-
PDF
- Split View
-
Views
-
Cite
Cite
Steven Habbous, Sebastian Przech, Rey Acedillo, Sisira Sarma, Amit X. Garg, Janet Martin, The efficacy and safety of sevelamer and lanthanum versus calcium-containing and iron-based binders in treating hyperphosphatemia in patients with chronic kidney disease: a systematic review and meta-analysis, Nephrology Dialysis Transplantation, Volume 32, Issue 1, January 2017, Pages 111–125, https://doi.org/10.1093/ndt/gfw312
Close - Share Icon Share
Background. It remains unclear which phosphate binders should be preferred for hyperphosphatemia management in chronic kidney disease (CKD).
Methods. We performed a systematic review and meta-analysis of randomized trials comparing sevelamer or lanthanum with other phosphate binders in CKD.
Results. Fifty-one trials (8829 patients) were reviewed. Compared with calcium-based binders, all-cause mortality was nonsignificantly lower with sevelamer {risk ratio [RR] 0.62 [95% confidence interval (CI) 0.35–1.08]} and lanthanum [RR 0.73 (95% CI 0.18–3.00)], but risk of bias was concerning. Compared with calcium-based binders, sevelamer reduced the risk of hypercalcemia [RR 0.27 (95% CI 0.17–0.42)], as did lanthanum [RR 0.12 (95% CI 0.05–0.32)]. Sevelamer reduced hospitalizations [RR 0.50 (95% CI 0.31–0.81)], but not lanthanum [RR 0.80 (95% CI 0.34–1.93)]. The presence/absence of other clinically relevant outcomes was infrequently reported. Compared with calcium-based binders, sevelamer reduced serum calcium, low-density lipoprotein and coronary artery calcification, but increased intact parathyroid hormone. The clinical relevance of these changes is unknown since corresponding clinical outcomes were not reported. Lanthanum had less favorable impact on biochemical parameters. Sevelamer hydrochloride and sevelamer carbonate were similar in three studies. Sevelamer was similar to lanthanum (three studies) and iron-based binders (three studies).
Conclusion. Sevelamer was associated with a nonsignificant reduction in mortality and significantly lower hospitalization rates and hypercalcemia compared with calcium-based binders. However, differences in important outcomes, such as cardiac events, fractures, calciphylaxis, hyperchloremic acidosis and health-related quality of life remain understudied. Lanthanum and iron-based binders did not show superiority for any clinically relevant outcomes. Future studies that fail to measure clinically important outcomes (the reason why phosphate binders are prescribed in the first place) will be wasteful.
INTRODUCTION
Chronic kidney disease (CKD) affects 5% of adults, is very costly and is associated with a high risk of mortality and hospitalization [1–3]. Some of the poor outcomes for patients with CKD have been attributed to the inability of diseased kidneys to excrete dietary phosphate, leading to complex mineral and bone disorders and arterial calcification, which is thought to lead to increased risk of adverse cardiac events and premature mortality [4–7]. Phosphate binders have become the mainstay of prevention and management of hyperphosphatemia among patients with CKD, particularly the calcium-based phosphate binders (CBPBs) calcium carbonate and calcium acetate [8, 9]. Although inexpensive and highly effective in reducing serum phosphorus levels, CBPBs may result in elevated serum calcium and adverse clinical events related to hypercalcemia, potentially increasing the risk of vascular calcification and arterial stiffening. This prompted the development of calcium-free phosphate binders, including sevelamer hydrochloride, sevelamer carbonate, lanthanum carbonate and iron-based binders [10, 11].
Whether calcium-free binders improve clinically important outcomes compared with CBPBs still remains a matter of debate [12]. Recent systematic reviews failed to adequately address all clinically important outcomes (cardiac events, bone fractures, hypercalcemia, hospitalization, all-cause mortality) and failed to adequately address missing data and losses to follow-up [13–15, 13, 16, 17]. Moreover, clinical relevance of comparisons among the non-calcium-containing binders also need to be determined [11, 18]. The purpose of this systematic review and meta-analysis is to reevaluate the evidence reporting the safety and efficacy of calcium-free phosphate binders in CKD patients and to make recommendations for future research in this area.
