-
PDF
- Split View
-
Views
-
Cite
Cite
Masafumi Fukagawa, Keitaro Yokoyama, Takashi Shigematsu, Takashi Akiba, Akifumi Fujii, Takuto Kuramoto, Motoi Odani, Tadao Akizawa, the ONO-5163 Study Group, A phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of etelcalcetide (ONO-5163/AMG 416), a novel intravenous calcimimetic, for secondary hyperparathyroidism in Japanese haemodialysis patients, Nephrology Dialysis Transplantation, Volume 32, Issue 10, October 2017, Pages 1723–1730, https://doi.org/10.1093/ndt/gfw408
Close - Share Icon Share
Abstract
Secondary hyperparathyroidism (SHPT) is a major complication associated with chronic kidney disease. We evaluated the efficacy and safety of etelcalcetide (ONO-5163/AMG 416), a novel intravenous calcimimetic, in Japanese haemodialysis patients with SHPT.
In this phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel-group study, etelcalcetide was administered three times per week at an initial dose of 5 mg, and subsequently adjusted to doses between 2.5 and 15 mg at 4-week intervals for 12 weeks. A total of 155 SHPT patients with serum intact parathyroid hormone (iPTH) levels ≥300 pg/mL were assigned to receive etelcalcetide (n = 78) or placebo (n = 77). The primary endpoint was the proportion of patients with decreased serum iPTH to the target range proposed by the Japanese Society for Dialysis Therapy (60–240 pg/mL). The major secondary endpoint was the proportion of patients with ≥30% reductions in serum iPTH from baseline.
The proportion of patients meeting the primary endpoint was significantly higher for etelcalcetide (59.0%) versus placebo (1.3%). Similarly, the proportion of patients meeting the major secondary endpoint was significantly higher for etelcalcetide (76.9%) versus placebo (5.2%). Serum albumin-corrected calcium, phosphorus and intact fibroblast growth factor-23 levels were decreased in the etelcalcetide group. Nausea, vomiting and symptomatic hypocalcaemia were mild with etelcalcetide. Serious adverse events related to etelcalcetide were not observed.
This study demonstrated the efficacy and safety of etelcalcetide. As the only available intravenous calcium-sensing receptor agonist, etelcalcetide is likely to provide a new treatment option for SHPT in haemodialysis patients.
INTRODUCTION
Secondary hyperparathyroidism (SHPT) is a complex disorder associated with chronic kidney disease (CKD) and subsequently becomes a maladaptive process that develops in response to declining kidney function and impaired phosphate/calcium homeostasis [1–3]. Continuous stimulation of the parathyroid glands through a combination of elevated extracellular phosphorus (P), decreased serum ionised calcium and markedly decreased serum 1,25-dihydroxyvitamin D leads to increased parathyroid hormone (PTH) synthesis and release in dialysis patients [1–3], resulting in the development of parathyroid hyperplasia. Elevated P increases fibroblast growth factor-23 (FGF23), leading to downregulated renal production of 1,25-dihydroxyvitamin D [4–6]. This further exacerbates the deficiency of 1,25-dihydroxyvitamin D, acting as an additional driver for SHPT [7, 8]. Elevated serum PTH with parathyroid hyperplasia continues to stimulate bone resorption, thereby releasing calcium and P, and leading to not only bone and mineral disease but also vascular calcification [3], both of which are strongly associated with increased morbidity and mortality [9–11]. To prevent the progression of these changes in SHPT patients, control of serum PTH within an appropriate range is important.
Current treatment options for SHPT consist of oral and intravenous active vitamin D analogues, and the oral calcimimetic agent cinacalcet. Although active vitamin D analogues decrease PTH, they also increase calcium and P. Meanwhile, despite the beneficial clinical properties of cinacalcet, its use has been limited by gastrointestinal adverse events (AEs), including nausea and vomiting. Furthermore, most SHPT patients need to take other oral medications to treat complications, causing poor adherence.
Etelcalcetide (ONO-5163/AMG 416) is a new peptide calcium-sensing receptor (CaSR) agonist with a similar mechanism of action to cinacalcet [12, 13]. Unlike cinacalcet, etelcalcetide requires cysteine at position 482 in human CaSR for pharmacological activity, specifically for the formation of a disulphide bond with the d-cysteine in the peptide backbone of etelcalcetide [13]. In addition, etelcalcetide is injectable through the blood return after haemodialysis, thus lessening the pill burden on patients.
The present study aimed to evaluate the efficacy and safety profile of etelcalcetide in a phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel-group study for the treatment of SHPT in Japanese CKD patients on dialysis.
