is one of the most critical complications of DM and is associated with cardiovascular disease and increased mortality [4]. DN is characterized by a progressive loss of kidney function, increased albuminuria and characteristic morphological tissue changes [5]. Treatment is initiated to prevent later stages of chronic kidney disease (CKD) and to reduce the clinical manifestations of impaired renal function [6]. However, there are no treatments available to reverse the effects of DN [7, 8]. DM is the leading cause of end-stage renal disease, and it is estimated that 20–40% of patients with DM develop DN [9].
Low-intensity shockwave therapy (LI-SWT) is suggested as a novel modality to recover tissue vascularization and organ function. Shockwaves are biphasic acoustic waves consisting of initial high amplitude-positive pressure followed by a tensile phase propagating in the 3-dimensional space, and conduct through different kinds of tissues [10, 11]. At the cellular level, the shockwaves generate a mechanical stimulation of the cytoskeleton and membrane-bound proteins [12, 13]. Compared with lithotripsy, shockwave therapy for regenerative purposes is applied with much lower energy.
In clinical studies, LI-SWT has been found to promote angiogenesis and to have regenerative effects in conditions such as coronary artery disease, musculoskeletal disorders and soft tissue wounds [14–16]. LI-SWT potentially ameliorates erectile dysfunction, but patient selection and indications are still debated after some studies demonstrated placebo to be equally effective [17, 18]. However, under the circumstances of chronic ischaemia, LI-SWT is reported to increase tissue perfusion and improve symptoms [19, 20]. As a means to restore vascularization and organ perfusion, we hypothesized that LI-SWT can be therapeutically useful in patients with DN in order to preserve renal function.
The aim of the present Phase 1 pilot study was to investigate the safety of using LI-SWT as a treatment for DN. Our objectives were to get the first data on glomerular filtration rate (GFR), albuminuria, safety and side effects following LI-SWT-treatment in patients with DM and Stage 3 CKD.
MATERIALS AND METHODS
Fourteen patients were consecutively recruited and enrolled from the Department of Endocrinology, Odense University Hospital, Denmark between 27 May 2015 and 14 November 2016. The study was performed in accordance with the Helsinki II declaration on clinical trials and the participants provided informed written consent before entering the study. Approval was obtained from the Regional Committees on Health Research Ethics for Southern Denmark (project ID: S-20130150) and the Danish Data Protection Agency (common regional registration 2008-58-0035, journal no.: 15/34140). The trial was registered to ClinicalTrials.gov with identifying number NCT02515461.
Patients
Included patients were between 18 and 71 years old, diagnosed with DM and had estimated GFR (eGFR) between 30 and 60 mL/min/1.73 m2 on at least two occasions. Exclusion criteria for participation were any of the following: non-DM kidney disease, anticoagulant medical therapy (antiplatelet drugs were allowed for inclusion), bleeding disorder, blood pressure >140/90 mmHg, abnormal renography (kidney with <30% of total renal function), kidney or ureteric stone, obstructive uropathy, kidney tumour, chronic urinary tract infection, single kidney, coronary infarction within 1 year, severe psychiatric disorder, kidney transplant recipient and pregnancy. Before the treatment was initiated, participants had ultrasound performed to exclude obstructive uropathy, kidney/ureteric stone and tumour. In each patient, kidney biopsy was taken in order to characterize the pathological type and degree of nephropathy. Renography was carried out if there was any clinical suspicion or ultrasound finding of unilateral affection of the kidney function.
LI-SWT
The patients received LI-SWT treatment six times during a 3-week period. At every session, blood pressure and urine sample were taken before and after LI-SWT. In addition, ultrasound examination of the kidneys was performed before each treatment. For LI-SWT treatment, we used the Modulith SLX-2 (Storz Medical AG, Switzerland). The patients were placed in the supine position on the back or side while treatment was carried out. Water was poured on the table to secure optimal coupling and was refilled whenever needed during treatment. The kidneys were localized in focus using the in-line ultrasound of the lithotripter. No analgesics were administered. The patients were instructed to notify the staff if they felt any pain or discomfort during the treatment. We applied 3000 shockwaves to each kidney using 4 Hz and extended focal size. The kidneys were divided into three segments (upper, middle, lower) and 1000 shockwaves were applied to each segment. In order to accustom the patients to the treatment, the energy level was gradually increased from 0.1 (0.136 mJ/mm2) to 0.7 (0.265 mJ/mm2) within the first 200 shockwaves. Subsequently, we used energy level 0.7 for the final 800 shockwaves at each segment of the kidney. The clinical setting is depicted in Figure 1.