METHODS
Search strategy and inclusion criteria
PubMed, Embase and Cochrane Central were searched on 19 January 2015 using the search terms ‘sevelamer’ OR ‘renagel’ OR ‘renvela’, supplemented with ‘lanthanum carbonate’ on 9 February 2016; ‘phosphate binder’ AND ‘iron’ was added as an addendum to our original protocol (PROSPERO CRD42015024667). Reference lists from publications were also reviewed for additional citations. Screening was performed by a single author (S.H.) and data extraction was performed independently by S.H. and S.P. Eligible studies were randomized trials on adults (>18 years of age) published in peer-reviewed journals (i.e. not abstracts) that compared sevelamer, lanthanum or iron-based binders with any other phosphate binder (excluding studies where only a nonactive placebo control was used or where a combination of active controls was used). Studies were not restricted by language, year of publication or study size.
Data collection
Studies were classified by dialysis modality as chronic (>2 months) hemodialysis (HD), incident HD, chronic peritoneal dialysis (PD) and non-dialysis-dependent (NDD)-CKD. Information collected included ethnicity (by patient country of origin), follow-up time, study size, age at randomization, untreated serum phosphorus levels for patient inclusion (i.e. after washout), and study design (crossover versus parallel-arm trial; single versus multicentered; double-blind versus open-label; fixed dosing versus treat to target). Results from crossover trials were combined with noncrossover trials. If numeric data were unavailable, graphical representations were digitized (http://arohatgi.info/WebPlotDigitizer/).
Risk of bias assessment
Study bias was assessed using the Cochrane Risk of Bias tool by considering the possibility of selection bias, measurement bias (blinding of subjects and study personnel ascertaining subjective outcomes such as like coronary artery calcification), number and reason for participant withdrawal, method of randomization and clear reporting of outcomes [19]. Other bias was considered ‘unclear’ if sample size was small (<100 patients, or <50 if crossover), or if the sample size was <200 (100 if crossover) and the trial was not registered. Double-blind studies were not considered to have a low risk of bias if the method of blinding was not described.
Study outcomes
The primary outcome was all-cause mortality. Secondary analyses included major adverse cardiovascular events, bone-related events (i.e. fractures, osteoporosis), calciphylaxis and biochemical events (hypercalcemia, hyperchloremic acidosis). Other secondary outcomes included loss to follow-up (as this may be a source of undocumented adverse events attributable to treatment) and hospitalization rates. Although they are of uncertain clinical relevance, we also extracted biochemical parameters at the end of the study, including serum phosphorus, corrected serum calcium, low-density lipoprotein (LDL), intact parathyroid hormone (iPTH) and coronary artery calcification (CAC).
Statistical methods and subgroup analyses
Risk ratios (RRs) with 95% confidence intervals (CIs) were calculated for discrete outcomes. Mean differences (MDs) were used to compare continuous outcomes (biochemical values). The number needed to treat (NNT) was calculated by pooling studies with similar follow-up time [20, 21]. The random effects model was used for all analyses. Review Manager 5.3 was used to prepare meta-analyses, present risk of bias tables, generate forest plots and calculate pooled estimates. Review Manager applies a continuity correction of 0.5 to all cells of binary outcomes for studies with single zeros (double-zero studies are omitted) [22]. The methodology for incorporating double-zero studies has been provided without the need for continuity correction [23]. Thus, we supplemented the pooled estimates generated by Review Manager with these beta-binomial regression methods using the macro provided by Kuss in SAS version 9.4 [23]. Trials that reported the absence of events were included, while those that failed to report whether or not events occurred were omitted.
A priori–defined subgroup analyses were conducted if substantive (significant and important) heterogeneity was present. Mortality was also evaluated in subgroups by the length of follow-up (post hoc comparison). Subgroups included CBPBs (CaCO3, calcium acetate), ethnicity (White, Asian, other), dialysis status (chronic HD, incident HD, NDD-CKD, PD) and nature of dosing (treat to target/variable, fixed). Heterogeneity across studies and between subgroups was assessed using Cochrane's Q (P-values) and Higgin's I2, together with visual inspection of forest plots [24]. When necessary, standard deviations (SDs) were calculated by multiplying standard errors by the square root of the sample size or estimated by single imputation using values from a similar study [19]. Publication bias was assessed using funnel plots and Egger's regression using Stata version 13.0. Meta-regression was conducted with Stata version 13.0 using log RR as the outcome. Regression coefficients were exponentiated for interpretability.
