-
PDF
- Split View
-
Views
-
Cite
Cite
Menso J. Nubé, Sanne A.E. Peters, Peter J. Blankestijn, Bernard Canaud, Andrew Davenport, Muriel P.C. Grooteman, Gulay Asci, Francesco Locatelli, Francisco Maduell, Marion Morena, Ercan Ok, Ferran Torres, Michiel L. Bots, on behalf of the HDF Pooling Project investigators, Mortality reduction by post-dilution online-haemodiafiltration: a cause-specific analysis, Nephrology Dialysis Transplantation, Volume 32, Issue 3, March 2017, Pages 548–555, https://doi.org/10.1093/ndt/gfw381
Close - Share Icon Share
Abstract
Background. From an individual participant data (IPD) meta-analysis from four randomized controlled trials comparing haemodialysis (HD) with post-dilution online-haemodiafiltration (ol-HDF), previously it appeared that HDF decreases all-cause mortality by 14% (95% confidence interval 25; 1) and fatal cardiovascular disease (CVD) by 23% (39; 3). Significant differences were not found for fatal infections and sudden death. So far, it is unclear, however, whether the reduced mortality risk of HDF is only due to a decrease in CVD events and if so, which CVD in particular is prevented, if compared with HD.
Methods. The IPD base was used for the present study. Hazard ratios and 95% confidence intervals for cause-specific mortality overall and in thirds of the convection volume were calculated using the Cox proportional hazard regression models. Annualized mortality and numbers needed to treat (NNT) were calculated as well.
Results. Besides 554 patients dying from CVD, fatal infections and sudden death, 215 participants died from ‘other causes’, such as withdrawal from treatment and malignancies. In this group, the mortality risk was comparable between HD and ol-HDF patients, both overall and in thirds of the convection volume. Subdivision of CVD mortality in fatal cardiac, non-cardiac and unclassified CVD showed that ol-HDF was only associated with a lower risk of cardiac casualties [0.64 (0.61; 0.90)]. Annual mortality rates also suggest that the reduction in CVD death is mainly due to a decrease in cardiac fatalities, including both ischaemic heart disease and congestion. Overall, 32 and 75 patients, respectively, need to be treated by high-volume HDF (HV-HDF) to prevent one all-cause and one CVD death, respectively, per year.
Conclusion. The beneficial effect of ol-HDF on all-cause and CVD mortality appears to be mainly due to a reduction in fatal cardiac events, including ischaemic heart disease as well as congestion. In HV-HDF, the NNT to prevent one CVD death is 75 per year.
INTRODUCTION
Compared with the healthy population, haemodialysis (HD) patients have a greatly increased mortality risk [1]. Since retention of uraemic toxins in the middle-molecular weight (MMW) range has been implicated in the poor clinical prospects of these patients, removal by convection through high-permeable (high-flux) dialysers may improve survival. Three large randomized controlled trials (RCT), however, did not demonstrate an overall favourable effect of high-flux dialysers over low-permeable (low-flux) devices, which remove only small compounds by diffusion [2–4]. Yet, in these RCTs, a trend was observed towards a better clinical outcome for selected patient groups, who were treated with high-flux HD. Hence, it is possible that the amount of convective transport, which occurs within the dialyser in high-flux HD (<10 L per session) [5], was too small to obtain a noticeable clinical effect.
To retain the diffusive capacity of HD and augment the convection volume, haemodiafiltration (HDF) was developed [6]. In this modality, plasma water is extracted from the blood, which, however, must be replaced by a sterile endotoxin-free substitution fluid. Originally, replacement fluid was administered in bags, which restricted its volume (<13 L per session) [7] and prevented implementation on a large scale. With the introduction of modern HDF machines and water treatment systems, which allow the online production [online-haemodiafiltration (ol-HDF)] of large amounts of sterile and endotoxin-free substitution fluid [8], much larger convection volumes (>23 L per session) became accessible for everyday clinical practice.
Based on several observational studies [7, 9–11] suggesting a superior clinical outcome for patients who were treated with HDF, three large RCTs, comparing HD with ol-HDF, were conducted [12–14]. Despite inconsistent outcomes of the individual studies, a recently published individual participant data (IPD) analysis, based on these three studies and a fourth not yet published French investigation, indicated that both the all-cause and cardiovascular disease (CVD) mortality risks were significantly reduced by ol-HDF [15]. These results were confirmed in the latest survey of the French REIN Registry [16]. The IPD analysis also showed that the mortality risks for infections and sudden death were not different for HD and ol-HDF patients. Moreover, it appeared that the relative risk reduction in the all-cause and CVD mortality risks was greatest for patients who were treated with high-volume HDF (HV-HDF).
Since in our first IPD analysis the total number of deaths was markedly higher than the summed mortality from CVD, infections and sudden death, a considerable number of patients died from other causes. Hence, it is not quite clear whether the reduction in all-cause mortality is exclusively due to a decline in fatal CVD events or also to a decrease in non-CVD fatalities. In case of a selected reduction in fatal CVD events, the question arises whether the beneficial effect of ol-HDF is caused by a decrease in cardiac or non-cardiac fatalities, or both. If, for example, the risk reduction in all-cause mortality is largely due to a decrease in fatal cardiac events, it might be helpful for prevention and management to know which heart disease in particular benefits from treatment with ol-HDF. Finally, from a public health care perspective, it is also important to know the number of patients needed to be treated (NNT) by ol-HDF to prevent one death. The aim of the present study was therefore to address these issues and to examine the relationship between the effects of ol-HDF versus HD on cause-specific mortality endpoints.
MATERIALS AND METHODS
Study design
For the present analysis, data were used from the IPD base of four large multicentre RCTs, comparing the effects of post-dilution ol-HDF with HD in adult patients [15]. A detailed description of the study designs, patient eligibility criteria and treatment procedures of each of the individual studies has been provided elsewhere [12–14].
Study population, study endpoint and follow-up
Besides the subdivision of all-cause mortality in CVD mortality, fatal infections and sudden death, a group ‘other causes’ was created, including death due to malignancies and patients who discontinued dialysis treatment, as defined within the individual studies. In addition, we repeated the analysis by adding sudden death to the group of CVD mortality [17]. Sudden death was defined as a sudden unexpected fatality occurring within an hour of symptom onset, or un-witnessed, unexpected death in patients thought to be well in the past 24 h. Then we investigated CVD mortality, by splitting up this fraction in ‘all cardiac events’, including myocardial infarction (MI), arrhythmia (AR), ‘chronic heart failure and/or fluid overload’ and non-further specified fatal cardiac events, ‘non-cardiac’ fatalities, including stroke and peripheral arterial disease and ‘unclassified CVD’ mortality, consisting of fatalities that were denoted in the RCTs as CVD, but without any further classification. As congestive heart failure and fluid overload are difficult to distinguish in HD patients, in the present analysis both conditions were brought together under the heading ‘congestion’. Subsequently, we compared the individual cardiac death causes between HD and ol-HDF. The hazard ratios (HRs) for cardiac causes were assessed (i) by calculating the HR of all cardiac causes combined, and (ii) by subsequently removing congestion and (iii) AR, thus ending up with MIs alone. Next, we estimated the annualized all-cause and CVD mortality data per 100 patient-years in both groups and lastly, the patient NNT by ol-HDF to prevent one HD death per year, overall and in the CVD subgroup.
