-
PDF
- Split View
-
Views
-
Cite
Cite
Ken Farrington, Adrian Covic, Ionut Nistor, Filippo Aucella, Naomi Clyne, Leen De Vos, Andrew Findlay, Denis Fouque, Tomasz Grodzicki, Osasuyi Iyasere, Kitty J. Jager, Hanneke Joosten, Juan Florencio Macias, Andrew Mooney, Evi Nagler, Dorothea Nitsch, Maarten Taal, James Tattersall, Marijke Stryckers, Dieneke van Asselt, Nele Van den Noortgate, Sabine van der Veer, Wim van Biesen, Clinical Practice Guideline on management of older patients with chronic kidney disease stage 3b or higher (eGFR<45 mL/min/1.73 m2): a summary document from the European Renal Best Practice Group, Nephrology Dialysis Transplantation, Volume 32, Issue 1, January 2017, Pages 9–16, https://doi.org/10.1093/ndt/gfw411
Close - Share Icon Share
The population of patients with moderate and severe CKD is growing. Frail and older patients comprise an increasing proportion. Many studies still exclude this group, so the evidence base is limited. In 2013 the advisory board of ERBP initiated, in collaboration with European Union of Geriatric Medicine Societies (EUGMS), the development of a guideline on the management of older patients with CKD stage 3b or higher (eGFR >45 mL/min/1.73 m2). The full guideline has recently been published and is freely available online and on the website of ERBP (www.european-renal-best-practice.org). This paper summarises main recommendations of the guideline and their underlying rationales.
Introduction
Despite the growing number of frail and older patients with estimated glomerular filtration rate (eGFR) <45 mL/min/1.73 m2, most studies still exclude this population, so providing guidance on best practice in this setting remains problematic. Nevertheless, there is a clear need to support patients, their families and healthcare professionals with evidence-based guidance to enhance the quality of patient care and experience and to establish a transparent framework for service provision and development. A joint initiative of the European Renal Association–European Dialysis Transplant Association (ERA-EDTA) and the European Union Geriatric Medicine Society (EUGMS) was established to address this issue. Expert groups were set up to scope the project, prioritize topics, search the literature, critically examine the evidence and produce recommendations. The methods used have been fully described [1–3]. The current document summarizes the main recommendations and their underlying rationales. The full guideline is freely available online and on the website of European Renal Best Practice Group (ERBP) (www.european-renal-best-practice.org) [1]. In the following sections, we have used the term ‘older’ to refer to people aged over 65 years.
Proposed management pathway for older patients with advanced CKD (eGFR <45 mL/min/1.73 m2) (FIGURE 1)
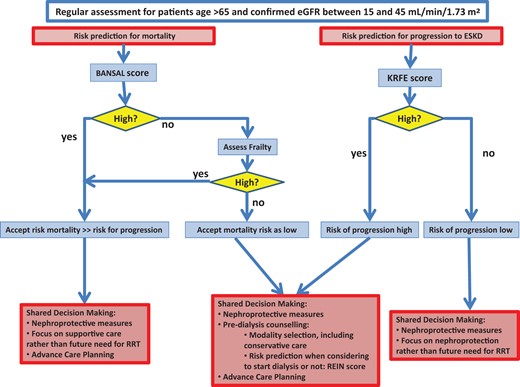
Proposed management pathway for older patients with advanced CKD. KRFE score is the 4-variable Kidney Failure Risk Equation (see Question 2). For Bansal and REIN score see Question 3.
Deciding which older patients with advanced chronic kidney disease (CKD) may benefit from closer nephrological follow-up requires consideration of factors including the likelihood of progression of CKD (considered in Question 2) and the probability of survival to end-stage (considered in Question 3).
The guideline development group considers that the Kidney Failure Risk Equation score [4, 5] provides reasonable predictions of the risk of progression of kidney failure in older patients. Management options for those with a low predicted progression should focus on nephroprotection rather than preparation for dialysis or conservative care. The Bansal score [6] was considered to provide acceptable risk prediction of mortality in this setting. For those with a high Bansal score—i.e. at high risk of dying—management should focus on advance care planning and on nephroprotection, if considered appropriate. Since the Bansal score was developed in cohorts with a low prevalence of frailty, in those patients with low Bansal scores, frailty should be formally assessed, and if present the patient should be considered to be at higher risk and managed accordingly.