RESULTS
| Reference . | Washouta . | Follow-up time . | Crossover . | Centres . | Blinding . | Ethnicity . | Random (n) . | Baseline (n) . | End-of-study (n) . | Age, years (SD) . | Percent diabetic . | Dialysis vintage . | Inclusion (phosphorus mg/dL) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sevelamer versus CaCO3 | |||||||||||||
| Braun (2004) [25] | 2 weeks | 2 years | No | M | OL | Europe | 114 | 55/59 | 42/40 | 56.5 (14.1) | 13/17 | Stable HD | ≥5.5 |
| Caravaca (2007) [37] | 2 weeks | 3 weeks | Yes | S | OL | Spain | 20 | 20 | 17 | 54 (17) | NR | CKD stages 3–4 | None |
| Chennasamudram (2013) [26]b | 2 weeks | 8 weeks | Yes | S | OL | USA | 15 | 7/8 | 7/8 | 54 (9) | 100 | Chronic PD | ≥5.5 |
| De Santo (2006) [27] | 2 weeks | 24 weeks | Yes | S | OL | Italian | 16 | 8/8 | 8/8 | 35–50 years | 0 | HD 6–10 months | ≥5.5 |
| Di Iorio (2012) [28] | None | 24 months | No | M | OL | Italian | 239 | 121/118 | 107/105 | 57.9 (12.2) | 27/29 | CKD stage 3–4 | None |
| Di Iorio (2013) [29] | None | 36 months | No | M | OL | Italian | 466 | 232/234 | 199/198 | 65.6 (14.8) | 30/29 | New to HD | None |
| Ferreira (2008) [30] | 0 weeks | 12 months | No | M | OL | Portugal | 91 | 44/47 | 33/35 | 54.7 (14.5) | 6/23 | HD >3 months | <8.1 |
| Kakuta (2011) [31] | 0 weeks | 12 months | No | M | OL | Japan | 183 | 91/92 | 79/84 | 58.0 (12.0) | 23/19 | Stable HD | None |
| Koiwa (2005) [38] | 0 weeks | 4 weeks | No | M | OL | Japan | 56 | 29/27 | 16/20 | 57.1 (10.6) | 23 | HD >12 months | None |
| Lin (2014) [32] | 2 weeks | 48 weeks | No | M | OL | Taiwan | 75 | 36/39 | 23/27 | 58.2 (8.0) | NR | HD >3 months | ≥5.5 |
| Russo (2007) [36] | 0 weeks | 24 months | No | M | OL | Italy | 60 | 30/30 | 27/28 | 54.7 (12.7) | 0 | CKD stages 3–5 | None |
| Sadek (2003) [33] | 0 weeks | 5 months | No | S | OL | France | 42 | 21/21 | 15/16 | NR | NR | Chronic HD | NR |
| Shaheen (2004) [34] | 2 weeks | 8 weeks | Yes | S | OL | Saudi Arabia | 20 | 10/10 | 19/18 | 42.7 (9.9) | 20 | HD >3 months | ≥5.5 |
| Vlassara (2012) [35]b | 0 weeks | 8 weeks | Yes | S | OL | USA | 20 | 10/10 | 10/10 | 61.1 (11.5) | 100 | CKD stage 2–4 | NR |
| Sevelamer versus Ca-acetate | |||||||||||||
| Barreto (2008) [41] | 2 weeks | 12 months | No | M | OL | Brazil | 101 | 52/49 | 41/30 | 47 (13.3) | 15/13 | HD >3 months | ≥5.5 |
| Bleyer (1999) [39] | 2 weeks | 8 weeks | Yes | M | OL | USA | 83 | 83 | 80 | 54.5 (15) | 29 | Stable HD | ≥6 |
| Block (2012) [49]b | 0 weeks | 9 months | No | S | DBc | USA | 90 | 30/30 | 30/30 | 68 (11) | 53/57 | CKD < stage 5D | 10.8–18.6 |
| Caglar (2008) [40] | 2 weeks | 8 weeks | No | S | NR | Turkey | 50 | 25/25 | 25/25 | 40.4 (13.0) | 0 | CKD stage 4 | ≥5.5 |
| Evenepoel (2009) [42] | 2 weeks | 12 weeks | No | M | OL | Europe | 143 | 97/46 | 74/30 | 54.4 (15.7) | 20/26 | PD >6 months | ≥5.5 |
