-
PDF
- Split View
-
Views
-
Cite
Cite
Sokratis Stoumpos, Patrick B. Mark, Emily P. McQuarrie, Jamie P. Traynor, Colin C. Geddes, Continued monitoring of acute kidney injury survivors might not be necessary in those regaining an estimated glomerular filtration rate >60 mL/min at 1 year, Nephrology Dialysis Transplantation, Volume 32, Issue 1, January 2017, Pages 81–88, https://doi.org/10.1093/ndt/gfw413
Close - Share Icon Share
Background. Severe acute kidney injury (AKI) among hospitalized patients often necessitates initiation of short-term dialysis. Little is known about the long-term outcome of those who recover to normal renal function. The aim of this study was to determine the long-term renal outcome of patients experiencing AKI requiring dialysis secondary to hypoperfusion injury and/or sepsis who recovered to apparently normal renal function.
Methods. All adult patients with AKI requiring dialysis in our centre between 1 January 1980 and 31 December 2010 were identified. We included patients who had estimated glomerular filtration rate (eGFR) >60 mL/min/1.73 m2 12 months or later after the episode of AKI. Patients were followed up until 3 March 2015. The primary outcome was time to chronic kidney disease (CKD) (defined as eGFR persistently <60 mL/min/1.73 m2) from first dialysis for AKI.
Results. Among 2922 patients with a single episode of dialysis-requiring AKI, 396 patients met the study inclusion criteria. The mean age was 49.8 (standard deviation 16.5) years and median follow-up was 7.9 [interquartile range (IQR) 4.8–12.7] years. Thirty-five (8.8%) of the patients ultimately developed CKD after a median of 5.3 (IQR 2.8–8.0) years from first dialysis for AKI giving an incidence rate of 1 per 100 person-years. Increasing age, diabetes and vascular disease were associated with higher risk of progression to CKD [adjusted hazard ratios (95% confidence interval): 1.06 (1.03, 1.09), 3.05 (1.41, 6.57) and 3.56 (1.80, 7.03), respectively].
Conclusions. Recovery from AKI necessitating in-hospital dialysis was associated with a very low risk of progression to CKD. Most of the patients who progressed to CKD had concurrent medical conditions meriting monitoring of renal function. Therefore, it seems unlikely that regular follow-up of renal function is beneficial in patients who recover to eGFR >60 mL/min/1.73 m2 by 12 months after an episode of AKI.
INTRODUCTION
Severe acute kidney injury (AKI) requiring initiation of dialysis is associated with in-hospital mortality rates from 45% to 60% [1–3]. Among those who survive, as many as 15% require dialysis at the time of discharge [1, 4].
Little is known about the long-term sequelae of AKI requiring in-hospital dialysis, especially in patients who recover sufficient kidney function. Reports of long-term renal function in survivors of dialysis-requiring AKI are sparse and only a few studies have observed patients for more than 1 year [5, 6]. In a recent prospective study of 19 survivors of AKI needing dialysis, 60% of participants did not have any reduction of kidney function and only one patient required maintenance haemodialysis after 5 years [7]. Recently, there has been increasing recognition that patients with AKI with apparent complete recovery remain at risk for long-term renal complications [8–10] and may benefit from longitudinal follow-up for primary chronic kidney disease (CKD) prevention. Current guidelines suggest that people should be monitored for the development of CKD for at least 2–3 years after AKI, even if serum creatinine has returned to baseline [11].
This study aimed to examine the long-term renal outcome among hospitalized patients who sustained AKI requiring acute dialysis, i.e. the severest form of AKI, and had normal renal function 12 months or later after the episode of AKI, to help determine if this group of patients merits long-term follow-up of kidney function.
MATERIALS AND METHODS
Design and participants
This was a single-centre population-based cohort study of all adult patients in our catchment area (serving a population of approximately 1.5 million) with AKI who required in-hospital dialysis in the nephrology unit. We identified all adults aged 18 years and older who had a first episode of dialysis-requiring AKI between 1 January 1980 and 31 December 2010 through a structured query language interrogation from the prospectively maintained electronic patient record. We included patients who had an estimated glomerular filtration rate (eGFR) >60 mL/min/1.73 m2 at least 12 months after the index hospitalization discharge. We excluded individuals with underlying nephropathy (glomerulonephritis, vasculitis with kidney involvement, haemolytic-uremic syndrome, polycystic kidney disease and multiple myeloma), receipt of a kidney transplant, dialysis specifically for drug toxicity or more than one episode of dialysis-requiring AKI.
Baseline data
Age, sex, aetiology of AKI, admission in intensive care unit (ICU) before transfer to the renal unit, duration of dialysis, length of hospital admission and the presence of comorbidity prior to the index hospitalization admission date (diabetes, vascular disease and heart failure) were recorded. Vascular disease included documented coronary artery disease, peripheral vascular disease or cerebrovascular disease.