For patients with a high predicted risk for progression and with a low predicted risk for mortality, and in all cases of clinical equipoise, a shared decision approach should consider options for renal replacement therapy (RRT) and conservative management (considered in Question 6). The Renal Epidemiology and Information Network (REIN) score [7] provides a reasonable estimate of short-term mortality risk should dialysis be commenced.
Older patients with advanced CKD (eGFR <45 mL/min/1.73 m2) should be screened regularly for functional impairment (considered in Question 4) and malnutrition (considered in Question 5) to identify those likely to benefit from more in-depth assessment and intervention. Interventions to improve nutritional and functional status were evaluated and recommendations formulated.
Q1: What parameter should be used in older patients (a) to estimate kidney function AND (b) for dose adaptation purposes?
1.1 We recommend using estimating equations that correct for differences in creatinine generation rather than plain serum creatinine measurements to assess kidney function in older patients (1A)
1.2 We recommend that there is insufficient evidence to prefer one estimating equation over another since all perform equally and substantial misclassification can occur with any of these equations when used in older patients with differing body composition (1B)
1.3 We recommend formal measurement of kidney function if more accurate and precise estimation of GFR is required (1B). We suggest use of CKD-EPICr-Cys may be an acceptable alternative (2C)
1.4 We recommend taking account of kidney function when prescribing drugs whose active forms or metabolites are renally cleared (1A)
1.5 We suggest that for drugs with a narrow toxic/therapeutic range, regular measurement of serum concentrations can provide useful information. Differences in protein binding in relation to uraemia may necessitate use of different target levels of total drug concentration (2C)
Advice for clinical practice
Kidney function can vary over time and should be monitored serially using the same equation.
Estimating equations can not be reliably used in patients with acute changes in kidney function.
Use of different equations, even if well established, can result in different classifications of CKD stage for the same creatinine value from the same patient.
Serum levels of drugs depend upon absolute rather than body size-corrected clearance.
Formulae other than Cockcroft and Gault return eGFR, already corrected for body surface area (BSA), in units of mL/min/1.73 m2. Drug dosing requires adjustment in proportion to absolute clearance in units of mL/min. To convert eGFR to absolute clearance, multiply eGFR by BSA/1.73.
Rationale
Methods to accurately assess true GFR (Cr-EDTA, Inulin clearance or Tc-DPTA) are impractical for use in routine clinical practice. Various formulae, based on creatinine and/or cystatin, are in widespread use but there is no consensus about which formula should be used in older patients with advanced CKD. As aging is associated not only with declining GFR, but also with reduced creatinine generation due to loss of muscle mass, reduced physical activity and decreased food intake, recommendations for the general population cannot necessarily be extrapolated to this subgroup. In addition, use of prescription drugs also tends to be high in older patients with advanced CKD. CKD management, referral practices and safe use of renally excreted drugs may be compromised if renal function is incorrectly estimated.
Evidence suggests that, though serum creatinine concentration alone is insufficient to allow correct estimation of GFR in older people without some correction for creatinine generation, none of the established formulae consistently outperforms the others. Substantial reclassification in CKD stages has been demonstrated when different formulae are used to correct the same patient’s serum creatinine estimate. Relative performance is influenced by the methodology of creatinine measurement and the case-mix of the cohort (age, CKD stage and prevalence of frailty). If more exact knowledge of kidney function is sought, formal GFR measurement should be considered, though such testing may be laborious and expensive. Use of the CKD-EpiCr-Cys equation may be a useful alternative since this may improve the eGFR estimate. For drugs or their active drug metabolites that are cleared by the kidneys, dosing should be adapted to renal function. Hypoalbuminaemia associated with malnutrition/inflammation and uraemia-related changes in protein binding may increase serum levels of the unbound (active) form of some drugs, which may require lower total concentrations to be targeted.