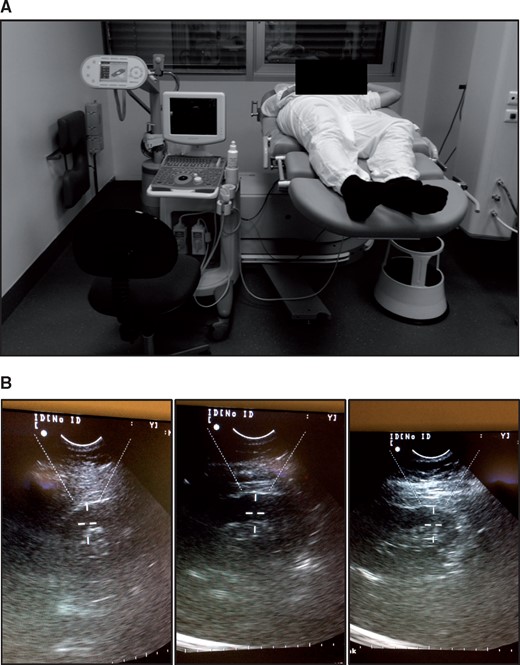
LI-SWT treatment with the Modulith SLX-2 lithotripter. (A) Patients were laid on their back or side while treatment was carried out. The shockwave source is found beneath the table under the patient’s lumbar region. Water was used as coupling between the patient and the lithotripter. (B) The kidneys were treated with 1000 shockwaves at the upper, middle and lower part (3000 in total for each kidney). Energy level was increased from 0.136 mJ/mm2 to 0.265 mJ/mm2 during the first 200 shocks at each part of the kidney. The final 800 shockwaves were applied with 0.265 mJ/mm2. The shockwaves were administered with 4 Hz and extended focal size. Both kidneys were treated at each session.
Other procedures
An overview of investigations conducted during the study is provided in Figure 2. A complete methods description can be found for GFR measurement, 24-h urine sample, ambulatory blood pressure (ABP), blood samples and kidney biopsy in Supplementary data, Appendix SI.
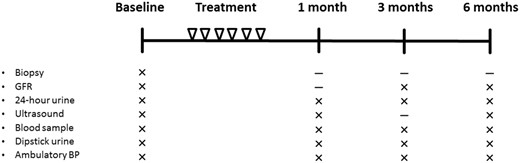
Overview of treatments and investigations. The patients were treated with LI-SWT six times during a 3-week period. Investigations were performed at baseline and at 1, 3 and 6 months follow-up.
Statistics
Data were evaluated statistically using IBM SPSS Statistics version 24 for Microsoft Windows. Descriptive statistics are presented as median and interquartile ranges (IQRs). Follow-up data were analysed for statistical significance in comparison with baseline by performing related samples Wilcoxon Signed rank test with 95% confidence level. No significant differences were detected between baseline and follow-up visits. Accordingly, only descriptive statistics are displayed.
RESULTS
Fourteen patients with DN were recruited for the study (Table 1). All patients completed the treatment with LI-SWT as per protocol. The patients were characterized by a median (IQR) age of 61.5 years (52.5–69.0 years) and 23.0 years (10.0–29.5 years) diabetes duration. Most of the patients were male (79%) and diagnosed with type 2 DM (93%). Kidney biopsy was available in 12 patients and revealed mild diabetic glomerular lesions in the majority of the patients. Pathologic classification of the kidney biopsies is included in Supplementary data, Appendix SII.
| Variables . | Obtained at baseline . |
|---|---|
| Sex | |
| Male | 11 (79) |
| Female | 3 (21) |
| Age (years) | 61.5 (52.5–69.0) |
| Body mass index (kg/m2) | 30.9 (26.7–34.3) |
| Smoking | |
| Current | 0 (0) |
| Former | 10 (71) |
| Never | 4 (29) |
| Package years, non-smokers excluded | 28.5 (6.8–50.3) |
| Alcohol consumption (units/week) (1 unit = 12 g) | 2.0 (0.0–5.3) |
| Diabetes mellitus | |
| Type 1 | 1 (7) |
| Type 2 | 13 (93) |
| Duration of diabetes (years) | 23.0 (10.0–29.5) |
| Medical treatment of diabetes mellitus | |
| None | 1 (7) |
| Metformin | 4 (29) |
| Insulin | 11 (79) |
| DPP-4 inhibitor/GLP1-analogue | 3 (21) |
| Medical treatment of hypertension | |
| None | 2 (14) |
| ACE inhibitor/AT2 antagonist | 11 (79) |
| Thiazide | 7 (50) |
| Furosemide | 6 (43) |
| Beta-blocker | 5 (36) |
| Calcium antagonist | 7 (50) |
| Aldosterone antagonist | 3 (21) |
| Other | 2 (14) |
| Receiving lipid-lowering medication | 12 (86) |
| Charlson Index Scorea | |
| 2 | 7 (50) |
| 3–4 | 5 (36) |
| 5 or more | 2 (14) |
| Variables . | Obtained at baseline . |
|---|---|
| Sex | |
| Male | 11 (79) |
| Female | 3 (21) |
| Age (years) | 61.5 (52.5–69.0) |
| Body mass index (kg/m2) | 30.9 (26.7–34.3) |
| Smoking | |
| Current | 0 (0) |
| Former | 10 (71) |
| Never | 4 (29) |
| Package years, non-smokers excluded | 28.5 (6.8–50.3) |
| Alcohol consumption (units/week) (1 unit = 12 g) | 2.0 (0.0–5.3) |
| Diabetes mellitus | |
| Type 1 | 1 (7) |
| Type 2 | 13 (93) |
| Duration of diabetes (years) | 23.0 (10.0–29.5) |
| Medical treatment of diabetes mellitus | |
| None | 1 (7) |
| Metformin | 4 (29) |
| Insulin | 11 (79) |
| DPP-4 inhibitor/GLP1-analogue | 3 (21) |
| Medical treatment of hypertension | |
| None | 2 (14) |
| ACE inhibitor/AT2 antagonist | 11 (79) |
| Thiazide | 7 (50) |
| Furosemide | 6 (43) |
| Beta-blocker | 5 (36) |
| Calcium antagonist | 7 (50) |
| Aldosterone antagonist | 3 (21) |
| Other | 2 (14) |
| Receiving lipid-lowering medication | 12 (86) |
| Charlson Index Scorea | |
| 2 | 7 (50) |
| 3–4 | 5 (36) |
| 5 or more | 2 (14) |
Data are n (%) or median (25–75% quartile).