We also retrieved data on the last serum creatinine prior to the index hospitalization (if it was known), serum creatinine on admission and discharge from hospital, the first serum creatinine recorded 12 months after the episode of AKI and subsequent serum creatinine levels up to the last creatinine recorded in the system. Our electronic patient record automatically imports all subsequent lab results from the point of installation of a patient at the time of referral, thus allowing complete capture of results for analysis even if the patient is not attending the renal services.
Follow-up data included early nephrology clinic appointment defined as a visit with a nephrologist within 90 days of hospital discharge. For the patients that developed CKD, follow-up data in any outpatient clinics for comorbid conditions were retrieved.
Causes of AKI included decreased effective plasma volume (volume contraction, heart failure, hypotension and cardiac arrest), sepsis, medication-related, radiocontrast media, post-operative and others (mainly rhabdomyolysis, acute pancreatitis, suspected thrombotic microangiopathy and uncertain aetiology).
Comorbidities were defined as follows: (i) diabetes—if a patient had ever required hypoglycaemic agents or insulin or when the diagnosis had been noted in the medical records; (ii) coronary artery disease—documented coronary stenosis by angiography or history of myocardial infarction or previous coronary revascularization by angioplasty, stenting or bypass surgery; (iii) peripheral vascular disease—history of lower extremity revascularization or digit or extremity amputation or when the diagnosis had been noted in the medical records; (iv) cerebrovascular disease—history of stroke or transient ischaemic attack documented by computed tomography scan, magnetic resonance imaging or when the diagnosis had been noted in the medical records; and (v) heart failure—classic signs and symptoms and either documentation by echocardiography or when the diagnosis had been noted in the medical records.
The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used for estimation of eGFR [12].
Outcome assessment
Patients were tracked for outcomes beginning 12 months after hospital discharge. The main study outcome was progression to CKD from first dialysis for AKI. This was defined as the date of the first sustained drop in eGFR defined as two outpatient eGFR of <60 mL/min/1.73 m2 at least 90 days apart. Normal renal function was defined as an outpatient eGFR of ≥60 mL/min/1.73 m2 on at least one occasion 1 year or later after the episode of AKI.
Statistical analyses
We examined differences in demographic and clinical factors, stratified by ever progressing to CKD or not. Continuous variables were expressed with means and standard deviations (SDs) or medians and interquartile ranges (IQRs) (for non-parametric data), and compared by Student’s t-test or Mann–Whitney U test as appropriate. Categorical variables were reported as frequencies and percentages and proportions compared by chi-squared test or Fisher’s exact test.
Incidence rates of progression to CKD were determined for study participants.
Because of the very long inclusion period of 30 years, we also compared outcomes in three eras: 1980–90, 1991–2000 and 2001–10. Mean eGFR on admission, discharge, 12 months after the AKI and at time of last creatinine recorded between patients that progressed to CKD and patients with normal renal function were compared by t-test.
Time-to-event analysis was performed until 3 March 2015 by Kaplan–Meier estimate with time to CKD as the outcome variable. Follow-up was censored at the date of the last serum creatinine recorded before 3 March 2015. Univariate analysis was performed to test the association of baseline variables with future CKD. Statistically significant variables were tested in a Cox proportional hazards model with time from first dialysis for AKI to progression to CKD as the dependent variable.
For all analyses, a P < 0.05 was considered significant. The IBM SPSS Statistics Package (version 21.0; SPSS, Inc., Armonk, NY, USA) was used for all analyses.
RESULTS
Study population
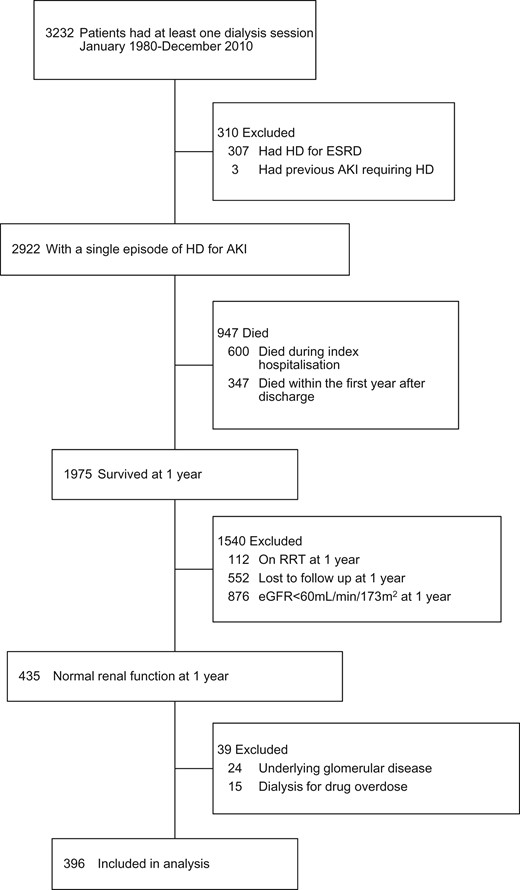
Creation of the dialysis-requiring AKI study cohort. HD, haemodialysis; RRT, renal replacement therapy.