MATERIALS AND METHODS
Patients
This was a phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel-group study. The study population comprised Japanese patients with SHPT who were aged ≥20 years and had been treated with haemodialysis three times per week for at least 90 days. The primary inclusion criteria were mean serum intact PTH (iPTH) ≥300 pg/mL within 14 days of the first dose, serum albumin-corrected calcium (cCa) ≥8.4 mg/dL and dialysate calcium level ≥2.25 mEq/L. The major exclusion criteria were primary hyperparathyroidism, parathyroidectomy within 90 days before the start of screening, symptomatic angina pectoris or chronic heart failure, and uncontrollable diabetes or hypertension.
The protocol was reviewed and approved by the institutional review board at each study site and written informed consent was obtained from all patients prior to enrolment. The study was conducted in conformity with the International Council for Harmonisation – Good Clinical Practice guidelines, and the Declaration of Helsinki. The study was registered as JapicCTI-142664.
Study design
Using a dynamic allocation method, the patients were randomly assigned through a centralized randomization system to receive either etelcalcetide or placebo. To balance certain patient characteristics between the two groups, the randomization was stratified according to serum iPTH (iPTH <500 pg/mL; iPTH ≥500 and <700 pg/mL; iPTH ≥700 pg/mL) and cCa (cCa ≥ 8.4 and ≤10.0 mg/dL; cCa >10.0 mg/dL) measured at screening, and cinacalcet pre-treatment (with or without washout).
Etelcalcetide was administered three times per week at an initial dose of 5 mg, and the dose was subsequently adjusted to between 2.5 and 15 mg at 4-week intervals for 12 weeks. The dose was increased by 5 mg if serum iPTH was >240 pg/mL, serum cCa was ≥8.4 mg/dL and there were no clinically significant AEs, including symptomatic hypocalcaemia, that prevented dose escalation. Drug administration was interrupted if serum cCa prior to dialysis was <7.5 mg/mL, or symptomatic hypocalcaemia developed.
Blood samples were collected prior to dialysis at the start of the first dialysis session of the week and on the day of discontinuation. Serum iPTH, albumin, calcium and P levels were determined every week. Serum intact FGF23 (iFGF23), bone alkaline phosphatase (BAP) and tartrate-resistant acid phosphatase (TRACP)-5b levels were determined every 4 weeks and on the day of discontinuation. Anti-etelcalcetide antibodies were determined on Days 1 and 85 and on the day of discontinuation. All samples were analysed by SRL (Tokyo, Japan) for serum iPTH using ECLIA on an Elecsys PTH (Roche Diagnostics, Tokyo, Japan) (normal range 10–65 pg/mL), and FGF23 using ELISA on an FGF23 ELISA Kit (Kainos, Tokyo, Japan) (normal range 14.7–40.5 pg/mL). BAP was determined by CLEIA (Access Ostase; Beckman Coulter, Tokyo, Japan) [normal range male, 3.7–20.9 µg/L; female, 2.9–14.5 µg/L (before menopause), 3.8–22.6 µg/L (after menopause)] and TRACP-5b was determined by EIA (Osteolinks TRAP-5b; Nittobo Medical, Fukushima, Japan) [normal range male, 170–590 mU/dL; female (young adult mean), 120–420 mU/dL]. Anti-etelcalcetide antibodies were determined by Amgen Biological Sample Management (Thousand Oaks, CA, USA) using surface plasmon resonance.
Bisphosphonates, human PTH and denosumab were prohibited from 24 weeks before the start of screening to the final examination, and the following medications were prohibited from 14 days prior to iPTH determination at screening and from 28 days before the first dose to the final examination: calcitonin, oestrogens, synthetic oestrogens, selective oestrogen receptor modulators and cinacalcet. Phosphate binders, calcium supplements and active vitamin D analogues were allowed if the dosage and administration schedules remained unchanged from 14 days prior to screening until the final examination.
Efficacy
The primary endpoint of the study was the proportion of patients with serum iPTH between 60 and 240 pg/mL [14] on Day 85, and the superiority of etelcalcetide over placebo was tested using this parameter. The following parameters were determined as secondary efficacy endpoints: measured and per cent changes from baseline in serum iPTH, cCa and P at each time point. Serum iFGF23, BAP and TRACP-5b levels at each time point were determined as exploratory efficacy endpoints.
Safety
The safety and tolerability profiles of etelcalcetide were assessed based on AEs, vital signs, laboratory measurements, 12-lead electrocardiograms (ECGs) and anti-etelcalcetide antibodies. All AEs were summarized by frequency and severity according to the Medical Dictionary for Regulatory Activities. The 12-lead ECGs were analyzed by Suzuken Co. Ltd (Nagoya, Japan).
Statistical analysis
Under the assumption that 10.0% of patients in the placebo group and 35.0% of patients in the etelcalcetide group would have serum iPTH between 60 and 240 pg/mL on Day 85, 67 patients per group needed to be enrolled for the study to have 94% power to detect a statistical significance at a two-sided alpha level of 5%. Anticipating approximately 10% exclusion from the analyses, the aim was to recruit 75 patients per group.