Minimum Charlson Index Score is 2 according to the diagnosis of diabetic nephropathy in all patients.
| Variables . | Obtained at baseline . |
|---|---|
| Sex | |
| Male | 11 (79) |
| Female | 3 (21) |
| Age (years) | 61.5 (52.5–69.0) |
| Body mass index (kg/m2) | 30.9 (26.7–34.3) |
| Smoking | |
| Current | 0 (0) |
| Former | 10 (71) |
| Never | 4 (29) |
| Package years, non-smokers excluded | 28.5 (6.8–50.3) |
| Alcohol consumption (units/week) (1 unit = 12 g) | 2.0 (0.0–5.3) |
| Diabetes mellitus | |
| Type 1 | 1 (7) |
| Type 2 | 13 (93) |
| Duration of diabetes (years) | 23.0 (10.0–29.5) |
| Medical treatment of diabetes mellitus | |
| None | 1 (7) |
| Metformin | 4 (29) |
| Insulin | 11 (79) |
| DPP-4 inhibitor/GLP1-analogue | 3 (21) |
| Medical treatment of hypertension | |
| None | 2 (14) |
| ACE inhibitor/AT2 antagonist | 11 (79) |
| Thiazide | 7 (50) |
| Furosemide | 6 (43) |
| Beta-blocker | 5 (36) |
| Calcium antagonist | 7 (50) |
| Aldosterone antagonist | 3 (21) |
| Other | 2 (14) |
| Receiving lipid-lowering medication | 12 (86) |
| Charlson Index Scorea | |
| 2 | 7 (50) |
| 3–4 | 5 (36) |
| 5 or more | 2 (14) |
| Variables . | Obtained at baseline . |
|---|---|
| Sex | |
| Male | 11 (79) |
| Female | 3 (21) |
| Age (years) | 61.5 (52.5–69.0) |
| Body mass index (kg/m2) | 30.9 (26.7–34.3) |
| Smoking | |
| Current | 0 (0) |
| Former | 10 (71) |
| Never | 4 (29) |
| Package years, non-smokers excluded | 28.5 (6.8–50.3) |
| Alcohol consumption (units/week) (1 unit = 12 g) | 2.0 (0.0–5.3) |
| Diabetes mellitus | |
| Type 1 | 1 (7) |
| Type 2 | 13 (93) |
| Duration of diabetes (years) | 23.0 (10.0–29.5) |
| Medical treatment of diabetes mellitus | |
| None | 1 (7) |
| Metformin | 4 (29) |
| Insulin | 11 (79) |
| DPP-4 inhibitor/GLP1-analogue | 3 (21) |
| Medical treatment of hypertension | |
| None | 2 (14) |
| ACE inhibitor/AT2 antagonist | 11 (79) |
| Thiazide | 7 (50) |
| Furosemide | 6 (43) |
| Beta-blocker | 5 (36) |
| Calcium antagonist | 7 (50) |
| Aldosterone antagonist | 3 (21) |
| Other | 2 (14) |
| Receiving lipid-lowering medication | 12 (86) |
| Charlson Index Scorea | |
| 2 | 7 (50) |
| 3–4 | 5 (36) |
| 5 or more | 2 (14) |
Data are n (%) or median (25–75% quartile).
Minimum Charlson Index Score is 2 according to the diagnosis of diabetic nephropathy in all patients.