The mean age of the enrolled participants was 49.8 (SD 16.5) years, 34.8% were women and the median length of index hospital admission was 13 (IQR 9–20) days. Of the participants, 13.4% had documented diabetes and 18.2% had vascular disease in the period before the index hospital admission. During hospitalization, 25.0% of patients with AKI requiring dialysis received ICU care before transfer to the renal unit. More than a quarter of participants had sepsis and 110 (27.8%) patients had multifactorial AKI. Fewer than half of the patients were followed up in the nephrology clinic within 90 days after discharge from the index admission (Table 1).
Patients who recovered from dialysis-requiring AKI, stratified by progression to CKD
| . | All patients (n = 396) . | CKD (n = 35) . | No CKD (n = 361) . | P-valuea . |
|---|---|---|---|---|
| Age, mean (SD) | 49.8 (16.5) | 60.8 (11.6) | 48.8 (16.5) | <0.001 |
| Male sex, n (%) | 258 (65.2) | 22 (62.9) | 236 (65.4) | 0.83 |
| Comorbidity | ||||
| Diabetes, n (%) | 53 (13.4) | 11 (31.4) | 42 (11.6) | 0.005 |
| Vascular disease (coronary artery disease, cerebrovascular disease, peripheral vascular disease), n (%) | 72 (18.2) | 17 (48.6) | 55 (15.2) | <0.001 |
| Heart failure, n (%) | 10 (2.5) | 3 (8.6) | 7 (1.9) | 0.04 |
| None of the above, n (%) | 283 (71.5) | 12 (34.3) | 271 (75.1) | 0.006 |
| Cause of AKI | ||||
| Decreased renal perfusion (volume contraction, heart failure, hypotension, cardiac arrest), n (%) | 103 (19.3) | 13 (25.0) | 90 (18.6) | 0.32 |
| Sepsis, n (%) | 143 (26.7) | 15 (28.9) | 128 (26.5) | 0.78 |
| Medication-related, n (%) | 78 (14.6) | 13 (25.0) | 65 (13.5) | 0.06 |
| Radiocontrast media, n (%) | 5 (0.9) | 1 (1.9) | 4 (0.8) | 1.00 |
| Post-operative, n (%) | 39 (7.3) | 5 (9.6) | 34 (7.0) | 0.60 |
| Others [rhabdomyolysis (n = 94), acute pancreatitis (n = 17), suspected TMA (n = 12), acute liver failure (n = 8), obstructive uropathy (n = 4), pregnancy related (n = 3), hypercalcaemia (n = 1), tumour lysis syndrome (n = 1), unclear (n = 27)], n (%) | 167 (31.2) | 5 (9.6) | 162 (33.6) | 0.004 |
| Era of AKI | ||||
| 1980 to 1990 | 126 (31.8) | 9 (25.8) | 117 (32.4) | 0.53 |
| 1991 to 2000 | 110 (27.8) | 13 (37.1) | 97 (26.9) | 0.32 |
| 2001 to 2010 | 160 (40.4) | 13 (37.1) | 147 (40.7) | 0.78 |
| Days on dialysis, median (IQR) | 6 (3–11) | 5 (2–9) | 6 (3–11) | 0.14 |
| Length of admission, days; median (IQR) | 13 (9–20) | 12 (7–20) | 14 (9–20) | 0.38 |
| Admission in ICU, n (%) | 99 (25.0) | 9 (25.7) | 90 (24.9) | 1.00 |
| eGFR at hospital discharge, mL/min/1.73m2; | ||||
| mean (SD) | 30.0 (22.3) | 37.9 (23.7) | 29.3 (22.1) | 0.03 |
| <15, n (%) | 116 (29.3) | 7 (20.0) | 109 (30.2) | 0.32 |
| 15–29, n (%) | 128 (32.3) | 5 (14.3) | 123 (34.1) | 0.06 |
| 30–59, n (%) | 108 (27.3) | 16 (45.7) | 92 (25.5) | 0.05 |
| ≥60, n (%) | 44 (11.1) | 7 (20.0) | 37 (10.2) | 0.12 |
| Early nephrology outpatient follow up, n (%) | 163 (41.2) | 11 (31.4) | 152 (42.1) | 0.40 |
| Died, n (%) | 113 (28.5) | 16 (45.7) | 97 (26.9) | 0.05 |
| Follow-up, year; median, (IQR) | 7.9 (4.8–12.7) | 8.0 (5.1–9.9) | 7.9 (4.8–12.9) | 0.41 |
| . | All patients (n = 396) . | CKD (n = 35) . | No CKD (n = 361) . | P-valuea . |
|---|---|---|---|---|
| Age, mean (SD) | 49.8 (16.5) | 60.8 (11.6) | 48.8 (16.5) | <0.001 |
| Male sex, n (%) | 258 (65.2) | 22 (62.9) | 236 (65.4) | 0.83 |
| Comorbidity | ||||
| Diabetes, n (%) | 53 (13.4) | 11 (31.4) | 42 (11.6) | 0.005 |