Data analysis
Cox proportional hazard regression models with a random effect for study were used to estimate HR and 95% confidence intervals (CIs) comparing the effect of ol-HDF versus HD on the study endpoints. The dose–response association between achieved convection volume in ol-HDF versus HD and clinical outcomes was examined in thirds of the actual (on-treatment) delivered, 1.73 m2 body surface area-standardized convection volume, and was adjusted for age, sex, albumin, creatinine, history of diabetes and previous CVD. All analyses were performed in the statistical environment R (version 2.15.3). Two-sided P-values and 95% CI were used for statistical inferences. Annual mortality rates per 100 patient-years were calculated by dividing the number of deaths by the total follow-up in years and multiplying by 100. NNTs per year were calculated by the fraction 100/(annualized mortality rate HD − annualized mortality rate ol-HDF).
RESULTS
In Table 1, the demographical and clinical patient characteristics, laboratory data and treatment-related parameters are summarized. During a mean follow-up of 2.5 (interquartile range 1.9–3.0) years, 292 participants died of CVD, 150 of infections, 112 of sudden death and 215 of ‘other causes’, see Table 2.
| . | All (n = 2793) . | HD (n = 1393) . | HDF (n = 1400) . |
|---|---|---|---|
| Patient characteristics | |||
| Females (%) | 1045 (37) | 528 (38) | 517 (37) |
| Age (years) | 64.1 (14.7) | 64.4 (14.8) | 63.8 (14.3) |
| BMI (after dialysis, kg/m2) | 25.2 (4.7) | 25.2 (4.6) | 25.2 (4.9) |
| BSA (m2) | 1.76 (0.22) | 1.77 (0.22) | 1.76 (0.22) |
| Systolic blood pressure (mmHg) | 137.4 (22.4) | 137.5 (22.8) | 137.3 (22.1) |
| Diastolic blood pressure (mmHg) | 73.7 (13.3) | 73.5 (13.6) | 73.9 (13.1) |
| Diabetes mellitus (%) | 814 (30) | 402 (29) | 412 (29) |
| History of CVD (%) | 989 (35) | 499 (36) | 490 (35) |
| Laboratory data | |||
| Haemoglobin (g/dL) | 11.7 (1.6) | 11.7 (1.6) | 11.7 (1.6) |
| PTH (pmol/L) | 312 (145; 774) | 307 (147; 789) | 318 (142; 752) |
| Calcium (mg/dL) | 36.2 (4.4) | 36.0 (4.8) | 36.4 (4.0) |
| Bicarbonate (mmol/L) | 22.2 (3.5) | 22.2 (3.6) | 22.3 (3.5) |
| Creatinine (mg/dL), predialysis | 8.39 (2.57) | 8.42 (2.63) | 8.35 (2.51) |
| Phosphate (mg/dL) | 4.76 (1.53) | 4.71 (1.52) | 4.79 (1.53) |
| β-2-microglobulin (mg/L) | 27.2 (11.6) | 27.7 (11.9) | 26.8 (11.3) |
| Albumin (g/dL) | 3.98 (0.41) | 3.98 (0.41) | 3.98 (0.41) |
| Cholesterol (mg/dL) | 159 (48) | 160 (49) | 158 (47) |
| CRP (mg/L) | 3.48 (0.90; 8.60) | 3.47 (0.89; 8.50) | 3.50 (0.94; 8.70) |
| Treatment-related parameters | |||
| Dialysis vintage (months) | 33 (15; 64) | 34 (15; 65) | 32 (14; 64) |
| Vascular access (AVF) | 2376 (85) | 1175 (84) | 1201 (86) |
| Dialysis single-pool Kt/V | 1.52 (0.31) | 1.50 (0.30) | 1.51 (0.32) |
| Duration of session (min) | 233 (20) | 233 (20) | 233 (20) |
| Blood flow (mL/min) | 337 (66) | 335 (66) | 336 (66) |
| . | All (n = 2793) . | HD (n = 1393) . | HDF (n = 1400) . |
|---|---|---|---|
| Patient characteristics | |||
| Females (%) | 1045 (37) | 528 (38) | 517 (37) |
| Age (years) | 64.1 (14.7) | 64.4 (14.8) | 63.8 (14.3) |
| BMI (after dialysis, kg/m2) | 25.2 (4.7) | 25.2 (4.6) | 25.2 (4.9) |
| BSA (m2) | 1.76 (0.22) | 1.77 (0.22) | 1.76 (0.22) |
| Systolic blood pressure (mmHg) | 137.4 (22.4) | 137.5 (22.8) | 137.3 (22.1) |
| Diastolic blood pressure (mmHg) | 73.7 (13.3) | 73.5 (13.6) | 73.9 (13.1) |
| Diabetes mellitus (%) | 814 (30) | 402 (29) | 412 (29) |
| History of CVD (%) | 989 (35) | 499 (36) | 490 (35) |
| Laboratory data | |||
| Haemoglobin (g/dL) | 11.7 (1.6) | 11.7 (1.6) | 11.7 (1.6) |
| PTH (pmol/L) | 312 (145; 774) | 307 (147; 789) | 318 (142; 752) |
| Calcium (mg/dL) | 36.2 (4.4) | 36.0 (4.8) | 36.4 (4.0) |
| Bicarbonate (mmol/L) | 22.2 (3.5) | 22.2 (3.6) | 22.3 (3.5) |
| Creatinine (mg/dL), predialysis | 8.39 (2.57) | 8.42 (2.63) | 8.35 (2.51) |
| Phosphate (mg/dL) | 4.76 (1.53) | 4.71 (1.52) | 4.79 (1.53) |
| β-2-microglobulin (mg/L) | 27.2 (11.6) | 27.7 (11.9) | 26.8 (11.3) |
| Albumin (g/dL) | 3.98 (0.41) | 3.98 (0.41) | 3.98 (0.41) |
| Cholesterol (mg/dL) | 159 (48) | 160 (49) | 158 (47) |
| CRP (mg/L) | 3.48 (0.90; 8.60) | 3.47 (0.89; 8.50) | 3.50 (0.94; 8.70) |
| Treatment-related parameters | |||
| Dialysis vintage (months) | 33 (15; 64) | 34 (15; 65) | 32 (14; 64) |
| Vascular access (AVF) | 2376 (85) | 1175 (84) | 1201 (86) |
| Dialysis single-pool Kt/V | 1.52 (0.31) | 1.50 (0.30) | 1.51 (0.32) |
| Duration of session (min) | 233 (20) | 233 (20) | 233 (20) |
| Blood flow (mL/min) | 337 (66) | 335 (66) | 336 (66) |
Values are n (%) for categorical variables, and mean (standard deviation) or median (Q1; Q3) for continuous variables.