The primary endpoint was assessed using a Mantel–Haenszel test stratified according to serum iPTH, cCa and cinacalcet pre-treatment (primary analysis). The analysis was performed based on the intention-to-treat principle. Patients who received at least one administration and who were evaluated at least once for efficacy endpoints were included in the efficacy analysis population (full analysis set, FAS). The proportion of patients meeting the secondary endpoint of serum iPTH reductions of ≥30% from baseline on Day 85 was analyzed in a similar manner to the primary endpoint. For the secondary endpoints, pre-specified analyses in subgroups defined according to serum iPTH at screening, cinacalcet pre-treatment and dialysis history were also performed. Continuous efficacy outcomes were analyzed in a descriptive manner and, for the mean per cent changes in serum iPTH, cCa and P from baseline, a repeated-measures analysis of covariance method was used. This repeated-measures model included: terms of treatment, serum iPTH at screening, serum cCa at screening, cinacalcet pre-treatment; time; and interactions of time with treatment, time with serum iPTH at screening, time with serum cCa at screening and time with cinacalcet pre-treatment. The treatment differences in terms of mean per cent changes from baseline at a given time point were estimated and tested using this model. No adjustments for multiplicity were made, because there was only one primary efficacy hypothesis to be verified.
The safety analysis population (safety set, SAF) included patients who received at least one administration. Dose distributions and safety endpoints were analyzed in a descriptive manner.
Results
Patients
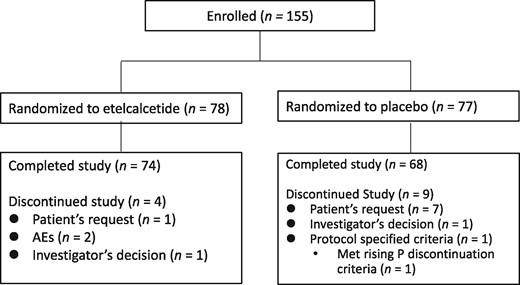
A total of 155 patients were enrolled in the study, and randomly allocated to the etelcalcetide group (n = 78) or placebo group (n = 77) (Figure 1). All patients received the study drug at least once, and were therefore considered to comprise both the SAF and FAS populations. There were no apparent differences in the demographic and baseline characteristics between the two treatment groups (Table 1). The mean etelcalcetide dose on Day 82 (last dose) was 7.80 ± 4.93 mg.
| Patient characteristics . | Classification . | Etelcalcetide . | Placebo . |
|---|---|---|---|
| Number of patients, n | 78 | 77 | |
| Sex, n (%) | Male | 48 (61.5) | 52 (67.5) |
| Female | 30 (38.5) | 25 (32.5) | |
| Age (years) | Mean ± SD | 62.1 ± 9.9 | 60.6 ± 12.3 |
| Median | 64.0 | 61.0 | |
| Range | 37–83 | 22–86 | |
| Body weight (kg) (post-dialysis) | Mean ± SD | 59.27 ± 11.63 | 62.22 ± 13.07 |
| Median | 58.40 | 61.20 | |
| Range | 39.2–97.2 | 39.1–104.0 | |
| BMI (kg/m2) | Mean ± SD | 22.67 ± 3.55 | 23.12 ± 3.55 |
| Median | 22.15 | 22.85 | |
| Range | 16.3–34.2 | 14.8–33.2 | |
| Duration of dialysis (years) | Mean ± SD | 11.9 ± 7.3 | 11.8 ± 7.8 |
| Median | 11.0 | 11.0 | |
| Range | 1–41 | 1–32 | |
| Use of active vitamin D, n (%) | Yes | 63 (80.8) | 67 (87.0) |
| No | 15 (19.2) | 10 (13.0) | |
| Use of phosphate binder, n (%) | Yes | 74 (94.9) | 72 (93.5) |
| No | 4 (5.1) | 5 (6.5) | |
| Serum iPTH (pg/mL) | Mean ± SD | 536.2 ± 245.7 | 568.1 ± 354.1 |
| Median | 484.0 | 504.0 | |
| Range | 222–1470 | 209–2620 | |
| Serum cCa (mg/dL) | Mean ± SD | 9.58 ± 0.65 | 9.48 ± 0.76 |
| Median | 9.60 | 9.50 | |