Q2: What is the most reliable Risk Model Score to predict progression of chronic kidney disease in older patients with advanced CKD (eGFR <45 mL/min/1.73 m2)?
We recommend that the 4-variable Kidney Failure Risk Equation performs sufficiently well for use in older patients with advanced CKD and eGFR <45 mL/min/1.73 m2 (1B)
Rationale
The purpose of this question is to provide guidance to clinicians on how best to estimate the risk of progression of CKD to end-stage kidney disease (ESKD) in older patients. This is important because the prevalence of CKD increases sharply with age [8] such that almost 50% of people aged over 70 years have CKD stage 3–5, though only a minority progress to ESKD [9–11]. We therefore need robust methods to identify those at high risk of progression so that they can be offered optimal nephroprotective therapy and timely preparation for RRT. Preparation for RRT in older people may be protracted due to multi-morbidity and frailty. Risk prediction is challenging because GFR decline may not be linear [12] and rapid decline may occur due to relatively unpredictable episodes of acute kidney injury [13] for which older people are at greater risk.
It is also important to consider the competing risk of death in older people. In those aged 65 years and more, the risk of ESKD exceeds that of death only in those with eGFR <15 mL/min/1.73 m2 [14]. Hence identification of the majority who are at low risk of progression could avoid the morbidity and stress associated with unnecessary interventions in preparation for RRT. Older people are often excluded from studies to evaluate nephroprotective interventions or develop risk prediction scores for CKD, so it is not clear whether scores developed in younger people will perform adequately well in older people.
We found that the 4-variable Kidney Failure Risk Equation developed by Tangri et al. [4, 5] performed well in younger and older groups and was well-validated, and we recommend it for clinical use. A correction factor may need to be applied in non-North American populations. The 8-variable score performed only marginally better than the 4-variable. Only basic demographic and laboratory data are required for the 4-variable score, enabling a risk estimate to be generated automatically by laboratory computer systems.
Q3: What is the most reliable risk prediction model to predict mortality in older and/or frail patients with advanced CKD (eGFR <45 mL/min/1.73 m2)
3.1 We suggest using the Bansal score to predict individual 5 year risk of death before ESKD in older people with CKD stage 3–5 (2C)
3.2 We suggest that in patients at low risk on the Bansal score, a formal assessment of frailty be carried out as stated in 4a. Frail patients should be managed as high risk (2C)
3.3 We suggest the REIN score be used to predict the short term/6-month risk for mortality in older patients with CKD stage 5 should dialysis be embarked on (2B)
Rationale
Counselling older people with advanced CKD on treatment options requires reliable estimates of an individual’s absolute probability of death within a given time frame, both with and without starting dialysis. Correctly identifying those people likely to die within the next few months, regardless of whether RRT is started, may avoid their being subjected to the added burden of the dialysis pathway. On the other hand, identifying those likely to live longer may inform shared decisions, balancing quality versus quantity of life. Few available risk prediction models have targeted older people with advanced CKD. Fewer still have been tested in populations outside those used to develop them. Hence it is unclear whether existing models reliably help estimate risk of death in older people with advanced CKD.
We found that the Bansal risk prediction model had the best credentials to be recommended as a tool for predicting the absolute probability of death within 5 years for older people with CKD stage 3–5 not on dialysis [6]. The model includes nine readily available demographic, clinical and biochemical predictors: age, sex, ethnicity, eGFR, urinary albumin-to-creatinine ratio, diabetes, smoking, history of heart failure and stroke. Model discrimination was moderate in both development and validation cohorts (c-statistic 0.72 and 0.69, respectively). External validation is lacking in cohorts including a substantial proportion of frail older patients. Since frailty is an independent risk factor for mortality [15], we hesitate to recommend the score as the sole means of predicting mortality in this population. A high Bansal score will deliver a reliable prediction irrespective of the presence of frailty, but in those with a low score, a validated frailty score is likely to contribute useful additional information on mortality.