| Variables . | Baselinea . | 1 montha . | 3 monthsa . | 6 monthsb . |
|---|---|---|---|---|
| 51-chromium-EDTA clearance | ||||
| Standard-GFR, mL/min/1.73 m2 | 33.5 (27.8–43.8) | – | 36.5 (29.3–47.0) | 36.0 (27.5–52.0) |
| Albuminuriac | ||||
| Albumin (total), mg/24 h | 256 (79–619) | 219 (57–335) | 220 (56–351) | 137 (41–404) |
| 24-h urine | ||||
| Volume, mL | 2152 (1198–2633) | 2032 (1274–2571) | 1895 (1424–2359) | 1919 (1545–2670) |
| Creatinine clearance, mL/min | 49.1 (35.1–65.8) | 49.7 (34.6–72.4) | 49.9 (37.3–64.6) | 51.3 (29.2–74.2) |
| Sodium, mmol/24 h | 175 (79–207) | 169 (91–218) | 148 (100–188) | 171 (109–208) |
| Potassium, mmol/24 h | 58 (43–80) | 65 (52–84) | 65 (48–93) | 63 (50–77) |
| Ambulatory blood pressure | ||||
| Systolic, mmHg | 122 (112–131) | 120 (116–126) | 124 (115–132) | 116 (121–128) |
| Diastolic, mmHg | 68 (65–78) | 72 (62–75) | 73 (66–79) | 69 (62–78) |
| Blood samples | ||||
| Haemoglobin, mmol/L | 8.0 (7.0–8.7) | 7.9 (7.3–8.4) | 7.9 (7.1–8.5) | 7.8 (7.5–8.6) |
| Albumin, g/L | 43 (40–45) | 43 (40–45) | 42 (40–44) | 43 (42–45) |
| Sodium, mmol/L | 138 (135–141) | 138 (136–140) | 138 (136–141) | 138 (136–141) |
| Potassium, mmol/L | 4.2 (3.8–4.5) | 4.2 (4.0–4.7) | 4.3 (4.0–4.6) | 4.3 (4.0–4.8) |
| Ionised calcium mmol/L | 1.28 (1.23–1.30) | 1.25 (1.23–1.28) | 1.24 (1.22–1.28) | 1.25 (1.25–1.29) |
| Phosphate, mmol/L | 1.00 (0.90–1.15) | 1.09 (0.90–1.19) | 1.09 (0.94–1.16) | 1.05 (0.81–1.26) |
| Creatinine, µmol/L | 141 (129–197) | 154 (121–209) | 161 ( 122–199) | 136 (118–209) |
| eGFR, mL/min/1.73m2 | 42 ( 31–48) | 36 (28–49) | 35 (29–51) | 45 (24–54) |
| BUN, mmol/L | 10.4 (9.1–12.8) | 12.9 (8.6–15.0) | 11.9 (8.2–12.8) | 13.9 (7.4–16.9) |
| Glycated haemoglobin, mmol/mol | 59 (50–80) | 59 (55–79) | 60 (53–79) | 57 (55–68) |
| Variables . | Baselinea . | 1 montha . | 3 monthsa . | 6 monthsb . |
|---|---|---|---|---|
| 51-chromium-EDTA clearance | ||||
| Standard-GFR, mL/min/1.73 m2 | 33.5 (27.8–43.8) | – | 36.5 (29.3–47.0) | 36.0 (27.5–52.0) |
| Albuminuriac | ||||
| Albumin (total), mg/24 h | 256 (79–619) | 219 (57–335) | 220 (56–351) | 137 (41–404) |
| 24-h urine | ||||
| Volume, mL | 2152 (1198–2633) | 2032 (1274–2571) | 1895 (1424–2359) | 1919 (1545–2670) |
| Creatinine clearance, mL/min | 49.1 (35.1–65.8) | 49.7 (34.6–72.4) | 49.9 (37.3–64.6) | 51.3 (29.2–74.2) |
| Sodium, mmol/24 h | 175 (79–207) | 169 (91–218) | 148 (100–188) | 171 (109–208) |
| Potassium, mmol/24 h | 58 (43–80) | 65 (52–84) | 65 (48–93) | 63 (50–77) |
| Ambulatory blood pressure | ||||
| Systolic, mmHg | 122 (112–131) | 120 (116–126) | 124 (115–132) | 116 (121–128) |
| Diastolic, mmHg | 68 (65–78) | 72 (62–75) | 73 (66–79) | 69 (62–78) |
| Blood samples | ||||
| Haemoglobin, mmol/L | 8.0 (7.0–8.7) | 7.9 (7.3–8.4) | 7.9 (7.1–8.5) | 7.8 (7.5–8.6) |
| Albumin, g/L | 43 (40–45) | 43 (40–45) | 42 (40–44) | 43 (42–45) |
| Sodium, mmol/L | 138 (135–141) | 138 (136–140) | 138 (136–141) | 138 (136–141) |
| Potassium, mmol/L | 4.2 (3.8–4.5) | 4.2 (4.0–4.7) | 4.3 (4.0–4.6) | 4.3 (4.0–4.8) |
| Ionised calcium mmol/L | 1.28 (1.23–1.30) | 1.25 (1.23–1.28) | 1.24 (1.22–1.28) | 1.25 (1.25–1.29) |
| Phosphate, mmol/L | 1.00 (0.90–1.15) | 1.09 (0.90–1.19) | 1.09 (0.94–1.16) | 1.05 (0.81–1.26) |
| Creatinine, µmol/L | 141 (129–197) | 154 (121–209) | 161 ( 122–199) | 136 (118–209) |
| eGFR, mL/min/1.73m2 | 42 ( 31–48) | 36 (28–49) | 35 (29–51) | 45 (24–54) |
| BUN, mmol/L | 10.4 (9.1–12.8) | 12.9 (8.6–15.0) | 11.9 (8.2–12.8) | 13.9 (7.4–16.9) |
| Glycated haemoglobin, mmol/mol | 59 (50–80) | 59 (55–79) | 60 (53–79) | 57 (55–68) |
Data are presented as median (25–75% quartile). aN = 14, bN = 13, cPatients with non-significant albuminuria are excluded (three patients with <30 mg/24 h at all visits).