| Vascular disease (coronary artery disease, cerebrovascular disease, peripheral vascular disease), n (%) | 72 (18.2) | 17 (48.6) | 55 (15.2) | <0.001 |
| Heart failure, n (%) | 10 (2.5) | 3 (8.6) | 7 (1.9) | 0.04 |
| None of the above, n (%) | 283 (71.5) | 12 (34.3) | 271 (75.1) | 0.006 |
| Cause of AKI | ||||
| Decreased renal perfusion (volume contraction, heart failure, hypotension, cardiac arrest), n (%) | 103 (19.3) | 13 (25.0) | 90 (18.6) | 0.32 |
| Sepsis, n (%) | 143 (26.7) | 15 (28.9) | 128 (26.5) | 0.78 |
| Medication-related, n (%) | 78 (14.6) | 13 (25.0) | 65 (13.5) | 0.06 |
| Radiocontrast media, n (%) | 5 (0.9) | 1 (1.9) | 4 (0.8) | 1.00 |
| Post-operative, n (%) | 39 (7.3) | 5 (9.6) | 34 (7.0) | 0.60 |
| Others [rhabdomyolysis (n = 94), acute pancreatitis (n = 17), suspected TMA (n = 12), acute liver failure (n = 8), obstructive uropathy (n = 4), pregnancy related (n = 3), hypercalcaemia (n = 1), tumour lysis syndrome (n = 1), unclear (n = 27)], n (%) | 167 (31.2) | 5 (9.6) | 162 (33.6) | 0.004 |
| Era of AKI | ||||
| 1980 to 1990 | 126 (31.8) | 9 (25.8) | 117 (32.4) | 0.53 |
| 1991 to 2000 | 110 (27.8) | 13 (37.1) | 97 (26.9) | 0.32 |
| 2001 to 2010 | 160 (40.4) | 13 (37.1) | 147 (40.7) | 0.78 |
| Days on dialysis, median (IQR) | 6 (3–11) | 5 (2–9) | 6 (3–11) | 0.14 |
| Length of admission, days; median (IQR) | 13 (9–20) | 12 (7–20) | 14 (9–20) | 0.38 |
| Admission in ICU, n (%) | 99 (25.0) | 9 (25.7) | 90 (24.9) | 1.00 |
| eGFR at hospital discharge, mL/min/1.73m2; | ||||
| mean (SD) | 30.0 (22.3) | 37.9 (23.7) | 29.3 (22.1) | 0.03 |
| <15, n (%) | 116 (29.3) | 7 (20.0) | 109 (30.2) | 0.32 |
| 15–29, n (%) | 128 (32.3) | 5 (14.3) | 123 (34.1) | 0.06 |
| 30–59, n (%) | 108 (27.3) | 16 (45.7) | 92 (25.5) | 0.05 |
| ≥60, n (%) | 44 (11.1) | 7 (20.0) | 37 (10.2) | 0.12 |
| Early nephrology outpatient follow up, n (%) | 163 (41.2) | 11 (31.4) | 152 (42.1) | 0.40 |
| Died, n (%) | 113 (28.5) | 16 (45.7) | 97 (26.9) | 0.05 |
| Follow-up, year; median, (IQR) | 7.9 (4.8–12.7) | 8.0 (5.1–9.9) | 7.9 (4.8–12.9) | 0.41 |
TMA, thrombotic microangiopathy.
t-test or chi-squared test or Mann–Whitney U test where appropriate.
Patients who recovered from dialysis-requiring AKI, stratified by progression to CKD
| . | All patients (n = 396) . | CKD (n = 35) . | No CKD (n = 361) . | P-valuea . |
|---|---|---|---|---|
| Age, mean (SD) | 49.8 (16.5) | 60.8 (11.6) | 48.8 (16.5) | <0.001 |
| Male sex, n (%) | 258 (65.2) | 22 (62.9) | 236 (65.4) | 0.83 |
| Comorbidity | ||||
| Diabetes, n (%) | 53 (13.4) | 11 (31.4) | 42 (11.6) | 0.005 |
| Vascular disease (coronary artery disease, cerebrovascular disease, peripheral vascular disease), n (%) | 72 (18.2) | 17 (48.6) | 55 (15.2) | <0.001 |
| Heart failure, n (%) | 10 (2.5) | 3 (8.6) | 7 (1.9) | 0.04 |
| None of the above, n (%) | 283 (71.5) | 12 (34.3) | 271 (75.1) | 0.006 |
| Cause of AKI | ||||
| Decreased renal perfusion (volume contraction, heart failure, hypotension, cardiac arrest), n (%) | 103 (19.3) | 13 (25.0) | 90 (18.6) | 0.32 |
| Sepsis, n (%) | 143 (26.7) | 15 (28.9) | 128 (26.5) | 0.78 |
| Medication-related, n (%) | 78 (14.6) | 13 (25.0) | 65 (13.5) | 0.06 |
| Radiocontrast media, n (%) | 5 (0.9) | 1 (1.9) | 4 (0.8) | 1.00 |
| Post-operative, n (%) | 39 (7.3) | 5 (9.6) | 34 (7.0) | 0.60 |
| Others [rhabdomyolysis (n = 94), acute pancreatitis (n = 17), suspected TMA (n = 12), acute liver failure (n = 8), obstructive uropathy (n = 4), pregnancy related (n = 3), hypercalcaemia (n = 1), tumour lysis syndrome (n = 1), unclear (n = 27)], n (%) | 167 (31.2) | 5 (9.6) | 162 (33.6) | 0.004 |