AVF, arteriovenous fistula; BMI, body mass index; BSA, body surface area; PTH, parathyroid hormone; CRP, C-reactive protein.
| . | All (n = 2793) . | HD (n = 1393) . | HDF (n = 1400) . |
|---|---|---|---|
| Patient characteristics | |||
| Females (%) | 1045 (37) | 528 (38) | 517 (37) |
| Age (years) | 64.1 (14.7) | 64.4 (14.8) | 63.8 (14.3) |
| BMI (after dialysis, kg/m2) | 25.2 (4.7) | 25.2 (4.6) | 25.2 (4.9) |
| BSA (m2) | 1.76 (0.22) | 1.77 (0.22) | 1.76 (0.22) |
| Systolic blood pressure (mmHg) | 137.4 (22.4) | 137.5 (22.8) | 137.3 (22.1) |
| Diastolic blood pressure (mmHg) | 73.7 (13.3) | 73.5 (13.6) | 73.9 (13.1) |
| Diabetes mellitus (%) | 814 (30) | 402 (29) | 412 (29) |
| History of CVD (%) | 989 (35) | 499 (36) | 490 (35) |
| Laboratory data | |||
| Haemoglobin (g/dL) | 11.7 (1.6) | 11.7 (1.6) | 11.7 (1.6) |
| PTH (pmol/L) | 312 (145; 774) | 307 (147; 789) | 318 (142; 752) |
| Calcium (mg/dL) | 36.2 (4.4) | 36.0 (4.8) | 36.4 (4.0) |
| Bicarbonate (mmol/L) | 22.2 (3.5) | 22.2 (3.6) | 22.3 (3.5) |
| Creatinine (mg/dL), predialysis | 8.39 (2.57) | 8.42 (2.63) | 8.35 (2.51) |
| Phosphate (mg/dL) | 4.76 (1.53) | 4.71 (1.52) | 4.79 (1.53) |
| β-2-microglobulin (mg/L) | 27.2 (11.6) | 27.7 (11.9) | 26.8 (11.3) |
| Albumin (g/dL) | 3.98 (0.41) | 3.98 (0.41) | 3.98 (0.41) |
| Cholesterol (mg/dL) | 159 (48) | 160 (49) | 158 (47) |
| CRP (mg/L) | 3.48 (0.90; 8.60) | 3.47 (0.89; 8.50) | 3.50 (0.94; 8.70) |
| Treatment-related parameters | |||
| Dialysis vintage (months) | 33 (15; 64) | 34 (15; 65) | 32 (14; 64) |
| Vascular access (AVF) | 2376 (85) | 1175 (84) | 1201 (86) |
| Dialysis single-pool Kt/V | 1.52 (0.31) | 1.50 (0.30) | 1.51 (0.32) |
| Duration of session (min) | 233 (20) | 233 (20) | 233 (20) |
| Blood flow (mL/min) | 337 (66) | 335 (66) | 336 (66) |
| . | All (n = 2793) . | HD (n = 1393) . | HDF (n = 1400) . |
|---|---|---|---|
| Patient characteristics | |||
| Females (%) | 1045 (37) | 528 (38) | 517 (37) |
| Age (years) | 64.1 (14.7) | 64.4 (14.8) | 63.8 (14.3) |
| BMI (after dialysis, kg/m2) | 25.2 (4.7) | 25.2 (4.6) | 25.2 (4.9) |
| BSA (m2) | 1.76 (0.22) | 1.77 (0.22) | 1.76 (0.22) |
| Systolic blood pressure (mmHg) | 137.4 (22.4) | 137.5 (22.8) | 137.3 (22.1) |
| Diastolic blood pressure (mmHg) | 73.7 (13.3) | 73.5 (13.6) | 73.9 (13.1) |
| Diabetes mellitus (%) | 814 (30) | 402 (29) | 412 (29) |
| History of CVD (%) | 989 (35) | 499 (36) | 490 (35) |
| Laboratory data | |||
| Haemoglobin (g/dL) | 11.7 (1.6) | 11.7 (1.6) | 11.7 (1.6) |
| PTH (pmol/L) | 312 (145; 774) | 307 (147; 789) | 318 (142; 752) |
| Calcium (mg/dL) | 36.2 (4.4) | 36.0 (4.8) | 36.4 (4.0) |
| Bicarbonate (mmol/L) | 22.2 (3.5) | 22.2 (3.6) | 22.3 (3.5) |
| Creatinine (mg/dL), predialysis | 8.39 (2.57) | 8.42 (2.63) | 8.35 (2.51) |
| Phosphate (mg/dL) | 4.76 (1.53) | 4.71 (1.52) | 4.79 (1.53) |
| β-2-microglobulin (mg/L) | 27.2 (11.6) | 27.7 (11.9) | 26.8 (11.3) |
| Albumin (g/dL) | 3.98 (0.41) | 3.98 (0.41) | 3.98 (0.41) |
| Cholesterol (mg/dL) | 159 (48) | 160 (49) | 158 (47) |
| CRP (mg/L) | 3.48 (0.90; 8.60) | 3.47 (0.89; 8.50) | 3.50 (0.94; 8.70) |
| Treatment-related parameters | |||
| Dialysis vintage (months) | 33 (15; 64) | 34 (15; 65) | 32 (14; 64) |
| Vascular access (AVF) | 2376 (85) | 1175 (84) | 1201 (86) |
| Dialysis single-pool Kt/V | 1.52 (0.31) | 1.50 (0.30) | 1.51 (0.32) |
| Duration of session (min) | 233 (20) | 233 (20) | 233 (20) |
| Blood flow (mL/min) | 337 (66) | 335 (66) | 336 (66) |
Values are n (%) for categorical variables, and mean (standard deviation) or median (Q1; Q3) for continuous variables.
AVF, arteriovenous fistula; BMI, body mass index; BSA, body surface area; PTH, parathyroid hormone; CRP, C-reactive protein.
Ol-HDF and all-cause and cause-specific mortality
In Table 2, both the absolute patient numbers and risk ratios are presented. As reported before [15], ol-HDF reduced the risk of all-cause and CVD mortality by 14% [95% confidence interval (CI) 25; 1] and 23% (95% CI 39; 3), respectively. In absolute numbers, CVD death accounted for 71% (36/51) of the difference in all-cause mortality between the two groups. The number of fatalities due to infections, sudden death and the combined group ‘other causes’ was comparable. The conclusions for CVD mortality did not materially alter when the group ‘sudden death’ was included in this group.