| Range | 8.2–11.1 | 7.8–11.1 | |
| Serum P (mg/dL) | Mean ± SD | 5.94 ± 1.44 | 6.33 ± 1.41 |
| Median | 5.80 | 6.20 | |
| Range | 3.4–10.7 | 3.4–11.4 | |
| Serum BAP (µg/L) | Mean ± SD | 19.35 ± 8.96 | 19.56 ± 10.62 |
| Median | 16.80 | 16.60 | |
| Range | 7.5–64.5 | 7.9–78.3 | |
| Serum TRACP-5b (mU/dL) | Mean ± SD | 854.5 ± 351.5 | 873.3 ± 344.5 |
| Median | 791.5 | 864.0 | |
| Range | 279–1990 | 244–1780 | |
| Serum iFGF23 (pg/mL) | Mean ± SD | 22 769.7 ± 39 726.9 | 23 928.5 ± 38 857.2 |
| Median | 9050.0 | 11800.0 | |
| Q1, Q3 | 3300.0, 26 100.0 | 4500.0, 28 400.0 |
| Patient characteristics . | Classification . | Etelcalcetide . | Placebo . |
|---|---|---|---|
| Number of patients, n | 78 | 77 | |
| Sex, n (%) | Male | 48 (61.5) | 52 (67.5) |
| Female | 30 (38.5) | 25 (32.5) | |
| Age (years) | Mean ± SD | 62.1 ± 9.9 | 60.6 ± 12.3 |
| Median | 64.0 | 61.0 | |
| Range | 37–83 | 22–86 | |
| Body weight (kg) (post-dialysis) | Mean ± SD | 59.27 ± 11.63 | 62.22 ± 13.07 |
| Median | 58.40 | 61.20 | |
| Range | 39.2–97.2 | 39.1–104.0 | |
| BMI (kg/m2) | Mean ± SD | 22.67 ± 3.55 | 23.12 ± 3.55 |
| Median | 22.15 | 22.85 | |
| Range | 16.3–34.2 | 14.8–33.2 | |
| Duration of dialysis (years) | Mean ± SD | 11.9 ± 7.3 | 11.8 ± 7.8 |
| Median | 11.0 | 11.0 | |
| Range | 1–41 | 1–32 | |
| Use of active vitamin D, n (%) | Yes | 63 (80.8) | 67 (87.0) |
| No | 15 (19.2) | 10 (13.0) | |
| Use of phosphate binder, n (%) | Yes | 74 (94.9) | 72 (93.5) |
| No | 4 (5.1) | 5 (6.5) | |
| Serum iPTH (pg/mL) | Mean ± SD | 536.2 ± 245.7 | 568.1 ± 354.1 |
| Median | 484.0 | 504.0 | |
| Range | 222–1470 | 209–2620 | |
| Serum cCa (mg/dL) | Mean ± SD | 9.58 ± 0.65 | 9.48 ± 0.76 |
| Median | 9.60 | 9.50 | |
| Range | 8.2–11.1 | 7.8–11.1 | |
| Serum P (mg/dL) | Mean ± SD | 5.94 ± 1.44 | 6.33 ± 1.41 |
| Median | 5.80 | 6.20 | |
| Range | 3.4–10.7 | 3.4–11.4 | |
| Serum BAP (µg/L) | Mean ± SD | 19.35 ± 8.96 | 19.56 ± 10.62 |
| Median | 16.80 | 16.60 | |
| Range | 7.5–64.5 | 7.9–78.3 | |
| Serum TRACP-5b (mU/dL) | Mean ± SD | 854.5 ± 351.5 | 873.3 ± 344.5 |
| Median | 791.5 | 864.0 | |
| Range | 279–1990 | 244–1780 | |
| Serum iFGF23 (pg/mL) | Mean ± SD | 22 769.7 ± 39 726.9 | 23 928.5 ± 38 857.2 |
| Median | 9050.0 | 11800.0 | |
| Q1, Q3 | 3300.0, 26 100.0 | 4500.0, 28 400.0 |
BMI, body mass index; SD, standard deviation.
| Patient characteristics . | Classification . | Etelcalcetide . | Placebo . |
|---|---|---|---|
| Number of patients, n | 78 | 77 | |
| Sex, n (%) | Male | 48 (61.5) | 52 (67.5) |
| Female | 30 (38.5) | 25 (32.5) | |
| Age (years) | Mean ± SD | 62.1 ± 9.9 | 60.6 ± 12.3 |
| Median | 64.0 | 61.0 | |
| Range | 37–83 | 22–86 | |
| Body weight (kg) (post-dialysis) | Mean ± SD | 59.27 ± 11.63 | 62.22 ± 13.07 |
| Median | 58.40 | 61.20 | |
| Range | 39.2–97.2 | 39.1–104.0 | |
| BMI (kg/m2) | Mean ± SD | 22.67 ± 3.55 | 23.12 ± 3.55 |
| Median | 22.15 | 22.85 | |
| Range | 16.3–34.2 | 14.8–33.2 | |
| Duration of dialysis (years) | Mean ± SD | 11.9 ± 7.3 | 11.8 ± 7.8 |
| Median | 11.0 | 11.0 | |
| Range | 1–41 | 1–32 | |
| Use of active vitamin D, n (%) | Yes | 63 (80.8) | 67 (87.0) |
| No | 15 (19.2) | 10 (13.0) | |
| Use of phosphate binder, n (%) | Yes | 74 (94.9) | 72 (93.5) |
| No | 4 (5.1) | 5 (6.5) | |
| Serum iPTH (pg/mL) | Mean ± SD | 536.2 ± 245.7 | 568.1 ± 354.1 |
| Median | 484.0 | 504.0 | |