| Variables . | Baselinea . | 1 montha . | 3 monthsa . | 6 monthsb . |
|---|---|---|---|---|
| 51-chromium-EDTA clearance | ||||
| Standard-GFR, mL/min/1.73 m2 | 33.5 (27.8–43.8) | – | 36.5 (29.3–47.0) | 36.0 (27.5–52.0) |
| Albuminuriac | ||||
| Albumin (total), mg/24 h | 256 (79–619) | 219 (57–335) | 220 (56–351) | 137 (41–404) |
| 24-h urine | ||||
| Volume, mL | 2152 (1198–2633) | 2032 (1274–2571) | 1895 (1424–2359) | 1919 (1545–2670) |
| Creatinine clearance, mL/min | 49.1 (35.1–65.8) | 49.7 (34.6–72.4) | 49.9 (37.3–64.6) | 51.3 (29.2–74.2) |
| Sodium, mmol/24 h | 175 (79–207) | 169 (91–218) | 148 (100–188) | 171 (109–208) |
| Potassium, mmol/24 h | 58 (43–80) | 65 (52–84) | 65 (48–93) | 63 (50–77) |
| Ambulatory blood pressure | ||||
| Systolic, mmHg | 122 (112–131) | 120 (116–126) | 124 (115–132) | 116 (121–128) |
| Diastolic, mmHg | 68 (65–78) | 72 (62–75) | 73 (66–79) | 69 (62–78) |
| Blood samples | ||||
| Haemoglobin, mmol/L | 8.0 (7.0–8.7) | 7.9 (7.3–8.4) | 7.9 (7.1–8.5) | 7.8 (7.5–8.6) |
| Albumin, g/L | 43 (40–45) | 43 (40–45) | 42 (40–44) | 43 (42–45) |
| Sodium, mmol/L | 138 (135–141) | 138 (136–140) | 138 (136–141) | 138 (136–141) |
| Potassium, mmol/L | 4.2 (3.8–4.5) | 4.2 (4.0–4.7) | 4.3 (4.0–4.6) | 4.3 (4.0–4.8) |
| Ionised calcium mmol/L | 1.28 (1.23–1.30) | 1.25 (1.23–1.28) | 1.24 (1.22–1.28) | 1.25 (1.25–1.29) |
| Phosphate, mmol/L | 1.00 (0.90–1.15) | 1.09 (0.90–1.19) | 1.09 (0.94–1.16) | 1.05 (0.81–1.26) |
| Creatinine, µmol/L | 141 (129–197) | 154 (121–209) | 161 ( 122–199) | 136 (118–209) |
| eGFR, mL/min/1.73m2 | 42 ( 31–48) | 36 (28–49) | 35 (29–51) | 45 (24–54) |
| BUN, mmol/L | 10.4 (9.1–12.8) | 12.9 (8.6–15.0) | 11.9 (8.2–12.8) | 13.9 (7.4–16.9) |
| Glycated haemoglobin, mmol/mol | 59 (50–80) | 59 (55–79) | 60 (53–79) | 57 (55–68) |
| Variables . | Baselinea . | 1 montha . | 3 monthsa . | 6 monthsb . |
|---|---|---|---|---|
| 51-chromium-EDTA clearance | ||||
| Standard-GFR, mL/min/1.73 m2 | 33.5 (27.8–43.8) | – | 36.5 (29.3–47.0) | 36.0 (27.5–52.0) |
| Albuminuriac | ||||
| Albumin (total), mg/24 h | 256 (79–619) | 219 (57–335) | 220 (56–351) | 137 (41–404) |
| 24-h urine | ||||
| Volume, mL | 2152 (1198–2633) | 2032 (1274–2571) | 1895 (1424–2359) | 1919 (1545–2670) |
| Creatinine clearance, mL/min | 49.1 (35.1–65.8) | 49.7 (34.6–72.4) | 49.9 (37.3–64.6) | 51.3 (29.2–74.2) |
| Sodium, mmol/24 h | 175 (79–207) | 169 (91–218) | 148 (100–188) | 171 (109–208) |
| Potassium, mmol/24 h | 58 (43–80) | 65 (52–84) | 65 (48–93) | 63 (50–77) |
| Ambulatory blood pressure | ||||
| Systolic, mmHg | 122 (112–131) | 120 (116–126) | 124 (115–132) | 116 (121–128) |
| Diastolic, mmHg | 68 (65–78) | 72 (62–75) | 73 (66–79) | 69 (62–78) |
| Blood samples | ||||
| Haemoglobin, mmol/L | 8.0 (7.0–8.7) | 7.9 (7.3–8.4) | 7.9 (7.1–8.5) | 7.8 (7.5–8.6) |
| Albumin, g/L | 43 (40–45) | 43 (40–45) | 42 (40–44) | 43 (42–45) |
| Sodium, mmol/L | 138 (135–141) | 138 (136–140) | 138 (136–141) | 138 (136–141) |
| Potassium, mmol/L | 4.2 (3.8–4.5) | 4.2 (4.0–4.7) | 4.3 (4.0–4.6) | 4.3 (4.0–4.8) |
| Ionised calcium mmol/L | 1.28 (1.23–1.30) | 1.25 (1.23–1.28) | 1.24 (1.22–1.28) | 1.25 (1.25–1.29) |
| Phosphate, mmol/L | 1.00 (0.90–1.15) | 1.09 (0.90–1.19) | 1.09 (0.94–1.16) | 1.05 (0.81–1.26) |
| Creatinine, µmol/L | 141 (129–197) | 154 (121–209) | 161 ( 122–199) | 136 (118–209) |
| eGFR, mL/min/1.73m2 | 42 ( 31–48) | 36 (28–49) | 35 (29–51) | 45 (24–54) |
| BUN, mmol/L | 10.4 (9.1–12.8) | 12.9 (8.6–15.0) | 11.9 (8.2–12.8) | 13.9 (7.4–16.9) |
| Glycated haemoglobin, mmol/mol | 59 (50–80) | 59 (55–79) | 60 (53–79) | 57 (55–68) |
Data are presented as median (25–75% quartile). aN = 14, bN = 13, cPatients with non-significant albuminuria are excluded (three patients with <30 mg/24 h at all visits).