| Hervas (2003) [43] | 2 weeks | 34 weeks | No | NR | NR | Spain | 51 | 18/22 | 18/22 | 60.4 (15.1) | 15 | HD >3 months | ≥6 |
| Lin (2010) [44] | 2 weeks | 8 weeks | No | S | OL | Taiwan | 52 | 26/26 | 23/20 | 57.3 (12.0) | 42/27 | HD >3 months | ≥5.5 |
| Liu (2006) [71] | 2 weeks | 8 weeks | No | S | OL | Asian | 73 | 37/36 | 33/30 | 48.9 (11.5) | 8/15 | HD >3 months | ≥6 |
| Navarro-González (2011) [72] | 2–3 weeks | 12 weeks | No | S | OL | Spain | 65 | 33/32 | 30/29 | 61.2 (15.5) | 43/41 | HD >3 months | NR |
| Oliveira (2010) [45] | 0 weeks | 6 weeks | No | S | OL | Brazil | 40 | 21/19 | 21/17 | 50.38 (11.4) | 0 | CKD stage 3–4 | None |
| Qunibi (2008) [46] | 6 weeks | 12 months | No | M | OL | USA | 203 | 100/103 | 70/59 | 59.4 (12.5) | 57/57 | HD >3 months | ≥5.5 |
| Qunibi (2004) [47] | 1–3 weeks | 8 weeks | No | M | DB | USA | 100 | 50/48 | 45/46 | 53.1 (14.0) | NR | HD > 3 months | ≥6 |
| Yilmaz (2012) [48] | 2 weeks | 8 weeks | No | S | OL | Turkey | 100 | 47/53 | 47/53 | 46 (median) | 0 | CKD stage 4 | ≥6 |
| Sevelamer versus unspecified calcium-based binder | |||||||||||||
| Block (2005) [51] | 0 weeks | 18 months | No | M | OL | USA | 148 | 73/75 | 54/55 | 58.0 (15.0) | 63/56 | New to HD | None |
| Chertow (2002) [52] | 2 weeks | 12 months | No | M | OL | USA/Europe | 200 | 99/101 | 81/88 | 56.5 (15.0) | 32/33 | Stable HD | ≥5.5 |
| Suki (2007) [53] | 0 weeks | 36 months | No | M | OL | USA | 2013 | 1053/1068 | 551/517 | 60.0 (14.7) | 51/50 | HD >3 months | None |
| Sevelamer versus lanthanum | |||||||||||||
| Block (2012) [49]b | 0 weeks | 9 months | No | S | DBc | USA | 90 | 30/30 | 30/28 | 68 (11) | 53/57 | CKD < stage 5D | 10.8–18.6 |
| Kasai (2012) [54] | 9 weeks | 13 weeks | Yes | S | OL | Japan | 42 | 42 | 41 | 60.9 (11.9) | 31 | HD >3 months | NR |
| Sprague (2009) [55] | 3 weeks | 4 weeks | Yes | M | OL | Intl | 182 | 86/95 | 60/59 | 55.5 (13.1) | HD >2 months | ≥6 | |
| Sevelamer versus magnesium carbonate | |||||||||||||
| de Francisco (2010) [50]d | 2–3 weeks | 24 weeks | No | M | OL | Europe | 255 | 129/126 | 99/105 | 57.6 (12.9) | 20/25 | HD/HDF >3 months | ≥5.5 |
| Zwiech (2011) [56] | 2 weeks | 12 weeks | No | S | OL | Poland | 40 | 10/30 | 10/28 | 57.8 (13.6) | NR | HD >6 months | ≥5.5 |
| Sevalamer hydrochloride versus sevelamer carbonate | |||||||||||||
| Abraham (2012) [57] | 2 weeks | 6 weeks | No | M | OL | Indian | 97 | 48/49 | 44/44 | 47.7 (12.6) | 29/20 | Stable HD | ≥6 |
| Delmez (2007) [58] | 0 weeks | 8 weeks | Yes | M | DB | USA | 79 | 40/39 | 19/21 | 58.1 (12.3) | Chronic HD | None | |
| Fan (2009) [59] | 2 weeks | 4 weeks | Yes | M | OL | UK (London) | 31 | 14/17 | 24 | 59.2 (13.2) | 13 | HD >3 months | ≥5.5 |
| Sevalamer versus iron-based binder | |||||||||||||
| Chen (2011) [73] | 1–2 weeks | 12 weeks | No | M | OL | Japan/Taiwan | 203 | 68/135 | 54/119 | 58.6 (11.2) | 29 | HD >3 months | ≥6.0 |