| Range | 222–1470 | 209–2620 | |
| Serum cCa (mg/dL) | Mean ± SD | 9.58 ± 0.65 | 9.48 ± 0.76 |
| Median | 9.60 | 9.50 | |
| Range | 8.2–11.1 | 7.8–11.1 | |
| Serum P (mg/dL) | Mean ± SD | 5.94 ± 1.44 | 6.33 ± 1.41 |
| Median | 5.80 | 6.20 | |
| Range | 3.4–10.7 | 3.4–11.4 | |
| Serum BAP (µg/L) | Mean ± SD | 19.35 ± 8.96 | 19.56 ± 10.62 |
| Median | 16.80 | 16.60 | |
| Range | 7.5–64.5 | 7.9–78.3 | |
| Serum TRACP-5b (mU/dL) | Mean ± SD | 854.5 ± 351.5 | 873.3 ± 344.5 |
| Median | 791.5 | 864.0 | |
| Range | 279–1990 | 244–1780 | |
| Serum iFGF23 (pg/mL) | Mean ± SD | 22 769.7 ± 39 726.9 | 23 928.5 ± 38 857.2 |
| Median | 9050.0 | 11800.0 | |
| Q1, Q3 | 3300.0, 26 100.0 | 4500.0, 28 400.0 |
| Patient characteristics . | Classification . | Etelcalcetide . | Placebo . |
|---|---|---|---|
| Number of patients, n | 78 | 77 | |
| Sex, n (%) | Male | 48 (61.5) | 52 (67.5) |
| Female | 30 (38.5) | 25 (32.5) | |
| Age (years) | Mean ± SD | 62.1 ± 9.9 | 60.6 ± 12.3 |
| Median | 64.0 | 61.0 | |
| Range | 37–83 | 22–86 | |
| Body weight (kg) (post-dialysis) | Mean ± SD | 59.27 ± 11.63 | 62.22 ± 13.07 |
| Median | 58.40 | 61.20 | |
| Range | 39.2–97.2 | 39.1–104.0 | |
| BMI (kg/m2) | Mean ± SD | 22.67 ± 3.55 | 23.12 ± 3.55 |
| Median | 22.15 | 22.85 | |
| Range | 16.3–34.2 | 14.8–33.2 | |
| Duration of dialysis (years) | Mean ± SD | 11.9 ± 7.3 | 11.8 ± 7.8 |
| Median | 11.0 | 11.0 | |
| Range | 1–41 | 1–32 | |
| Use of active vitamin D, n (%) | Yes | 63 (80.8) | 67 (87.0) |
| No | 15 (19.2) | 10 (13.0) | |
| Use of phosphate binder, n (%) | Yes | 74 (94.9) | 72 (93.5) |
| No | 4 (5.1) | 5 (6.5) | |
| Serum iPTH (pg/mL) | Mean ± SD | 536.2 ± 245.7 | 568.1 ± 354.1 |
| Median | 484.0 | 504.0 | |
| Range | 222–1470 | 209–2620 | |
| Serum cCa (mg/dL) | Mean ± SD | 9.58 ± 0.65 | 9.48 ± 0.76 |
| Median | 9.60 | 9.50 | |
| Range | 8.2–11.1 | 7.8–11.1 | |
| Serum P (mg/dL) | Mean ± SD | 5.94 ± 1.44 | 6.33 ± 1.41 |
| Median | 5.80 | 6.20 | |
| Range | 3.4–10.7 | 3.4–11.4 | |
| Serum BAP (µg/L) | Mean ± SD | 19.35 ± 8.96 | 19.56 ± 10.62 |
| Median | 16.80 | 16.60 | |
| Range | 7.5–64.5 | 7.9–78.3 | |
| Serum TRACP-5b (mU/dL) | Mean ± SD | 854.5 ± 351.5 | 873.3 ± 344.5 |
| Median | 791.5 | 864.0 | |
| Range | 279–1990 | 244–1780 | |
| Serum iFGF23 (pg/mL) | Mean ± SD | 22 769.7 ± 39 726.9 | 23 928.5 ± 38 857.2 |
| Median | 9050.0 | 11800.0 | |
| Q1, Q3 | 3300.0, 26 100.0 | 4500.0, 28 400.0 |
BMI, body mass index; SD, standard deviation.
Efficacy
The proportions of patients meeting the primary endpoint of serum iPTH between 60 and 240 pg/mL on Day 85 were 59.0% (46/78) and 1.3% (1/77) in the etelcalcetide and placebo groups, respectively (Figure 2). The proportions differed between the two groups by 60.0% [95% confidence interval (CI): 49.7–70.4%], with statistical significance (P < 0.0001, Mantel–Haenszel test). Likewise, the proportions of patients meeting the secondary endpoint of serum iPTH reductions of ≥30% from baseline on Day 85 were 76.9% (60/78) and 5.2% (4/77), respectively (Figure 2), with a significant difference between the two groups (P < 0.0001, Mantel–Haenszel test). Similar results were obtained irrespective of serum iPTH at screening: the proportions were 76.2%, 78.3% and 76.9% in patients with serum iPTH at screening of <500 pg/mL, ≥500 and <700 pg/mL, and ≥700 pg/mL, respectively.