We found one validated risk prediction model developed from the REIN registry, estimating risk of death at 3 months following dialysis initiation in older people with ESKD (the REIN score) [7]. The model included nine demographic, clinical and biochemical predictors: age, sex, history of congestive heart failure, peripheral vascular disease, dysrhythmia, cancer, severe behavioural disorder, mobility and baseline serum albumin concentration. Model discrimination was moderate (c-statistic in the internal validation cohort was 0.75). A second risk prediction model estimating risk of death at 6 months following dialysis initiation in older people [16], developed and internally validated in smaller cohorts from the same registry, had slightly inferior model discrimination (c-statistic 0.7).
Q4A: What is the best alternative method to assess functional decline in older and/or frail patients with advanced CKD?
4a.1 We recommend a simple score be used on a regular basis to assess functional status in older patients with CKD stage 3b–5d with the intention to identify those who would benefit from more in-depth geriatric assessment and rehabilitation (1C)
4a.2 We recommend that most simple scores, including self-report scales and field tests (sit to stand, gait speed or 6-min walk test) have comparable and sufficient discriminating power to identify patients with decreased functional status (1C)
Advice for clinical practice
On a regular basis implies 6–8 weekly for dialysis patients and at least at every clinic visit for patients with CKD stage 3b–5 who are not yet on dialysis.
Frailty scores are interlinked with functional status and can provide additional information during assessment and shared decision making on management options.
Rationale
CKD is an independent risk factor for functional impairment and frailty and functional decline is associated with adverse outcomes including excess mortality and hospitalization [17]. There is also evidence that interventions may reduce functional decline [18]. Several tools have been developed to assess the various domains of physical function in patients with CKD [19]. These have been categorized into laboratory-based measures of physiologic impairment, measures of mobility and performance capacity, which are either self-reported or obtained from field tests, and measures of physical activity. There is, however, no consensus on the most appropriate tool for assessing physical function in older patients with advanced CKD.
Evidence suggests that functional decline in older patients with CKD can feasibly be assessed using a combination of self- reporting and field tests. Such screening can help identify patients at risk who should be further evaluated by an experienced physician and/or multi-disciplinary team. The evidence suggests that all simple scores and tests perform reasonably well. None stands out as being specifically relevant for this particular cohort. Self-report measures of physical performance are simple, easy to use, reliable with good internal consistency, and predictive of adverse outcomes including mortality and hospitalization. It is unclear though, how sensitive they are to changes over time. Field tests of mobility and physical performance such as sit to stand, gait speed and the 6-min walk have been validated in cohorts that include older CKD patients. They have been shown to have good test–retest and interrater reliability, while also being predictive of adverse outcomes. They have also been shown to respond to interventions aimed at improving functional status. Physiologic measures such as vO2 max are difficult to incorporate into practice and have a limited role in this setting.
Q4b: Are interventions aimed at increasing functional status in older patients with renal failure (eGFR <45 mL/min/1.73 m2 or on dialysis) of benefit?
4b.1 We recommend that exercise has a positive impact on the functional status of older patients with CKD stage 3b or higher (1C)
4b.2 We suggest that exercise training be offered in a structured and individualized manner to avoid adverse events (2C)
Advice for clinical practice
‘Individualized’ means that the prescription is tailored to the needs and capacities of the patient. This can ideally be achieved by involving a clinical physiotherapist to prescribe a mix of strength and endurance exercises on a regular basis within the physical limitations of the patient.
Combined strength and endurance exercise should be provided on a regular basis.
In patients on haemodialysis exercise training can be administered during the first 2 h of the dialysis session.
Regular follow-up is important in order to optimize adherence and adjust the exercise intensity.
The evidence on positive outcomes of exercise tends to originate from programmes benefitting from intensive involvement of motivated physiotherapy teams.
There is little evidence that augmented dialysis improves functional status in the absence of multidisciplinary physiotherapy and nutritional interventions.
Rationale
Due to the aging of the CKD population and the associated increase of frailty in this group, it is important to formulate guidelines on how to maintain or improve functional status in an older CKD population. This question explored evidence regarding interventions that effectively improve functional status in frail older people with advanced CKD stages 3B or higher (eGFR <45 mL/min/1.73 m2) or on maintenance dialysis.