Side effects
The patients did not experience any significant pain during the treatment with LI-SWT. In general, the treatment was felt as a tingling or stinging sensation on the skin. One patient reported pain when the treatment was aimed at the upper part of the left kidney, but the pain ended immediately when the focus was moved slightly in the anterior or posterior direction. We suspected a nerve or a costa was affected inasmuch as the ultrasound scan demonstrated no signs of haematoma, cysts or calcification on the patient’s kidneys.
Mild macroscopic haematuria was observed in three patients after the treatment. In one of the patients, an ultrasound scan prior to the treatment revealed extensive parenchymal calcification of both kidneys. Thus, the calcifications were likely to trigger the haematuria in connection with LI-SWT. The two other patients had macroscopic haematuria in one and two sessions of LI-SWT, respectively. The haematuria disappeared within a few hours and the patients were advised to drink extra water. The presence of macroscopic haematuria did not lead to further complications and was not associated with an adverse renal functional outcome. GFR was increasing at 6 months follow-up in two of the patients (>5 mL/min/1.73 m2) and remained stable in the third of these patients (increase of 2 mL/min/1.73 m2 at 6 months follow-up).
A total of 11 patients experienced mild to moderate lower back tenderness or pain lasting up to 2 days after the treatments. The patients recovered before a new session was initiated and the pain did not increase after repeated treatments. Accordingly, there was no need to cancel or postpone treatment sessions.
The events of tenderness/pain and macroscopic haematuria are reported as Grade I complications in accordance with the Clavien–Dindo classification system.
Adverse events not relating to intervention
During the conduct of the study, adverse events were recorded in three patients without relation to LI-SWT treatment. One patient had a vertebral (T12) osteoporotic fracture detected at 6 months follow-up; one patient presented with acute pre-renal kidney failure 6 months after the treatment; and one patient dropped out of the study shortly before 6 months follow-up due to a recent diagnosis of multiple myeloma. The adverse events are outlined in Supplementary data, Appendix SIII.
Secondary outcomes
There were no statistically significant differences between baseline and follow-up visits for the collected data (Table 2).
At the 3 months follow-up visit, median (IQR) GFR increased to 36.5 mL/min/1.73 m2 (29.3–47.0 mL/min/1.73 m2) compared with 33.5 mL/min/1.73 m2 (27.8–43.8 mL/min/1.73 m2) at baseline. Six months after LI-SWT treatment, median (IQR) GFR was stable at 36.0 mL/min/1.73 m2 (27.5–52.0 mL/min/1.73 m2). In relation to GFR, the lower quartile was mildly increased or stable in comparison with baseline and the upper quartile increased also after 3 months post-intervention.
Albuminuria gradually decreased after the treatment. In patients with albuminuria (>30 mg/24 h), the median (IQR) urine albumin excretion was 256 mg/24 h (79–619 mg/24 h) at baseline and declined to 219 mg/24 h (57–335 mg/24 h) after 1 month. In the ensuing 2 months, albuminuria remained stable but appeared to further decrease at 6 months follow-up, where the median (IQR) excretion was 113 mg/24 h (41–404 mg/24 h).
GFR and albuminuria for each patient are presented in Figure 3 including mean slope.
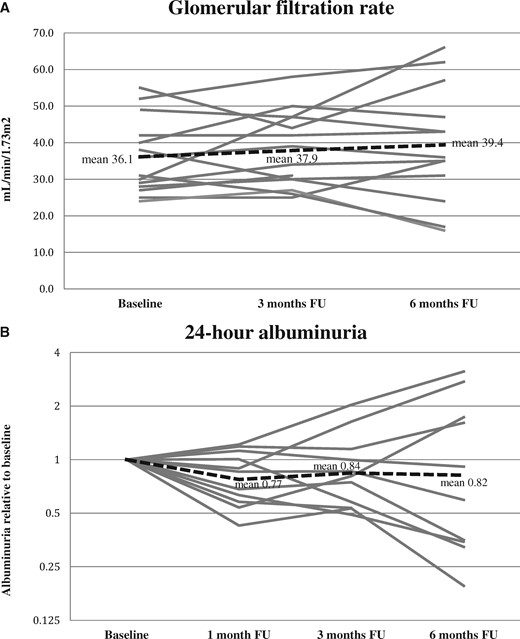
Individual curves of GFR. (A) GFR is presented with slopes for each patient at baseline, 3 and 6 months follow-up. Dashed line represents mean slope. (B) Individual slopes for albuminuria at 1, 3 and 6 months follow-up. Values were calculated as ratios between follow-up and baseline (values <1 indicate decreasing albuminuria). Dashed line represents mean slope.