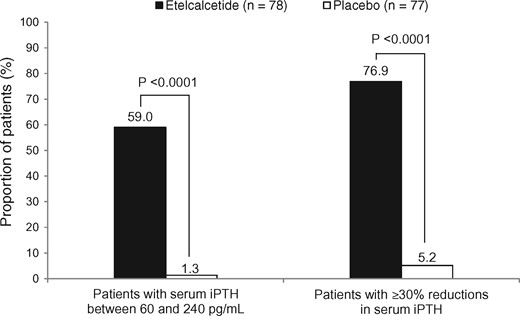
Proportions of patients meeting the primary and major secondary endpoints.
The serum iPTH, cCa and P levels decreased from baseline over time in the etelcalcetide group, but did not change appreciably in the placebo group (Figure 3). Significant reductions regarding per cent changes from baseline were observed from Day 8 (serum iPTH and cCa) and Day 15 (serum P) compared with the placebo group.

Serum iPTH (A), cCa (B) and P (C) levels during the study period. Data are expressed as means ± standard deviation. *P < 0.05 regarding per cent changes from baseline compared with placebo.
For the exploratory efficacy endpoints, the serum BAP levels increased just after the start of administration, and then returned to the baseline level on Day 85 (Figure 4). The serum TRACP-5b levels in the etelcalcetide group decreased from baseline over time, while those in the placebo group did not change appreciably (Figure 4).
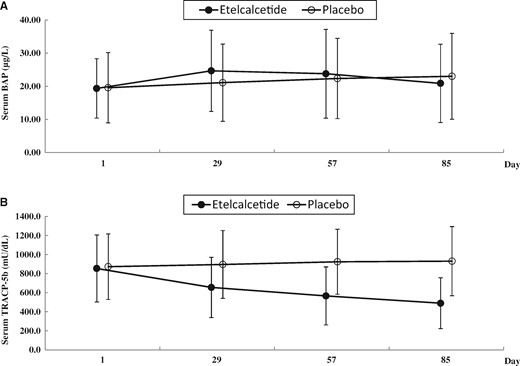
Serum BAP (A) and TRACP-5b (B) levels during the study period. Data are expressed as means ± standard deviation.
The serum iFGF23 levels in the etelcalcetide group decreased from baseline over time, while those in the placebo group did not change appreciably (Figure 5). The median per cent changes from baseline in iFGF23 on Day 85 were −72.0% (Q1, Q3: −83.5, −42.0%) for the etelcalcetide group and −3.7% (Q1, Q3: −33.3, 26.0%) for the placebo group.

Serum iFGF23 levels during the study period. Data are expressed as median (Q1, Q3).
It is well known that active vitamin D analogues can reduce the PTH level. In this study, changes in the doses of active vitamin D analogues were restricted throughout the study period. The mean dose of maxacalcitol, the most commonly used active vitamin D analogue, remained almost unchanged throughout the study period, as follows: 11.30 ± 8.72 µg/week on Day 1, 11.40 ± 8.63 µg/week on Day 85 for the etelcalcetide group; and 12.02 ± 8.79 µg/week on Day 1, 10.00 ± 5.34 µg/week on Day 85 for the placebo group.
Safety
At least one AE was reported in 65.4% (51/78) and 72.7% (56/77) of patients in the etelcalcetide and placebo groups, respectively. Drug-related AEs were reported in 19.2% (15/78) and 3.9% (3/77) of patients in the etelcalcetide and placebo groups, respectively. AE-induced treatment discontinuations were observed in 2.6% (2/78) of patients in the etelcalcetide group: cerebral infarction (1) and rash (1, drug-related AE). Serious AEs related to etelcalcetide were not reported. Drug-related AEs occurring in the etelcalcetide group, but not in the placebo group, included asymptomatic blood calcium decreased (6.4%), vomiting (3.8%), nausea (1.3%) and symptomatic hypocalcaemia (1.3%). All of these drug-related AEs were mild or moderate. No clinically relevant changes were reported in vital signs, body weight and laboratory tests, except for asymptomatic blood calcium decrease. The respective changes in QTc Fridericia (QTcF) interval from baseline at Days 29, 57 and 85 were 11.0 ± 14.1, 15.0 ± 14.0 and 12.7 ± 12.3 ms in the etelcalcetide group, and 3.2 ± 12.8, 2.2 ± 15.1 and 2.0 ± 13.3 ms in the placebo group. The QTcF prolongations occurring in the etelcalcetide group were related to blood calcium decrease. No clinically relevant cardiovascular AEs, including abnormal 12-lead ECGs, were observed.