| Era of AKI | ||||
| 1980 to 1990 | 126 (31.8) | 9 (25.8) | 117 (32.4) | 0.53 |
| 1991 to 2000 | 110 (27.8) | 13 (37.1) | 97 (26.9) | 0.32 |
| 2001 to 2010 | 160 (40.4) | 13 (37.1) | 147 (40.7) | 0.78 |
| Days on dialysis, median (IQR) | 6 (3–11) | 5 (2–9) | 6 (3–11) | 0.14 |
| Length of admission, days; median (IQR) | 13 (9–20) | 12 (7–20) | 14 (9–20) | 0.38 |
| Admission in ICU, n (%) | 99 (25.0) | 9 (25.7) | 90 (24.9) | 1.00 |
| eGFR at hospital discharge, mL/min/1.73m2; | ||||
| mean (SD) | 30.0 (22.3) | 37.9 (23.7) | 29.3 (22.1) | 0.03 |
| <15, n (%) | 116 (29.3) | 7 (20.0) | 109 (30.2) | 0.32 |
| 15–29, n (%) | 128 (32.3) | 5 (14.3) | 123 (34.1) | 0.06 |
| 30–59, n (%) | 108 (27.3) | 16 (45.7) | 92 (25.5) | 0.05 |
| ≥60, n (%) | 44 (11.1) | 7 (20.0) | 37 (10.2) | 0.12 |
| Early nephrology outpatient follow up, n (%) | 163 (41.2) | 11 (31.4) | 152 (42.1) | 0.40 |
| Died, n (%) | 113 (28.5) | 16 (45.7) | 97 (26.9) | 0.05 |
| Follow-up, year; median, (IQR) | 7.9 (4.8–12.7) | 8.0 (5.1–9.9) | 7.9 (4.8–12.9) | 0.41 |
| . | All patients (n = 396) . | CKD (n = 35) . | No CKD (n = 361) . | P-valuea . |
|---|---|---|---|---|
| Age, mean (SD) | 49.8 (16.5) | 60.8 (11.6) | 48.8 (16.5) | <0.001 |
| Male sex, n (%) | 258 (65.2) | 22 (62.9) | 236 (65.4) | 0.83 |
| Comorbidity | ||||
| Diabetes, n (%) | 53 (13.4) | 11 (31.4) | 42 (11.6) | 0.005 |
| Vascular disease (coronary artery disease, cerebrovascular disease, peripheral vascular disease), n (%) | 72 (18.2) | 17 (48.6) | 55 (15.2) | <0.001 |
| Heart failure, n (%) | 10 (2.5) | 3 (8.6) | 7 (1.9) | 0.04 |
| None of the above, n (%) | 283 (71.5) | 12 (34.3) | 271 (75.1) | 0.006 |
| Cause of AKI | ||||
| Decreased renal perfusion (volume contraction, heart failure, hypotension, cardiac arrest), n (%) | 103 (19.3) | 13 (25.0) | 90 (18.6) | 0.32 |
| Sepsis, n (%) | 143 (26.7) | 15 (28.9) | 128 (26.5) | 0.78 |
| Medication-related, n (%) | 78 (14.6) | 13 (25.0) | 65 (13.5) | 0.06 |
| Radiocontrast media, n (%) | 5 (0.9) | 1 (1.9) | 4 (0.8) | 1.00 |
| Post-operative, n (%) | 39 (7.3) | 5 (9.6) | 34 (7.0) | 0.60 |
| Others [rhabdomyolysis (n = 94), acute pancreatitis (n = 17), suspected TMA (n = 12), acute liver failure (n = 8), obstructive uropathy (n = 4), pregnancy related (n = 3), hypercalcaemia (n = 1), tumour lysis syndrome (n = 1), unclear (n = 27)], n (%) | 167 (31.2) | 5 (9.6) | 162 (33.6) | 0.004 |
| Era of AKI | ||||
| 1980 to 1990 | 126 (31.8) | 9 (25.8) | 117 (32.4) | 0.53 |
| 1991 to 2000 | 110 (27.8) | 13 (37.1) | 97 (26.9) | 0.32 |
| 2001 to 2010 | 160 (40.4) | 13 (37.1) | 147 (40.7) | 0.78 |
| Days on dialysis, median (IQR) | 6 (3–11) | 5 (2–9) | 6 (3–11) | 0.14 |
| Length of admission, days; median (IQR) | 13 (9–20) | 12 (7–20) | 14 (9–20) | 0.38 |
| Admission in ICU, n (%) | 99 (25.0) | 9 (25.7) | 90 (24.9) | 1.00 |
| eGFR at hospital discharge, mL/min/1.73m2; | ||||
| mean (SD) | 30.0 (22.3) | 37.9 (23.7) | 29.3 (22.1) | 0.03 |
| <15, n (%) | 116 (29.3) | 7 (20.0) | 109 (30.2) | 0.32 |
| 15–29, n (%) | 128 (32.3) | 5 (14.3) | 123 (34.1) | 0.06 |
| 30–59, n (%) | 108 (27.3) | 16 (45.7) | 92 (25.5) | 0.05 |
| ≥60, n (%) | 44 (11.1) | 7 (20.0) | 37 (10.2) | 0.12 |
| Early nephrology outpatient follow up, n (%) | 163 (41.2) | 11 (31.4) | 152 (42.1) | 0.40 |
| Died, n (%) | 113 (28.5) | 16 (45.7) | 97 (26.9) | 0.05 |
| Follow-up, year; median, (IQR) | 7.9 (4.8–12.7) | 8.0 (5.1–9.9) | 7.9 (4.8–12.9) | 0.41 |
TMA, thrombotic microangiopathy.