Absolute number of deaths in the HD and ol-HDF groups and differences between groups; HR with 95% CI in the complete HDF cohort and in thirds of the convection volume
| . | . | . | . | . | Ol-HDF: BSA-adjusted convection volume (L per session) . | |||
|---|---|---|---|---|---|---|---|---|
| . | All . | HD . | HDF . | HD-HDF . | Mean 22 . | <19 . | 19–23 . | >23 . |
| All-causes** | 769 | 410 | 359 | 51 | 0.86 (0.75; 0.99) | 0.83 (0.66; 1.03) | 0.93 (0.75; 1.16) | 0.78 (0.62; 0.98) |
| All CVD*** | 292 | 164 | 128 | 36 | 0.77 (0.61; 0.97) | 0.92 (0.65; 1.30) | 0.71 (0.49; 1.03) | 0.69 (0.47; 1.00) |
| Cardiac** | 135 | 81 | 54 | 27 | 0.64 (0.45; 0.90) | 0.95 (0.65; 1.39) | 0.69 (0.46; 1.04) | 0.70 (0.47; 1.05) |
| Non-cardiac* | 80 | 42 | 38 | 4 | 0.92 (0.60; 1.43) | 0.64 (0.26; 1.55) | 1.22 (0.67; 2.23) | 0.86 (0.47; 1.78) |
| Unclassified** | 77 | 41 | 36 | 5 | 0.90 (0.58; 1.42) | 0.82 (0.25; 1.50 | 1.20 (0.66; 2.20) | 0.85 (0.46; 1.55) |
| INFECTIONS* | 150 | 77 | 73 | 4 | 0.94 (0.68; 1.30) | 1.50 (0.92; 2.46) | 0.97 (0.54; 1.74) | 0.62 (0.32; 1.19) |
| SUDDEN death** | 112 | 56 | 56 | 0 | 0.99 (0.68; 1.43) | 1.09 (0.69; 1.74) | 1.04 (0.63; 1.70) | 0.69 (0.39; 1.20) |
| OTHER causes* | 215 | 113 | 102 | 9 | 0.88 (0.68; 1.13) | 0.67 (0.45; 1.01) | 1.13 (0.77; 1,67) | 0.87 (0.59; 1.30) |
| CVD including sudden death** | 404 | 220 | 184 | 36 | 0.81 (0.65;1.00) | 0.93 (0.66;1.30) | 0.82 (0.59;1.14) | 0.72 (0.51;1.00) |
| . | . | . | . | . | Ol-HDF: BSA-adjusted convection volume (L per session) . | |||
|---|---|---|---|---|---|---|---|---|
| . | All . | HD . | HDF . | HD-HDF . | Mean 22 . | <19 . | 19–23 . | >23 . |
| All-causes** | 769 | 410 | 359 | 51 | 0.86 (0.75; 0.99) | 0.83 (0.66; 1.03) | 0.93 (0.75; 1.16) | 0.78 (0.62; 0.98) |
| All CVD*** | 292 | 164 | 128 | 36 | 0.77 (0.61; 0.97) | 0.92 (0.65; 1.30) | 0.71 (0.49; 1.03) | 0.69 (0.47; 1.00) |
| Cardiac** | 135 | 81 | 54 | 27 | 0.64 (0.45; 0.90) | 0.95 (0.65; 1.39) | 0.69 (0.46; 1.04) | 0.70 (0.47; 1.05) |
| Non-cardiac* | 80 | 42 | 38 | 4 | 0.92 (0.60; 1.43) | 0.64 (0.26; 1.55) | 1.22 (0.67; 2.23) | 0.86 (0.47; 1.78) |
| Unclassified** | 77 | 41 | 36 | 5 | 0.90 (0.58; 1.42) | 0.82 (0.25; 1.50 | 1.20 (0.66; 2.20) | 0.85 (0.46; 1.55) |
| INFECTIONS* | 150 | 77 | 73 | 4 | 0.94 (0.68; 1.30) | 1.50 (0.92; 2.46) | 0.97 (0.54; 1.74) | 0.62 (0.32; 1.19) |
| SUDDEN death** | 112 | 56 | 56 | 0 | 0.99 (0.68; 1.43) | 1.09 (0.69; 1.74) | 1.04 (0.63; 1.70) | 0.69 (0.39; 1.20) |
| OTHER causes* | 215 | 113 | 102 | 9 | 0.88 (0.68; 1.13) | 0.67 (0.45; 1.01) | 1.13 (0.77; 1,67) | 0.87 (0.59; 1.30) |
| CVD including sudden death** | 404 | 220 | 184 | 36 | 0.81 (0.65;1.00) | 0.93 (0.66;1.30) | 0.82 (0.59;1.14) | 0.72 (0.51;1.00) |
The HD group is used as reference.
BSA, body surface area. Cardiac CVD includes: MI, AR and congestion; non-cardiac CVD includes: stroke, peripheral arterial disease; unclassified includes: CVD, but without any further specificity. P for trend *NS, **0.02–0.05, ***0.07. Part of this table was published in [15].
Absolute number of deaths in the HD and ol-HDF groups and differences between groups; HR with 95% CI in the complete HDF cohort and in thirds of the convection volume
| . | . | . | . | . | Ol-HDF: BSA-adjusted convection volume (L per session) . | |||
|---|---|---|---|---|---|---|---|---|
| . | All . | HD . | HDF . | HD-HDF . | Mean 22 . | <19 . | 19–23 . | >23 . |
| All-causes** | 769 | 410 | 359 | 51 | 0.86 (0.75; 0.99) | 0.83 (0.66; 1.03) | 0.93 (0.75; 1.16) | 0.78 (0.62; 0.98) |
| All CVD*** | 292 | 164 | 128 | 36 | 0.77 (0.61; 0.97) | 0.92 (0.65; 1.30) | 0.71 (0.49; 1.03) | 0.69 (0.47; 1.00) |
| Cardiac** | 135 | 81 | 54 | 27 | 0.64 (0.45; 0.90) | 0.95 (0.65; 1.39) | 0.69 (0.46; 1.04) | 0.70 (0.47; 1.05) |
| Non-cardiac* | 80 | 42 | 38 | 4 | 0.92 (0.60; 1.43) | 0.64 (0.26; 1.55) | 1.22 (0.67; 2.23) | 0.86 (0.47; 1.78) |
| Unclassified** | 77 | 41 | 36 | 5 | 0.90 (0.58; 1.42) | 0.82 (0.25; 1.50 | 1.20 (0.66; 2.20) | 0.85 (0.46; 1.55) |
| INFECTIONS* | 150 | 77 | 73 | 4 | 0.94 (0.68; 1.30) | 1.50 (0.92; 2.46) | 0.97 (0.54; 1.74) | 0.62 (0.32; 1.19) |
| SUDDEN death** | 112 | 56 | 56 | 0 | 0.99 (0.68; 1.43) | 1.09 (0.69; 1.74) | 1.04 (0.63; 1.70) | 0.69 (0.39; 1.20) |
| OTHER causes* | 215 | 113 | 102 | 9 | 0.88 (0.68; 1.13) | 0.67 (0.45; 1.01) | 1.13 (0.77; 1,67) | 0.87 (0.59; 1.30) |
| CVD including sudden death** | 404 | 220 | 184 | 36 | 0.81 (0.65;1.00) | 0.93 (0.66;1.30) | 0.82 (0.59;1.14) | 0.72 (0.51;1.00) |
| . | . | . | . | . | Ol-HDF: BSA-adjusted convection volume (L per session) . | |||
|---|---|---|---|---|---|---|---|---|
| . | All . | HD . | HDF . | HD-HDF . | Mean 22 . | <19 . | 19–23 . | >23 . |
| All-causes** | 769 | 410 | 359 | 51 | 0.86 (0.75; 0.99) | 0.83 (0.66; 1.03) | 0.93 (0.75; 1.16) | 0.78 (0.62; 0.98) |
| All CVD*** | 292 | 164 | 128 | 36 | 0.77 (0.61; 0.97) | 0.92 (0.65; 1.30) | 0.71 (0.49; 1.03) | 0.69 (0.47; 1.00) |
| Cardiac** | 135 | 81 | 54 | 27 | 0.64 (0.45; 0.90) | 0.95 (0.65; 1.39) | 0.69 (0.46; 1.04) | 0.70 (0.47; 1.05) |
| Non-cardiac* | 80 | 42 | 38 | 4 | 0.92 (0.60; 1.43) | 0.64 (0.26; 1.55) | 1.22 (0.67; 2.23) | 0.86 (0.47; 1.78) |
| Unclassified** | 77 | 41 | 36 | 5 | 0.90 (0.58; 1.42) | 0.82 (0.25; 1.50 | 1.20 (0.66; 2.20) | 0.85 (0.46; 1.55) |
| INFECTIONS* | 150 | 77 | 73 | 4 | 0.94 (0.68; 1.30) | 1.50 (0.92; 2.46) | 0.97 (0.54; 1.74) | 0.62 (0.32; 1.19) |
| SUDDEN death** | 112 | 56 | 56 | 0 | 0.99 (0.68; 1.43) | 1.09 (0.69; 1.74) | 1.04 (0.63; 1.70) | 0.69 (0.39; 1.20) |
| OTHER causes* | 215 | 113 | 102 | 9 | 0.88 (0.68; 1.13) | 0.67 (0.45; 1.01) | 1.13 (0.77; 1,67) | 0.87 (0.59; 1.30) |
| CVD including sudden death** | 404 | 220 | 184 | 36 | 0.81 (0.65;1.00) | 0.93 (0.66;1.30) | 0.82 (0.59;1.14) | 0.72 (0.51;1.00) |
The HD group is used as reference.