The available evidence is consistent in supporting a positive impact on the physical, functional and psychological well being of CKD patients who perform exercise. Older patients with CKD were able to respond with increased physical function to exercise training. None of the studies reported any adverse events or negative effects, which supports the safety and feasibility of exercise training in this setting. However all patients had been carefully screened by a physician before participation. Furthermore, studies were generally small, and there was a high risk for selection bias. In addition, it is noteworthy that exercise programmes were closely monitored by a team including a physiotherapist, and that most adapted the intensity of the exercise to the individual capacity of the patient. This may account for some of the benefits described and the lack of adverse events. The guideline development group therefore suggests that exercise programmes are supervised by a physiotherapist as a part of structured multi-disciplinary programme
Q5a: Which is the best alternative to evaluate nutritional status in older patients with advanced CKD 3b or higher (eGFR <45 mL/min/1.73 M2) or on dialysis
5a.1 We recommend the Subjective Global Assessment (SGA) as the gold standard to assess nutritional status of older patients with CKD stage 3b or higher (eGFR <45 mL/min/1.73 m2 (1C)
5a.2 We suggest that in older patients on haemodialysis, a score including serum albumin, body mass index, serum creatinine/body surface area and normalized Protein Nitrogen Appearance (nPNA) may be used to assess nutritional status (2D)
Rationale
Important nutritional deficiencies occur in patients with advanced CKD stage 3b or higher (eGFR <45 mL/min/1.732) as a result of metabolic defects, chronic inflammation, loss of appetite, repeated surgical interventions or infectious episodes [20]. This may lead to a state of protein-energy wasting, which is common in patients approaching the need for dialysis [21]. Further deterioration may occur post dialysis initiation and nutritional status is a strong predictor of survival in dialysis patients. Older patients are at high risk of wasting because of reduced appetite and a high prevalence of multi-morbidity, social isolation and depression. In an aging dialysis population, it is important to identify reliable, easy to use tools that allow routine assessment of nutritional status, so that patients at risk can be considered for further assessment and management.
We found a high degree of consensus among studies that SGA provides an acceptable estimate of nutritional status, is related to relevant patient outcomes (morbidity and mortality) and that it is sufficiently sensitive to reliably capture changes in nutritional status. SGA is reasonably easy to perform, relatively brief and can thus be used on a routine basis. The guideline development group suggests the use of SGA as a gold standard for routine assessment of nutritional status. For older patients on dialysis, a score including serum albumin, body mass index, serum creatinine normalized to body surface area and nPNA may be used to assess nutritional status [22]. It has been shown to have an acceptable predictive value for mortality and improvements in the score are associated with improved outcomes. External validation though is lacking.
Q5b: Which interventions are effective in improving nutritional status in older/frail patients with advanced CKD (eGFR <45 mL/min/1.73 m2) or on dialysis?
5b.1 We suggest a trial of structured dietary advice and support with the aim of improving nutritional status (2C)
Advice for clinical practice
Preserving nutritional status should prevail over any other dietary restriction.
There is insufficient evidence to prefer intravenous (intradialytic) nutritional support over oral nutritional support.
Correcting metabolic acidosis by oral supplementation is safe and cheap.
Rationale
Malnutrition and protein energy wasting are prevalent in older patients with advanced CKD (eGFR <45 mL/min/1.73 m2) and are associated with excess mortality [20–22]. Improvements in nutritional status have been reported to improve clinical outcomes, but though a variety of nutritional, pharmacological and dialytic interventions have been suggested, hard evidence from well-controlled and sufficiently powered randomized studies is lacking. Patients with advanced CKD (eGFR <45 mL/min/1.73 m2) are often placed on restrictive diets. For older patients these restrictions often come on top of many other factors that potentially compromise nutritional intake, such as social deprivation, functional and cognitive impairment, multi-morbidity, dental problems, depression and polypharmacy. For all these reasons, there remains uncertainty about optimal nutritional care for the older patient with advanced CKD, and a need for evidence-based guidelines on the prevention and management of malnutrition in this setting.