t-test or chi-squared test or Mann–Whitney U test where appropriate.
Progression to CKD
Thirty-five (8.8%) of the patients ultimately developed CKD after a median of 5.3 (IQR 2.8–8.0) years from first dialysis for AKI giving an incidence rate of progression to CKD of 1 per 100 person-years.
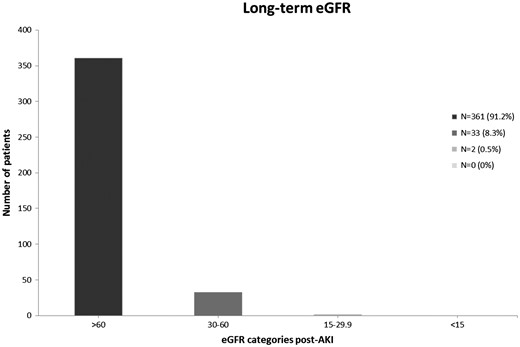
Long-term renal function after recovery from dialysis-requiring AKI.
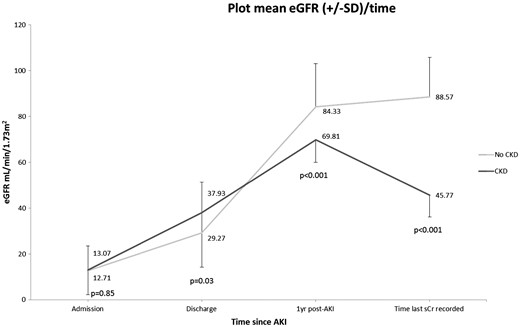
Estimated mean GFR and SD in patients that progressed to CKD versus those that maintained normal renal function. The difference in eGFR was statistically significant at discharge from hospital, at 1 year after the episode of AKI and at the time of last creatinine recorded. sCr, serum creatinine.
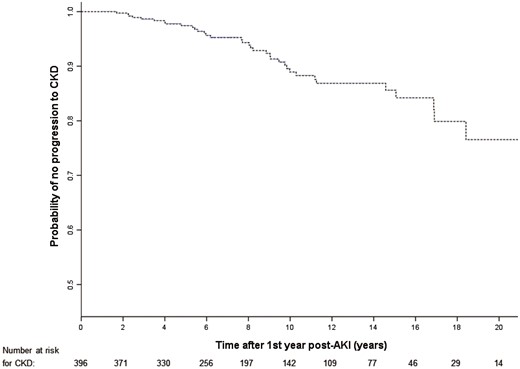
Kaplan–Meier plot of time to progression to CKD 1 year after an episode of dialysis-requiring AKI.
Predictors of progression to CKD
Univariate analysis for progression to CKD demonstrated age [hazard ratio (HR) 1.07 per year increase; P < 0.001], the presence of diabetes (HR 5.54; P < 0.001) and vascular disease (HR 6.60; P < 0.001) as independent predictors of progression to CKD. Due to the small number of CKD events, only three independent variables were tested in the proportional hazards regression model for multivariate analysis. By multivariate analysis age (HR 1.06; P < 0.001), the presence of diabetes (HR 3.05; P = 0.005) and vascular disease (HR 3.56; P < 0.001) were also independent predictors of progression to CKD (Table 2). There was no association between sex, early nephrology follow-up or the decade of AKI occurrence and progression to CKD.