Discussion
The currently available major therapeutic interventions for SHPT are active vitamin D analogues and calcimimetics [14, 15]. Although active vitamin D analogues suppress PTH secretion, they stimulate intestinal absorption of calcium and P and elevate serum FGF23, and higher doses frequently lead to hypercalcaemia and hyperphosphataemia, both of which are associated with ectopic calcification [16]. Furthermore, cinacalcet, the only approved oral calcimimetic, is an allosteric activator of the CaSR located on the parathyroid cell membrane. Cinacalcet binding induces a conformational change within the CaSR, thereby reducing its threshold for calcium and leading to reduced synthesis and secretion of PTH [17–19]. Cinacalcet treatment was shown to suppress serum iPTH, as well as serum calcium and P, in patients with SHPT [20–22]. Cinacalcet was also effective in patients refractory to active vitamin D analogues and capable of reducing serum iPTH in patients with severe SHPT, whose parathyroid gland hyperplasia was also reduced by the treatment [23, 24]. However, cinacalcet treatment is associated with gastrointestinal AEs, including nausea and vomiting, thereby limiting compliance with treatment [25–27]. Most SHPT patients also need to take other oral medications, including phosphate binders and antihypertensives, to treat complications [28]. Thus, new parenteral drugs leading to fewer gastrointestinal AEs and a lower pill burden are needed.
In this study, three times per week administration of etelcalcetide for 12 weeks reduced serum iPTH on Day 85 with statistical significance compared with placebo. Etelcalcetide reduced serum iPTH regardless of cinacalcet pre-treatment: 80.0% and 73.7% in patients with or without cinacalcet pre-treatment, respectively. In a post hoc subgroup analysis, the patients were divided into two groups by the median baseline serum iFGF23 level of 9050 pg/mL, and the patient groups with higher and lower serum iFGF23 levels had mean baseline serum iPTH levels of 610.9 pg/mL and 461.4 pg/mL, respectively. Etelcalcetide reduced these serum iPTH levels by 49.5% and 54.8% from baseline, respectively, on Day 85. Therefore, etelcalcetide is expected to reduce serum iPTH in SHPT patients regardless of their background. In addition, a phase 2 open-label, single-arm, dose-titration study in non-Japanese patients [29] demonstrated that the proportion of patients with serum iPTH reductions of ≥30% from baseline was 89% (95% CI: 73.9–96.9%). Japanese patients, in general, have a longer history of dialysis and lower baseline serum iPTH with well-controlled treatment compared with non-Japanese patients [30, 31]. Nevertheless, our data demonstrated that the serum iPTH-reducing effect of etelcalcetide was unaffected by these factors, suggesting that etelcalcetide is equally effective in Japanese and non-Japanese patients. In addition, as renal excretion is a major clearance route for etelcalcetide [12], its plasma levels should remain constant in haemodialysis patients, thereby enabling continuous reductions in serum iPTH during dialysis intervals. Indeed, a phase 1/2 study in Japanese patients (unpublished data) and phase 2 study in non-Japanese patients [32] demonstrated that etelcalcetide continuously reduced serum iPTH, indicating that etelcalcetide may be a useful new treatment option for SHPT in dialysis patients.
Elevated serum FGF23 has been implicated in CKD progression, with complications such as cardiac failure, increased cardiovascular events and mortality [9–11]. In this study, etelcalcetide reduced serum iFGF23 levels concomitantly with reductions in serum iPTH, cCa and P. In a post hoc analysis, the reductions in iFGF23 levels were associated with reductions in serum cCa and P, but not iPTH. Some possibilities for the mechanism of reduction in FGF23 with calcimimetics have been reported: one such possibility is an indirect pathway in which FGF23 decreases via the reductions in serum calcium and P. Similar results have been reported in a previous study with cinacalcet [33]. On the other hand, a pathway mediated by PTH reduction could also be considered, because parathyroidectomy for severe SHPT results in significant reductions in serum FGF23 [8]. Yet another possibility is a direct pathway via secretion of FGF23, because bone cells are known to express CaSR [34]. Although the precise mechanisms are still unclear, this effect is considered to have clinical impact. The data are in line with those of the EVOLVE trial [35] in which a post hoc analysis further demonstrated that cinacalcet-induced reductions in serum FGF23 were associated with lower rates of cardiovascular mortality and morbidity. Therefore, etelcalcetide may reduce the mortality of SHPT patients on dialysis.
SHPT patients are known to have high bone turnover, leading to reductions in bone mass and bone strength [1–3]. Chronic treatment with etelcalcetide may suppress or prevent these changes by inhibiting high bone turnover, since etelcalcetide reduced serum TRACP-5b, a marker of bone resorption, in this study.