Most studies of oral nutritional supplements reported statistically significant improvements of nutritional parameters including serum albumin and SGA. Similar improvements were demonstrated with intradialyic parenteral nutrition, though in one randomized controlled trial this therapy conferred no additional benefit over oral supplements [23]. Correcting metabolic acidosis by oral sodium bicarbonate improved albumin and/or SGA and appeared safe [24, 25]. Studies of other pharmacological interventions including recombinant growth hormone and nandrolone decanoate were largely anecdotal. There was only one study of the effect of care by dieticians, which suggested an independent association between >12 months pre-dialysis care by a dietician and improved survival during the first year on dialysis [26]. In general, the quality of evidence was poor, consisting largely of single-centre observational studies with low patient numbers and short follow-up. There were few randomized controlled trials. There was no consensus on the definition of nutritional status, inclusion criteria, or on which surrogate outcomes are relevant in this population. No studies addressed the impact of nutritional intervention on mortality. All these factors make it difficult to assess the effectiveness of these interventions.
Q6: What is the benefit of dialysis in frail and older patients?
6.1 We recommend use of validated tools as explained in Questions 2 and 3 to project likely outcomes and help decide the appropriateness of discussing options for RRT (see Figure 1)
6.2 We recommend that the option for conservative management be discussed during the shared decision-making process on different management options for ESKD (1D)
6.3 We recommend the REIN score can be useful to stratify short term/6 month mortality risk of patients intending to start RRT (1C)
Advice for clinical practice
Evidence on this topic derives from observational studies only.
For frail, older patients with stage 5 CKD the survival benefits of dialysis over conservative management are uncertain.
Probability, life expectancy, quality of life impact and experience of being on dialysis are difficult concepts. Use of patient-friendly tools to help visualize these concepts may be helpful in enhancing patient understanding of the implications of different treatment options.
Multidisciplinary assessment of older patients with stage 5 CKD should include cognitive function, frailty and comorbidities, and nutritional, functional and psychosocial factors.
Rationale
The number of older patients receiving dialysis treatment has increased dramatically over recent years. Mortality is particularly high in this group and a substantial part of this is due to dialysis withdrawal [27]. The extent to which dialysis improves survival in frail older patients over conservative management (CM), if at all, is unclear [28, 29]. Undertaking dialysis also impacts on quality of life. Providing some symptom relief comes at the cost of significant burdens for the patient, their families and carers. Hence difficult decisions have to be made about whether any potential survival benefits for a particular individual are acceptable to that individual taking into consideration the rigours of the treatment. Studies on decisions about the appropriateness of dialysis for patients with frailty, advanced age and high co-morbidity have demonstrated wide discrepancies in clinician, patient and carer choices. Hence this question was posed as part of the guideline to try to support clinicians in helping patients faced with this common, complex and challenging decision.
The guideline development group considers that there are sufficient data to indicate that CM may be a viable treatment for older patients and/or those with high comorbidity and/or those with poor functional status, and may not adversely effect survival or QoL. Choosing CM over dialysis may avoid hospital admissions and improve access to palliative care. However, the evidence derives from observational studies only. These were of variable size and quality. Populations were defined by different criteria, measuring different outcomes over different time periods in different eras. There was no consistent definition of CM. Most studies defined patients only according to age. Frailty was formally assessed in only one study [30]. Decisions about whether to opt for dialysis or not should take place some considerable time before dialysis may be necessary. There are validated tools that can guide shared decision making. The 4-variable Kidney Failure Risk Equation [4] and Bansal equation [6] (see Questions 2 and 3) inform assessment of the competing risks of progression of kidney failure and death in those with advanced CKD (Figure 1). The REIN score [7] (see Question 3) estimates short-term mortality risk should dialysis be embarked on. Tools to assist shared decision making are also available. Visual tools may help patients understand risks [31].
ACKNOWLEDGEMENT
This article was produced with the financial and logistical support of the European Renal Association – European Dialysis and Transplant Association.





Comments