BSA, body surface area. Cardiac CVD includes: MI, AR and congestion; non-cardiac CVD includes: stroke, peripheral arterial disease; unclassified includes: CVD, but without any further specificity. P for trend *NS, **0.02–0.05, ***0.07. Part of this table was published in [15].
Subdivision in thirds of the convection volume showed a distinct pattern for all-cause mortality, CVD mortality and sudden death: the larger the convection volume, the lower the associated risk (P for trend, respectively, 0.02, 0.07, 0.04). No such pattern was observed for the groups consisting of fatal infections and ‘other causes’ of death (P, respectively, 0.15 and 0.74).
Ol-HDF and CVD mortality
As also shown in Table 2, ol-HDF was associated with a lower risk of ‘cardiac’ CVD fatalities [HR 0.64 (0.61; 0.90), P = 0.01], but not of ‘non-cardiac’ CVD mortality [HR 0.92 (0.60; 1.43), P = 0.73] and ‘unclassified’ CVD mortality [HR 0.90 (0.58; 1.45), P = 0.65]. In absolute numbers, ‘cardiac’ death accounted for 77% (27/36) of the difference in total CVD mortality between the HD and ol-HDF groups.
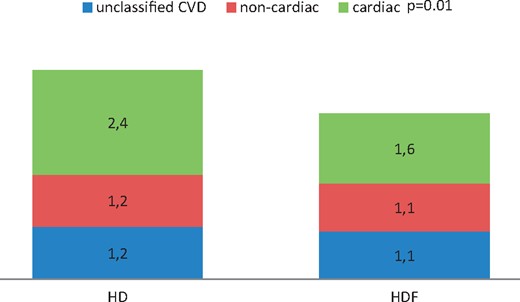
Annualized CVD mortality per 100 patient-years in the HD and ol-HDF groups. The numbers in boxes represent fatal CVD events per 100 patient-years. The difference in fatal cardiac events between HD and HDF is significant (P = 0.01).
Annualized all-cause and CVD mortality/100 patient-years and NNT/year in the complete ol-HDF cohort and in thirds of the convection volume
| . | HD . | Ol-HDF: BSA-adjusted convection volume (L per session) . | |||
|---|---|---|---|---|---|
| . | . | Mean 22 . | <19 . | 19–23 . | >23 . |
| All-cause/100 PY | 12.10 | 10.45 | 10.94 | 10.78 | 8.96 |
| CVD/100 PY | 4.84 | 3.73 | 4.28 | 3.66 | 3.51 |
| NNT/year: overall/CVD | n.a. | 61/90 | 86/178 | 75/84 | 32/75 |
| . | HD . | Ol-HDF: BSA-adjusted convection volume (L per session) . | |||
|---|---|---|---|---|---|
| . | . | Mean 22 . | <19 . | 19–23 . | >23 . |
| All-cause/100 PY | 12.10 | 10.45 | 10.94 | 10.78 | 8.96 |
| CVD/100 PY | 4.84 | 3.73 | 4.28 | 3.66 | 3.51 |
| NNT/year: overall/CVD | n.a. | 61/90 | 86/178 | 75/84 | 32/75 |
The HD group is used as reference
BSA, body surface area; PY, patient-years.
Annualized all-cause and CVD mortality/100 patient-years and NNT/year in the complete ol-HDF cohort and in thirds of the convection volume
| . | HD . | Ol-HDF: BSA-adjusted convection volume (L per session) . | |||
|---|---|---|---|---|---|
| . | . | Mean 22 . | <19 . | 19–23 . | >23 . |
| All-cause/100 PY | 12.10 | 10.45 | 10.94 | 10.78 | 8.96 |
| CVD/100 PY | 4.84 | 3.73 | 4.28 | 3.66 | 3.51 |
| NNT/year: overall/CVD | n.a. | 61/90 | 86/178 | 75/84 | 32/75 |
| . | HD . | Ol-HDF: BSA-adjusted convection volume (L per session) . | |||
|---|---|---|---|---|---|
| . | . | Mean 22 . | <19 . | 19–23 . | >23 . |
| All-cause/100 PY | 12.10 | 10.45 | 10.94 | 10.78 | 8.96 |
| CVD/100 PY | 4.84 | 3.73 | 4.28 | 3.66 | 3.51 |
| NNT/year: overall/CVD | n.a. | 61/90 | 86/178 | 75/84 | 32/75 |
The HD group is used as reference
BSA, body surface area; PY, patient-years.
Ol-HDF and pure ‘cardiac’ mortality
In Table 4, the absolute numbers and HRs (95% CI) are shown of the subgroups ‘all cardiac events’, ‘MI and AR’, and ‘MI alone’. ‘All cardiac events’ consisted of 38 patients with MI, 11 with AR, 31 with congestion and 2 patients with a non-classified cardiac death in the HD group. In the HDF group, these figures were 24, 5, 23 and 1, respectively. Thus, 80/82 in the subgroups ‘all cardiac events’ consisted of HD patients with MI, AR and congestion versus 52/53 in the HDF group. As for pure cardiac death, the lowest HR [0.58 (0.36; 0.91)], and hence a 42% mortality reduction, was found for the joint diagnoses MI and AR.