In a post hoc analysis, the proportions of patients whose serum iPTH, cCa and P levels were reduced to the corresponding target ranges recommended by the guideline [14] were significantly higher in the etelcalcetide group (28.4%) compared with the placebo group (1.5%). These proportions were comparable to the statistical data reported by the Japanese Society for Dialysis Therapy (32.3%) [36]. The doses and administration schedules of active vitamin D analogues and phosphate binders were kept constant according to the protocol of this study. However, these medications would usually be changed in a case-by-case manner depending on the responsiveness and severity of SHPT/CKD, which could improve the effects of etelcalcetide in such patients. In this regard, further data from long-term studies and clinical experience are required.
Etelcalcetide is a synthetic peptide and, therefore, potential concerns regarding efficacy and safety, such as hypersensitivity and infusion reactions, need to be addressed [37]. In an attempt to address these concerns, antibody detection was performed: only one patient was shown to have anti-etelcalcetide antibodies and these were pre-existing. Therefore, longer term or larger size studies are required to assess the clinical relevance of anti-etelcalcetide antibodies.
Nausea and vomiting, as specific AEs associated with the currently available calcimimetic cinacalcet, occurred in the etelcalcetide group. However, these events were mild and did not cause discontinuation of treatment, thereby demonstrating that etelcalcetide was well tolerated in Japanese patients.
In conclusion, etelcalcetide is safe, well tolerated and effective in reducing iPTH in Japanese haemodialysis patients with SHPT. In addition, intravenous etelcalcetide can be easily administered via the blood return after dialysis, thus enabling a decrease in the pill burden on patients, and providing a new alternative for the treatment of SHPT.
ACKNOWLEDGEMENTS
This study was funded by Ono Pharmaceutical Co., Ltd. The authors thank the Clinical Development Department, Ono Pharmaceutical Co., Ltd, for their assistance in preparing and writing this article. The authors gratefully acknowledge all the following investigators for their contribution to the trial: T. Chiba, Iwamizawa Clinic; I. Sasagawa, Yamagata Tokushukai Hospital; Y. Fukaya, Southen Tohoku General Hospital; T. Iitsuka, Ibaraki Seinan Medical Center Hospital; H. Kikuchi, Kikuchi Medical Clinic; M. Tomizawa, Hanyu General Hospital; J. Ooshima, Kubojima Clinic; T. Shinomiya, Yuainisshin Clinic; A. Watanabe, Yuainakagawa Clinic; K. Ootsuka, Ooshima Clinic; M. Kaneko, Naganuma Clinic; H. Terajima, Higashinarashino Clinic; T. Saito, IMS Memorial Hospital; M. Nishihara, Toshin Clinic; H. Moriya, Shonankamakura General Hospital; H. Degawa, Miyamaedairakenei Clinic; T. Kuji, Youkoudai Central Clinic; T. Nakanishi, Kurihama Clinic; S. Miyazaki, Shinrakuen Hospital; D. Inaguma, Japanese Red Cross Nagoya Daini Hospital; M. Tsuboi, Anjyo Kyoritsu Clinic; K. Nishikawa, Fuchu Hospital; J. Kim, Chibune Kidney and Dialysis Clinic; N. Takahara, Ako City Hospital; K. Ejiri, Koujukai Clinic; N. Kodama, Kodama Hospital; M. Oomoto, Saiseikai Imabari Hospital; K. Yuasa, Kochi Takasu Hospital; Y. Otsubo, Shinkoga Hospital; N. Itami, Higashimuroran Satellite Clinic; M. Enomoto, Ayase Ekimae Jin Clinic; S. Suzuki, Nerimatakanodai Clinic; K. Ozawa, Yokosuka Clinic; H. Yasuda, Sanko Hospital Satellite Clinic; J. Minakuchi, Kawashima Hospital; and S. Funakoshi, Nagasaki Jin Hospital.
AUTHORS’ CONTRIBUTIONS
Research idea, trial design, data analysis and interpretation: M.F., K.Y., T.S., T.Akiba, A.F., T.K., M.O., and T.Akizawa. Each author was involved in revising the manuscript critically for important intellectual content, and all authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
M.F., K.Y., T.S., Tak.A. and Tad.A. were the medical advisors of this study. A.F., T.K. and M.O. are employees of Ono Pharmaceutical Co., Ltd. The trial was funded by Ono Pharmaceutical Co., Ltd. M.F., T.S. and Tak.A. have received consultant fees from Ono Pharmaceutical Co., Ltd and Kyowa Hakko Kirin, and honoraria and research funding from Kyowa Hakko Kirin. K.Y. has received consultant fees from Ono Pharmaceutical Co., Ltd and honoraria from Kyowa Hakko Kirin. Tad.A. has received consultant fees from Ono Pharmaceutical Co., Ltd and Kyowa Hakko Kirin, and honoraria from Kyowa Hakko Kirin.
REFERENCES





Comments