| Factor . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | |
| Age, per year increase | 1.07 (1.04–1.10) | <0.001 | 1.06 (1.03–1.09) | <0.001 |
| Diabetes | 5.54 (2.60–11.80) | <0.001 | 3.05 (1.41–6.57) | 0.005 |
| Vascular disease | 6.60 (3.37–12.89) | <0.001 | 3.56 (1.80–7.03) | <0.001 |
| Factor . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | |
| Age, per year increase | 1.07 (1.04–1.10) | <0.001 | 1.06 (1.03–1.09) | <0.001 |
| Diabetes | 5.54 (2.60–11.80) | <0.001 | 3.05 (1.41–6.57) | 0.005 |
| Vascular disease | 6.60 (3.37–12.89) | <0.001 | 3.56 (1.80–7.03) | <0.001 |
| Factor . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | |
| Age, per year increase | 1.07 (1.04–1.10) | <0.001 | 1.06 (1.03–1.09) | <0.001 |
| Diabetes | 5.54 (2.60–11.80) | <0.001 | 3.05 (1.41–6.57) | 0.005 |
| Vascular disease | 6.60 (3.37–12.89) | <0.001 | 3.56 (1.80–7.03) | <0.001 |
| Factor . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | |
| Age, per year increase | 1.07 (1.04–1.10) | <0.001 | 1.06 (1.03–1.09) | <0.001 |
| Diabetes | 5.54 (2.60–11.80) | <0.001 | 3.05 (1.41–6.57) | 0.005 |
| Vascular disease | 6.60 (3.37–12.89) | <0.001 | 3.56 (1.80–7.03) | <0.001 |
DISCUSSION
Our findings expand on prior knowledge to provide clinicians with new information about the long-term effect of dialysis-requiring AKI. The data suggest that patients who recover from severe AKI have a low risk for CKD and ESRD. The relationship between AKI and CKD varied, with a greater risk associated with increasing age, diabetes and vascular comorbidity. Normal renal function at 1 year after the episode of AKI predicted a favourable long-term renal outcome. Strict adherence to current UK National Institute for Health and Care Excellence (NICE) guidelines [11] will lead to unnecessary follow-up for the majority of patients recovering from AKI.
Due in part to the ageing population, the incidence of AKI is increasing and is expected to double over the next decade [13]. Follow-up of all patients to detect the development of CKD and attempt to mitigate the risks of poor long-term outcomes requires staff resources, nephrologists’ time and patient time to attend clinic visits; therefore, these costs should be justified by demonstrable clinical benefits and there is no high quality evidence so far to prove that this will reduce morbidity or mortality. An unequivocal association between AKI and CKD/ESRD has been documented in a number of large, well-conducted studies [14] and a recent meta-analysis [15], but none of these studies focused on determining this relationship in patients who recover to normal renal function long after AKI. In a study looking at the long-term consequences of AKI in human immunodeficiency virus-infected persons, recovery from moderate to severe AKI markedly diminished the relationship between AKI and ESRD (those with recovery had a 2-fold risk for ESRD versus a 10-fold risk among those without recovery) [16].
The question is therefore which survivors of AKI warrant follow-up, who should follow these patients up and for how long. A multi-site clinical trial (NCT02483039) in Canada is currently recruiting adult AKI survivors to determine if structured post-AKI follow-up can improve outcomes compared with usual care; however, it will take some time to have the same length of follow-up as we have in this study.
Other data suggest that risk of progression to CKD may diminish over time [17] and when CKD develops after AKI, it occurs relatively early, within the first 3–6 months after hospital discharge [9]. Therefore, 6 months appears to be the minimum period of time during which kidney function should be monitored, with 12 months providing more reassurance that new or accelerated CKD will not be missed.
Interestingly, in this study eGFR at discharge did not predict long-term outcome as patients who subsequently developed CKD had higher eGFR at discharge. Sarcopenia is common in hospitalized acutely ill patients, ranging from 10% to 40%, and is associated with older age, prolonged hospital stay and adverse health outcomes [18–20]. This makes creatinine-based eGFR at discharge difficult to interpret as a higher eGFR may reflect loss of muscle mass and poor nutritional status. The same may hold true even a year after hospital discharge, especially for older AKI survivors, and may be a confounder. Future studies using cystatin C-derived eGFR, which is less dependent on muscle mass, may be more informative [21].
Several studies have previously evaluated the outcomes of survivors of AKI requiring dialysis; however, they often include patients with pre-existing CKD and patients who died or progressed to CKD or ESRD shortly following discharge. Wald et al. found that 3769 individuals with AKI requiring dialysis who survived free of dialysis for at least 30 days after discharge had a 3-fold increased risk of ESRD compared with 13 598 matches without AKI after a median follow-up of 3 years. Interestingly, the risk of chronic dialysis was 15 times higher when only patients without previous CKD were included in analysis [22]. Harel et al. showed that nephrology follow-up within 90 days of dialysis-requiring AKI was associated with a 24% lower risk of death at 2 years compared with patients who did not receive nephrology follow-up. Subjects with follow-up were more likely to progress to chronic dialysis, although they had a higher likelihood of having pre-existing CKD [23]. In another retrospective study of more than 100 000 ICU patients with or without pre-existing CKD in Sweden, the 5-year risk of progression to ESRD was 25.5% in the group of patients with acute on CKD, followed by 21.1% in the CKD group without AKI and 3.9% in the AKI group [24]. Comparable to our findings, recovery of renal function at 90 days after the AKI event was associated with four times lower probability of progression to CKD after a median follow-up of 2.8 years in a study of 3231 participants with AKI [25].