Absolute number of cardiac deaths in the HD and ol-HDF groups and differences between groups; HR and 95% CI for pure cardiac CVD mortality
| Cardiac cause . | HD . | HDF . | HD-HDF . | HR (95% CI) . |
|---|---|---|---|---|
| All cardiac events | 82 | 53 | 27 | 0.64 (0.45; 0.90) |
| MI, AR | 49 | 29 | 20 | 0.58 (0.36; 0.91) |
| MI | 38 | 24 | 14 | 0.61 (0.37; 1.02) |
| Cardiac cause . | HD . | HDF . | HD-HDF . | HR (95% CI) . |
|---|---|---|---|---|
| All cardiac events | 82 | 53 | 27 | 0.64 (0.45; 0.90) |
| MI, AR | 49 | 29 | 20 | 0.58 (0.36; 0.91) |
| MI | 38 | 24 | 14 | 0.61 (0.37; 1.02) |
The HD group is used as reference
Adjusted for age, sex, albumin, creatinine, history of CVD, history of diabetes.
All cardiac events includes MI, AR and congestion in both groups plus two non-classified cardiac events in the HD group, and one in the HDF group. For the absolute numbers of individual diagnoses, see text.
Absolute number of cardiac deaths in the HD and ol-HDF groups and differences between groups; HR and 95% CI for pure cardiac CVD mortality
| Cardiac cause . | HD . | HDF . | HD-HDF . | HR (95% CI) . |
|---|---|---|---|---|
| All cardiac events | 82 | 53 | 27 | 0.64 (0.45; 0.90) |
| MI, AR | 49 | 29 | 20 | 0.58 (0.36; 0.91) |
| MI | 38 | 24 | 14 | 0.61 (0.37; 1.02) |
| Cardiac cause . | HD . | HDF . | HD-HDF . | HR (95% CI) . |
|---|---|---|---|---|
| All cardiac events | 82 | 53 | 27 | 0.64 (0.45; 0.90) |
| MI, AR | 49 | 29 | 20 | 0.58 (0.36; 0.91) |
| MI | 38 | 24 | 14 | 0.61 (0.37; 1.02) |
The HD group is used as reference
Adjusted for age, sex, albumin, creatinine, history of CVD, history of diabetes.
All cardiac events includes MI, AR and congestion in both groups plus two non-classified cardiac events in the HD group, and one in the HDF group. For the absolute numbers of individual diagnoses, see text.
Ol-HDF and NNT in thirds of the convection volume
Note that the NNTs are presented per year. To prevent one all-cause death or one fatal CVD, 61 and 90 patients, respectively, need to be treated by ol-HDF. These figures are 32 and 75, respectively, in HV-HDF (Table 3).
DISCUSSION
Previously we reported that post-dilution ol-HDF reduces the all-cause mortality risk of dialysis patients by 14% (95% CI 25; 1) and the CVD mortality risk by 23% (95% CI 39; 3). These data appeared even more pronounced [22% (95% CI 38; 2)] and 31% (53; 0), respectively] when high convection volumes are applied, i.e. HV-HDF [15].
What this study adds
In the present extended analysis, we show first, that the beneficial effect of ol-HDF on survival indeed was most prominent for fatal CVD events. Other causes, including a combined group ‘others causes’ consisting of 215 fatalities of various diagnoses such as withdrawal from treatment and malignancies, did not differ between HD and ol-HDF patients. In absolute numbers, 71% of the lower all-cause mortality was due to a reduction in fatal CVD. Secondly, the risk of fatal pure cardiac CVD differed significantly between HD and ol-HDF, while the risk of non-cardiac CVD and un-classified CVD was comparable. In absolute numbers, 77% of the lower CVD mortality appeared to result from a reduction in cardiac deaths. Thirdly, considering only fatal heart diseases, it appeared that the associated risk reduction was not caused by a selective decrease in just one particular fatal heart disease, but more likely by a decrease in both ischaemic heart disease and congestion. Fourthly, our analysis showed that 32 and 75 patients, respectively, need to be treated by HV-HDF to prevent one all-cause and one CVD death, respectively, per year. For comparison, the NNT to prevent one CVD death by anti-hypertensive treatment in low-risk patients with mild hypertension has been estimated to be 82 per 10 years or 820 per year [18].
In our prior analysis, sudden death was considered separately because not only classical CVD events, such as cardiac ARs and severe stroke, may be underlying causes of death but also other diagnoses, including pulmonary embolism and septicaemia. In this respect, it should be noted, however, that, while several arrhythmic triggers, such as large extracellular volume changes, electrolyte disturbances and autonomic imbalance in chronic kidney disease (CKD) patients with uraemic cardiomyopathy, may contribute to the risk of sudden cardiac death (SCD), prevention with implantable cardioverter defibrillators (ICD) did not show unequivocal beneficial results. Although two meta-analyses [19, 20] and a recent large propensity-matched mortality analysis [21] suggested a survival advantage of ICD implantation in patients with mild CKD, benefit could not be demonstrated in advanced CKD and dialysis patients. Based on these data, it was speculated that SCD in the latter groups of patients is resistant to the effects of ICD. Alternatively, however, it seems possible that sudden death in CKD patients is caused less frequently by lethal ARs, as suggested in several papers [22, 23], but relatively more often by non-direct cardiac causes, such as severe stroke [13]. Unfortunately, scarce data are available on the reasons for sudden death in dialysis patients [24]. As shown in our study, the risk of sudden death was similar for HD and ol-HDF patients. Since sudden death in the general population is caused mainly by coronary heart disease-associated causes [17] and should hence be considered SCD, in a secondary analysis we included patients who were classified as ‘sudden death’ in the group ‘fatal CVD’. This modification did not materially change our main conclusions as outlined above.
Importance of ol-HDF on convective transport
Why is ol-HDF associated with an improved outcome when compared with HD? As mentioned before, retention of MMW and protein-bound uraemic toxins has been associated with morbidity and mortality in CKD patients [25, 26]. Until now, however, no specific uraemic toxin has been identified that is exclusively responsible for the poor clinical prospects of these patients. At this point, it might be noteworthy to mention that the levels of fibroblast growth factor 23, which are massively increased in patients with advanced CKD and correlate with left ventricular hypertrophy [27] and death [28], are highly efficiently removed by HDF [29]. Hence, lowering of this substance may favourably influence the clinical course in these patients. So far, however, definite proof is lacking that improvement of the ‘milieu intérieur’ will lead to a better clinical outcome. Yet, enhanced removal of MMW and other uraemic toxins by an augmented convective transport is an appealing explanation for the beneficial effects of ol-HDF on survival.
Importance of ol-HDF on intra-dialytic haemodynamic stability
Alternatively, HDF may improve intra-dialytic haemodynamic instability [30, 31], which is the most important acute CVD complication in intermittent dialysis therapies and is related to end-organ ischaemia and mortality [32]. Since micro-circulatory dysfunction is a common feature in end-stage kidney disease patients [33], intradialytic hypotension (IDH) may further compromise the perfusion and functioning of vital organs during treatment. Indeed, well-designed studies have shown that IDH contributes to reversible regional myocardial stunning [34, 35], splanchnic hypoperfusion and endotoxaemia [36], as well as cerebral dysfunctioning [37]. In studies comparing HD with HDF, IDH was mitigated or even absent during HDF [38]. Interestingly, when cooled dialysate was used in HD, the effect on IDH was comparable to HDF [39]. Thus, substantial evidence exists for an important effect of thermal balance as a contributing factor for the improved haemodynamic stability during HDF. Therefore, apart from enhanced uraemic toxin removal by convective transport, HDF may preserve vital organ functioning by a superior intra-dialytic haemodynamic stability [40].