The urinary output seemed slightly reduced after the treatment with LI-SWT as demonstrated by the median volume of the 24-h urine sample. We detected no influence of treatments on haemoglobin, albumin, phosphate and electrolytes in plasma. HbA1c was stable throughout the study with a median value approximately 60 mmol/mol. Median creatinine and median blood urea nitrogen (BUN) showed small increases after LI-SWT but IQRs were homogeneous for these outcomes. The creatinine clearance was unaffected by the treatment with LI-SWT.
ABP was not affected by the treatment with LI-SWT. There were no changes in the patients’ antihypertensive treatment at 1 month and 3 months follow-up. After 6 months, antihypertensive treatment was adjusted in four patients but only involved different types of diuretics or calcium blockers. Adjustments of antidiabetic treatment included switching from oral antiglycaemic agents to insulin, as well as dose increases and reductions in the same type of medication. The modifications of antihypertensive and antiglycaemic therapy during follow-up are outlined in Supplementary data, Appendix SV.
One patient was lost to follow-up 6 months after the treatment. We performed an additional analysis with the patient’s last observations carried forward. The analysis generated no relevant changes to the outcomes except for albuminuria and plasma creatinine levels. Potentially, at 6 months follow-up, albuminuria was lower [median (IQR) 99.6 mg/24 h (23.5–306 mg/24 h)], and the blood creatinine level was comparable to previous follow-up visits [median (IQR) 162 µmol/L (118–210 µmol/L)].
DISCUSSION
The present study indicates that LI-SWT is a safe treatment that could be evaluated as a potential adjunctive treatment of DN. A total of 14 patients received treatment with LI-SWT and were assigned to follow-up at 1, 3 and 6 months post-treatment. Macroscopic haematuria was observed in three patients immediately after the treatment and most patients experienced a light lower back pain. The presence of macroscopic haematuria was not associated with negative outcomes and ultrasound scans revealed no signs of renal haematoma. In most of the patients, GFR and albuminuria tended to improve after treatment with LI-SWT although these outcomes were non-significant. Currently, there are no accepted treatments to restore renal function in patients with DN although progression of nephropathy may be attenuated with strategies including blockade of the renin-angiotensin-aldosterone system as well as strict hypertensive and glycaemic control. These are the first clinical data suggesting LI-SWT as a safe treatment modality with a potential for restoring renal function.
It has previously been demonstrated that LI-SWT induces angiogenesis and tissue regeneration in other organs. In particular, LI-SWT upregulates proangiogenic factors such as vascular endothelial growth factor (VEGF) and endothelial nitric oxide synthase (eNOS) [21–23]. In diabetic eNOS knockout mice, shockwave-induced angiogenesis and wound healing are blunted, pointing to nitric oxide (NO) being the primary effector molecule of LI-SWT [21]. NO is released in response to shockwaves in patients undergoing lithotripsy for renal stones, and the NO-cGMP pathway is activated by shockwaves in rabbit kidneys [24, 25]. Application of LI-SWT subsequently leads to proliferation of endothelial cells and increased formation of microvessels [22]. Proliferation of the endothelial cells might be stimulated via VEGF activating the extracellular signal-regulated kinase mitogen-activated protein kinase pathway in an autocrine fashion [26]. On the other hand, high levels of VEGF are shown to be deleterious in DN and cause excessive endothelial proliferation, inflammation and vascular damage. This effect may be related to uncoupling of VEGF from its normal function where it stimulates NO production. NO, in turn, acts as an inhibitory factor to prevent excess endothelial proliferation [27]. In the setting of DN, where NO content in the kidneys is generally found to be low, increased NO bioavailability could work as a balancing factor to abnormal levels of VEGF and reduce the manifestations of microvascular disease [28].
The key role of the VEGF–NO axis is demonstrated in mice where combination of NO deficiency and VEGF overload is sufficient to induce nodular glomerulosclerosis [29]. As a new potential treatment for DN, LI-SWT could act as a non-pharmacological agent to locally increase NO and reduce endothelial dysfunction in the kidneys. Persistent expression and activity of eNOS could as well serve as a component to induce long-term effects after LI-SWT. At least, in our study, LI-SWT seems to have sustained effects on the patients’ GFR and albuminuria through follow-up. Ultimately, as indicated by improved perfusion in diabetic foot ulcers, the effect of LI-ESWT could be sustained up to 1 year after LI-SWT treatment [30].
So far, it is only partially established which cellular components transmit the mechanical stimulus associated with LI-SWT. LI-SWT is regarded as a non-specific physical stimulus, and many receptor types and downstream cellular pathways are potentially influenced. Ex vivo studies have identified some mechanosensory complexes in the endothelial cellular membrane that respond to LI-SWT and increase eNOS along with other factors involved in angiogenesis [31]. Other studies revealed that the permeability of the cellular membrane was affected by LI-SWT allowing flow of ribonucleic acid and adenosine triphosphate to the extracellular lumen and interaction of these molecules with various types of receptors [32, 33].