Studies assessing the risk of developing CKD have to take into account the association between CKD and age, and an estimate of the background population risk of developing CKD is essential to interpret results. The estimated probability of progression to CKD after an episode of dialysis-requiring AKI was 23.4% at 20 years, and this is similar to the prevalence of CKD in the general population for age-matched individuals. For example, large population studies have shown a prevalence of >35% for CKD stages 3–4 combined after the age of 70 years in USA [26], and a prevalence of CKD stages 3–5 of 18% for males and 28% for females aged between 65 and 74 years in UK [27]. It is therefore plausible that progression to CKD after prolonged follow-up relates to age, as well as to the common ancestors of both AKI and CKD, including a host of diverse metabolic and vascular abnormalities, rather than a direct consequence of previous AKI. The association of progression to CKD with vascular comorbid conditions supports this hypothesis, i.e. the patients may well have developed CKD, even without having had an episode of AKI. In a recent study of 1067 patients with AKI necessitating renal replacement therapy stratified by the presence of comorbidity, 10-year rates of progression to ESRD were 24.0% and 7.1% in patients with and without comorbidity, respectively [28].
In our study, 85.7% patients who progressed to CKD were already followed by multiple specialists for competing health problems and had regular blood tests including monitoring of renal function. The remaining 14.3% had a mean age of 68.4 years at time of CKD and a mean eGFR of 47 mL/min/1.73 m2 at time of last creatinine recorded. None of these patients had eGFR <30 mL/min/1.73 m2 at the end of follow-up. Thus, we contend that these patients do not need nephrology follow-up after the first year, but should be referred to nephrology for assessment if CKD develops.
Both human and animal studies have shown that a variety of intrinsic repair processes involving a host of growth factors and proliferative and other signalling cascades are activated rapidly after kidney injury [29, 30]. Sustained recovery might be attributed to adaptive repair, whereas progression may be secondary to maladaptive repair processes [31]. Several pathophysiologic processes constitute maladaptive repair with the potential to promote interstitial fibrosis. For example, ischaemia-reperfusion injury promotes the loss of renal vasculature, a reduction in the number of functioning nephrons and progressive renal fibrosis [32, 33]. Hence, evaluation of biomarkers indicative of adaptive and maladaptive repair represents the key to identify these patients who are most susceptible to progression. We have shown that serum creatinine concentration (and subsequent estimation of eGFR) at 1 year after the episode of AKI may provide a predictive insight as to the long-term consequences of AKI.
The strengths of this study include the rigorous assessments of recovery from AKI and progression to CKD by using outpatient creatinine measurements and actual creatinine changes instead of diagnostic codes. Also, the study population, which was a cohort of low risk individuals with inclusion only of patients that recovered to normal renal function, and the long duration of follow-up. Finally, only patients admitted to the renal unit were included to reduce potential selection bias.
The main limitation of our study is the single-centre study design that limits generalizability, so direct inferences must be interpreted in the context of the demographics of our study population. That said, in our cohort the patients were relatively young (mean age of 50 years) with low burden of comorbidity (13% diabetics and 18% vasculopaths), which is to be expected, as older, frail patients are less likely to survive long following an episode of AKI. Outcomes may have been influenced by centre-specific factors including time of initiation of dialysis following severe AKI, selection of patients for nephrology follow-up and duration of follow-up, and therapeutic interventions implemented to control blood pressure and proteinuria. Due to its retrospective design potential confounders may have not been recorded, though data were collected contemporaneously in the electronic record. Some data elements of interest may not be available in retrospective studies. It would have been interesting to analyse measures of kidney health other than serum creatinine at 1 year such as urinalysis, urine albumin to creatinine ratio, renal ultrasound and measurement of systolic and diastolic blood pressure, but analysis was limited by missing data. To minimize selection bias, we only included patients who received dialysis within our renal unit. Nonetheless, some selection bias may have occurred due to loss to follow-up as patients with fewer comorbid conditions and normal renal function in the long-term were less likely to get continued monitoring of their renal function. This may have overestimated the effect of dialysis-requiring AKI on the development of future CKD. Since the present study features a long inclusion period, practices may have changed substantially over this time period and this might have influenced our findings. Therefore, we analysed different eras but were unable to detect any era effect. The absence of a comparator group in our study prevents one from determining the degree to which AKI and dialysis is a predictor for long-term renal function in excess of global patient comorbidity before and during the index hospitalization. We did not retrieve data on subsequent episodes of non-dialysis-requiring AKI, which are associated with increased risk for developing CKD.
CONCLUSIONS
We have shown that recovery of renal function is a prognosticator of good long-term renal outcome in the most severe cases of AKI. If patients with AKI requiring dialysis survive to 12 months following onset of AKI and their renal function is normal, the risk of progression to CKD over the next 10 years is very low. Patients who would not otherwise require follow-up of renal function for comorbid conditions have very little to gain from ongoing monitoring of renal function.
CONFLICT OF INTEREST STATEMENT
None declared.





Comments