Notably, the results of this analysis were obtained despite the potential negative role of high-flow fistula on cardiac performance [41]. Previously [42], we described a mean blood flow of 387 mL/min in HV-HDF (versus 335 mL/min in the HD group), which was associated with the lowest mortality risk [15]. In this respect, it is noteworthy to refer to an echocardiographic evaluation in 326 CONTRAST participants. From this analysis it appeared that the functional and structural changes over time of the left ventricle that occurred in the group of HD patients were mitigated or virtually absent in patients who were treated with ol-HDF [43].
Is the reduced risk of cardiac mortality dependent on the convection volume?
While the mechanism(s) are uncertain, the beneficial effect of ol-HDF on mortality appears especially pronounced in HV-HDF [15]. Therefore, this extended cause-specific analysis was also performed in thirds of the convection volume. Apart from a significant trend for all-cause, CVD and pure cardiac mortality, a dose–response relationship was also observed for unclassified CVD mortality and sudden death: the higher the convection volume, the lower the associated risk. No such pattern was observed for fatal infections and the group consisting of ‘other causes’ of death. Assuming that a large proportion of the mortality in the groups ‘sudden death’ and ‘non-classified CVD’ originates from the heart as well, it appears that the beneficial effect of a high convection volume is indeed almost exclusively due to a lower ‘cardiac’ mortality risk.
Strengths and limitations
The strength of this study is the concise and meticulous data collection from four recent RCTs, as described previously [15]. As a consequence, informative censoring, due to transplantation or withdrawal alive from treatment, is negligible. As such, this IPD meta-analysis is unique, and quite different from meta-analyses based on aggregated patient data [44–46]. By contrast, our IPD meta-analysis cannot correct for selective inclusion in the individual RCTs, which is obviously a limitation of this study. In addition, we did not calculate changes over time in ultrafiltration rate, Kt/V, bicarbonate and Mg levels, which may be implicated in the life expectancy of our patients [47]. However, since not all these parameters are available over time in the individual studies and the association of these changes on outcome was not the goal of the present study, we refrained from such an analysis. Other limitations are the differences between trials in terms of study design and methodology, which we accounted for as much as possible in our statistical models. Some jargon, however, such as ‘unclassified’ CVD, which is already hard to interpret in one multicentre RCT, is even more delicate when four RCTs from different countries are combined. Furthermore, conclusions on the effects of different convection volumes on mortality are derived from post hoc analyses and should hence be considered with caution. Finally, the small absolute number of fatal ‘cardiac’ CVD events and hence the limited absolute difference between the study groups prevents firm conclusions on the protective effects of treatment with ol-HDF.
CONCLUSIONS
The most important finding of this large-scale IPD meta-analysis is the predominant risk reduction of fatal CVD in post-dilution ol-HDF. Other causes of death, including fatal infections and a group consisting of ‘other causes’ of death, such as withdrawal from treatment and malignancies, did not materially differ between HD and ol-HDF. The second most important outcome of this study is the finding that the risk of fatal non-cardiac CVD events, including stroke and peripheral artery disease, did not differ, while ol-HDF was associated with a lower risk of ‘cardiac’ deaths. The beneficial effect of HV-HDF appears especially apparent for this type of fatality. A lower risk of ischaemic heart disease, AR and congestion may play a role. Interestingly, only 32 patients need to be converted from HD to HV-HDF to prevent one death from all-causes and 75 to prevent one CVD death per year.
ACKNOWLEDGEMENTS
The HDF Pooling Project investigators:
ESHOL investigators: Francisco Maduell, Francesc Moreso, Mercedes Pons, Rosa Ramos, Josep Mora-Macià, Jordi Carreras, Jordi Soler, Ferran Torres, Josep M. Campistol, Alberto Martinez-Castelao, M. Pons, B. Insensé, C. Perez and T. Feliz (CETIRSA, Barcelona); R. Ramos, M. Barbetta and C. Soto (Hospital San Antonio Abad, Vilanova i la Geltru); J. Mora, A. Juan and O. Ibrik (Fresenius Medical Care, Granollers); A. Foraster and J. Carreras (DiaverumBaix Llobregat, Hospitalet); F. Moreso, J. Nin and A. Fernández (Fresenius Medical Care, Hospitalet); J. Soler, M. Arruche, C. Sánchez and J. Vidiella (Fresenius Medical Care, Reus); F. Barbosa, M. Chiné and S. Hurtado (Fresenius Medical Care Diagonal, Barcelona); J. Llibre, A. Ruiz, M. Serra, M. Salvó and T. Poyuelo (CETIRSA, Terrassa); F. Maduell, M. Carrera, N. Fontseré, M. Arias and Josep M. Campistol (Hospital Clínic, Barcelona); A. Merín and L. Ribera (FreseniusMedicalCare Julio Verne, Barcelona); J.M. Galceran, J. Mòdol, E. Moliner and A. Ramirez (Fundació Althaia, Manresa); J. Aguilera and M. Alvarez (Hospital Santa Tecla, Tarragona); B. de la Torre and M. Molera (Diaverum Bonanova, Barcelona); J. Casellas and G. Martín (Diaverum IHB, Barcelona); E. Andres and E. Coll (Fundació Puigvert, Barcelona); M. Valles and C. Martínez (Hospital Josep Trueta, Girona); E. Castellote (Hospital General, Vic); J.M. Casals, J. Gabàs and M. Romero (Diaverum, Mataró); A. Martinez-Castelao and X. Fulladosa (Hospital Universitari Bellvitge, Hospitalet); M. Ramirez-Arellano and M. Fulquet (Hospital de Terrassa); A. Pelegrí, M. el Manouari and N. Ramos (Diaverum Verge de Montserrat, Santa Coloma); J. Bartolomé (Centre Secretari Coloma, Barcelona); R. Sans (Hospital de Figueres); E. Fernández and F. Sarró (Hospital Arnau de Vilanova, Lleida); T. Compte (Hospital Santa Creu, Tortosa); F. Marco and R. Mauri (Diaverum Nephros, Barcelona); J. Bronsoms (Clínica Girona), J.A. Arnaiz, H. Beleta and A. Pejenaute (UASP Farmacología Clínica, Hospital Clínic Barcelona); and F. Torres, J. Ríos and J. Lara (Biostatistics Unit, School of Medicine, Universitat Autònoma de Barcelona).