In a recent study, Zhang et al. [34] investigated the effects of LI-SWT in a porcine model of renal artery stenosis. Their study demonstrated restoration of GFR and renal blood flow as well as decreasing proteinuria 4 weeks after LI-SWT was applied. Additionally, as revealed by micro-computed tomography scans, LI-SWT ameliorated the microvascular density in the renal cortex. Compared with the present study, LI-SWT was also administered six times over 3 weeks in the porcine model, but therapy was applied with lower frequency and energy level.
Strengths of our study include the evaluation of the most common side effects of LI-SWT. Furthermore, we implemented chrome-ethylenediaminetetraacetic acid (EDTA) clearance, ABP monitoring and 24-h urine specimens as high-quality investigations in order to study the outcomes of treatment with LI-SWT. Also, patients were followed for 6 months post-treatment with repeated measurements of the outcomes. For baseline characteristics, we used a kidney biopsy for characterization of the patients’ type and degree of kidney disease. At follow-up, we recorded antihypertensive and antidiabetic treatment along with HbA1c and ABP as potential confounding factors. Importantly, there were no changes in the patients’ antihypertensive medical therapy within 3 months after treatment. HbA1c and ABP were generally stable. At 6 months follow-up, antihypertensive therapy had been adjusted in four patients but did not include addition or regulation of angiotensin-converting-enzyme (ACE) inhibitors or angiotensin II (AT2) antagonists. There was no link between adjustment of antihypertensive therapy and improving outcomes. Conversely, we found reduced GFR at 6 months follow-up in two of the patients, which could relate to dysregulated blood pressure.
This study is limited as no control group was applied, and the results did not demonstrate significant improvements of GFR and albuminuria. We argue that there is a progressive loss of kidney function in DN although medical therapy and control of risk factors have improved the prognosis within recent years. In a Danish cohort consisting of patients with DN, it was found that annual GFR decline was 3.3 mL/min/1.73 m2 in patients with type 1 DM and 4.4 mL/min/1.73 m2 in patients with type 2 DM [35, 36]. Seen in this context, our results indicate that LI-SWT could promote restoration of kidney function since GFR was increased in the majority of the patients after the treatment. Another weakness of our study relates to the fact that other diseases than DM, i.e. hypertension, could affect the patients’ renal function. In some cases, the biopsies showed only mild affection of the kidneys due to DM. Indeed, clinical data revealed that 10 out of 14 patients received two or more types of antihypertensive medications excluding ACE inhibitors and AT2 antagonists. In the kidney biopsies, we detected no other specific signs of kidney disease other than DN. However, some vascular lesions in DN might be overlapping with hypertensive nephropathy—i.e. arteriolar amyloidosis that was documented in >80% of the patients. Finally, the number of included patients was relatively low, and it is likely that our study was underpowered for detection of significant effects. Also, due to the small number of patients, it was not statistically feasible to conduct analysis of subgroups.
In relation to LI-SWT, we believe that the intra- and intervariability in performing the treatment is low. The Modulith SLX-2 device is quite easy to operate, and we did not experience problems locating and targeting the patients’ kidneys. The treatment was carried out in a simple way with treatment only at three sites of each kidney (upper, middle and lower part). Due to the kidneys’ movement under respiration, most parts of the kidney parenchyma will be treated using this protocol. However, it was beyond the scope of this study to determine whether the treatment with LI-SWT can be improved. A number of variables may be adjusted in order to optimize LI-SWT for DN—for example, number of treatments, interval between treatments and treatment energy density. In the present study, the treatment protocol was based on previous studies using LI-SWT to improve perfusion and healing of diabetic foot ulcers (Wang 2011, Jeppesen 2016) and was almost identical to the treatment applied to porcine kidney by Zhang et al. [34]
In conclusion, our findings demonstrate LI-SWT as a clinically safe treatment modality for DN. We advocate for randomized studies to determine the effects of LI-SWT on GFR and albuminuria. Other perspectives include studying dose–response relationship and the relevance of LI-SWT in other types of CKD.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
ACKNOWLEDGEMENTS
The authors would like to thank Storz Medical AG for supplying and servicing the Modulith SLX-2 lithotripter throughout the study period. MLS Medical AS is acknowledged for technical assistance for the Modulith SLX-2 lithotripter. Dr Alessandro Conti is thanked for his kind support during the conduct of the study.
FUNDING
Funding was received from the Beckett-Foundation and the A.P. Møller Foundation for the Advancement of Medical Science. No part of the study was influenced by funding organizations.
AUTHORS’ CONTRIBUTIONS
S.M.S.-J., L.L., K.B.Y., C.B., B.L.J. and M.H. designed the study; S.M.S.-J., L.L., K.B.Y., C.B. and N.M. carried out treatments and investigations; S.M.S.-J. analysed the data; S.M.S.-J. and L.L. drafted and revised the article; all authors approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
S.M.S.-J. received a grant from Storz Medical AG during the conduct of the study. M.H. is advisor to Storz Medical AG and has received non-financial support and personal fees from Storz Medical AG. The remaining authors declare they have no conflicts of interest. The results presented in this article have not been published previously in whole or part, except in abstract format at the EAU18 meeting in Copenhagen 2018.
REFERENCES
American Diabetes Association.





Comments