CONTRAST investigators: executive committee, The Netherlands: P.J. Blankestijn, M.P.C. Grooteman, M.J. Nubé, P.M. ter Wee, M.L. Bots; M.A. van den Dorpel. Canada: Georges-L. Dumont Regional Hospital, Moncton: M. Dorval; CHUM St Luc Hospital, Montréal: R. Lévesque. The Netherlands: Academic Medical Center, Amsterdam: M.G. Koopman; Catharina Hospital, Eindhoven: C.J.A.M. Konings; Dialysis Clinic Noord, Beilen: W.P. Haanstra; Dianet Dialysis Centers, Utrecht: M. Kooistra and B. van Jaarsveld; Fransiscus Hospital, Roosendaal: T. Noordzij; Gelderse Vallei Hospital, Ede: G.W. Feith; Groene Hart Hospital, Gouda: H.G. Peltenburg; Haga Hospital, The Hague: M. van Buren; Isala Clinics, Zwolle: J.J.G. Offerman; Jeroen Bosch Hospital, Hertogenbosch: E.K. Hoogeveen; Maasland Hospital, Sittard: F. de Heer; Maasstad Hospital, Rotterdam: P.J. van de Ven; Martini Hospital, Groningen: T.K. Kremer Hovinga; Medical Center Alkmaar: W.A. Bax; Onze Lieve Vrouwe Gasthuis, Amsterdam: J.O. Groeneveld; Oosterschelde Hospital, Goes: A.T.J. Lavrijssen; Rijnland Hospital, Leiderdorp: A.M. Schrander-Van der Meer; Rijnstate Hospital, Arnhem: L.J.M. Reichert; Slingeland Hospital, Doetinchem: J. Huussen; St Elisabeth Hospital, Tilburg: P.L. Rensma; St Fransiscus Gasthuis, Rotterdam: Y. Schrama; University Medical Center St Radboud, Nijmegen: H.W. van Hamersvelt; University Medical Center Utrecht, Utrecht: W.H. Boer; VieCuri Medical Center, Venlo: W.H. van Kuijk; VU University Medical Center, Amsterdam: M.G. Vervloet; Zeeuws-Vlaanderen Hospital, Terneuzen: I.M.P.M.J. Wauters. Norway: Haukeland University Hospital, Bergen: I. Sekse.
Turkish HDF study investigators: Ercan Ok, Gulay Asci, Huseyin Toz, Ebru Sevinc Ok, Fatih Kircelli, Mumtaz Yilmaz, Ender Hur, Meltem Sezis Demirci, Cenk Demirci, Soner Duman, Ali Basci, Siddig Momin Adam, Ismet Onder Isik, Murat Zengin, Gultekin Suleymanlar, Mehmet Emin Yilmaz, Mehmet Ozkahya Pinar Ergin, Alfert Sagdic, Erkan Kayali, Can Boydak, Taskin Colak, Sihli Caliskan, Hakan Kaplan, Hasibe Ulas, Sait Kirbiyik, Hakan Berktas, Necati Dilbaz
French HDF study investigators: Bernard Canaud, Jean-Paul Cristol, Marion Morena, Hélène Leray-Moragues, Leïla Chenine, Marie-Christine Picot, Audrey Jaussent, Claire Belloc, Mélodie Lagarrigue (University Hospital Center, Montpellier), Lotfi Chalabi (AIDER, Montpellier), Alain Debure, Messaoud Ouziala, Jean-Jacques Lefevre (ATS, St Denis), Damien Thibaudin, Hesham Mohey, Christian Broyet, Aida Afiani, (University Hospital Center, Saint Etienne), Marie-Odile Serveaux, Laure Patrier (University Hospital Center, Nîmes), François Maurice, Jean-Pierre Rivory (CHL, Castelnau le Lez), Philippe Nicoud (La Vallée Blanche center, Chamonix), Claude Durand, Michel Normand (Saint Martin polyclinic, Pessac), Bruno Seigneuric (University Hospital Center, Toulouse), Eric Magnant (Centre Hémodialyse Provence, Aix en Provence), Lynda Azzouz (Artic 42, St Priest en Jarez), Mohamed Shariful Islam, Sandor Vido (University Hospital Center, Nice), Hilaire Nzeyimana, Danièle Simonin (ECHO Michel Ange center, Le Mans), Yamina Azymah, Ibrahim Farah, Jean-Philippe Coindre (Hospital center, Le Mans), Olivier Puyoo, Marie-Hélène Chabannier, Richard Ibos, Fabienne Rouleau (Centre Néphrologique d'Occitanie, Muret), Carlos Vela, Josiane Joule (Hospital center, Perpignan), François Combarnous (Tonkin clinic, Villeurbanne), Cécile Turc-Baron, Francis Ducret, Philippe Pointet, Isabelle Rey (Hospital center, Annecy), Jacky Potier (Hospital center, Cherbourg), Jean-Christophe Bendini, Franck Perrin (St Georges clinic, Nice), Kristian Kunz (University Hospital Center, Strasbourg), Gaëlle Lefrancois, Angélique Colin, Sophie Parahy, Irima Dancea, Stéphanie Coupel, Angelo Testa (ECHO center, Rezé), Philippe Brunet, Gaétan Lebrun, Dominique Jaubert (University Hospital Center AP-HM, Marseille), Catherine Delcroix, Frédéric Lavainne, Anne Lefebvre (University Hospital Center, Nantes), Marie-Paule Guillodo, Dominique Le Grignou (AUB, Brest), Assia Djema (Hospital center, Cholet), Mehadji Maaz, Sylvie Chiron (Hospital center, Colmar), Maxime Hoffmann, Pascale Depraetre (Louvière clinic, Lille), Atman Haddj-Elmrabet, Véronique Joyeux (University Hospital Center, Rennes), Dominique Fleury, Laurence Vrigneaud, Vincent Lemaitre (Hospital center, Valenciennes), Didier Aguilera, Abdallah Guerraoui (Hospital center, Vichy), Alain Cremault, Achour Laradi, Francois Babinet (ECHO Pole Santé Sud center, Le Mans).
AUTHORS’ CONTRIBUTIONS
M.J.N. drafted the report. S.A.E.P. did the analyses. All authors contributed to the interpretation of the data, the preparation of the manuscript and the decision to submit for publication. M.J.N., M.P.C.G., M.L.B. and S.A.E.P. vouch for the validity of the study and are responsible for the integrity of the work as a whole.
FUNDING STATEMENT
The HDF Pooling project was designed, conducted and analysed independently of the financial contributors of the individual studies as listed below. Study data were collected and retained by the investigators and were not available for the financial contributors of the individual studies. S.A.E.P. and the meetings of the representatives of the combined authors of the four studies were financially supported by the EuDial working group. EuDial is an official working group of the European Renal Association–European Dialysis Transplant Association (ERA-EDTA, http://era-edta.org/eudial/European_Dialysis_Working_Group.html). No industry funding was received for any part of or activity related to the present analysis.
The Turkish HDF study was supported by European Nephrology and Dialysis Institute with an unrestricted grant. The study was performed in Fresenius Medical Care haemodialysis clinics in Turkey. ESHOL was supported by The Catalan Society of Nephrology and by grants from Fresenius Medical Care and Gambro through the Catalan Society of Nephrology. The CONTRAST study was supported by a grant from the Dutch Kidney Foundation (Nierstichting Nederland Grant C02.2019), and unrestricted grants from Fresenius Medical Care, Netherlands, and Gambro Lundia AB, Sweden. Additional support was received from the Dr E.E. Twiss Fund, Roche Netherlands, the International Society of Nephrology/Baxter Extramural Grant Program, and the Netherlands Organization for Health Research and Development (ZONMw Grant 170882802). The French HDF study was supported by a national grant from the Health Ministry (Programme Hospitalier de Recherche Clinique, PHRC) as a means to improve care and outcome of elderly chronic disease patients.
CONFLICT OF INTEREST STATEMENT
None declared.





Comments