| Floege (2014) [74]b | 2–4 weeks | 12 weeks | No | M | OL | Intl | 1059 | 349/710 | 293/515 | 56 (14) | 28 | HD/PD | ≥6.0 |
| Yokoyama (2014) [75] | 2 weeks | 12 weeks | No | M | OL | Japan | 230 | 114/116 | 97/102 | 60.8 (10.1) | 26 | HD >3 months | ≥6.1 |
| Lanthanum versus calcium-based binder | |||||||||||||
| D'Haese (2003) [60] | 8–12 d | 12 months | No | M | OL | Europe | 98 | 49/49 | 34/34 | 55 (14.3) | 26 | HD >3 months | None |
| Hutchison (2005) [67] | 1–3 weeks | 5 weeks | No | M | OL | Europe | 800 | 533/267 | 453/209 | 57.5 (14.0) | NR | HD > 3 months | >5.6 |
| Lee (2013) [68] | 0 weeks | 24 weeks | No | M | NR | S Korea | 72 | 35/35 | 20/30 | 50.4 (11.4) | PD >6 months | >5.6 | |
| Ohtake (2013) [64] | 0 weeks | 6 months | No | S | OL | Japan | 52 | 26/26 | 19/23 | 67.8 (6.3) | 43 | Stable HD | None |
| Scaria (2009) [70]e | 4 weeks | 4 weeks | Yes | S | OL | India | 26 | 13/13 | 10/10 | 49.9 | NR | CKD stage 4 | >5.5 |
| Shigematsu (2008) [62] | 2 weeks | 8 weeks | No | M | DB | Japan | 259 | 126/132 | 122/126 | 57.4 (11.1) | 20 | Stable HD | 5.6–11.0 |
| Soriano (2013) [65] | 0 weeks | 4 months | No | S | NR | Spain | 32 | 16/16 | 16/16 | ∼60 | 25/12.5 | CKD stages 3–5 | >4 |
| Spasovski (2006) [61] | 0 weeks | 12 months | No | S | NR | Macedonia | 24 | 12/12 | 10/10 | 56 (9.8) | 20 | New to HD | None |
| Toussaint (2011) [66] | 1 week | 6 months | No | S | OL | Australia | 45 | 22/23 | 17/13 | 57.4 (14.9) | 36/39 | HD > 3 months | >5 |
| Toida (2012) [69] | 2 weeks | 3 months | Yes | S | OL | Japan | 50 | 25/25 | 18/24 | 65.6 (11.5) | Stable HD | None | |
| Wada (2014) [63] | 2 weeks | 12 months | No | S | OL | Japan | 43 | 21/22 | 19/22 | 65.7 (9.26) | 100 | HD >6 months | None |
| Reference . | Washouta . | Follow-up time . | Crossover . | Centres . | Blinding . | Ethnicity . | Random (n) . | Baseline (n) . | End-of-study (n) . | Age, years (SD) . | Percent diabetic . | Dialysis vintage . | Inclusion (phosphorus mg/dL) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sevelamer versus CaCO3 | |||||||||||||
| Braun (2004) [25] | 2 weeks | 2 years | No | M | OL | Europe | 114 | 55/59 | 42/40 | 56.5 (14.1) | 13/17 | Stable HD | ≥5.5 |
| Caravaca (2007) [37] | 2 weeks | 3 weeks | Yes | S | OL | Spain | 20 | 20 | 17 | 54 (17) | NR | CKD stages 3–4 | None |
| Chennasamudram (2013) [26]b | 2 weeks | 8 weeks | Yes | S | OL | USA | 15 | 7/8 | 7/8 | 54 (9) | 100 | Chronic PD | ≥5.5 |
| De Santo (2006) [27] | 2 weeks | 24 weeks | Yes | S | OL | Italian | 16 | 8/8 | 8/8 | 35–50 years | 0 | HD 6–10 months | ≥5.5 |
| Di Iorio (2012) [28] | None | 24 months | No | M | OL | Italian | 239 | 121/118 | 107/105 | 57.9 (12.2) | 27/29 | CKD stage 3–4 | None |
| Di Iorio (2013) [29] | None | 36 months | No | M | OL | Italian | 466 | 232/234 | 199/198 | 65.6 (14.8) | 30/29 | New to HD | None |
| Ferreira (2008) [30] | 0 weeks | 12 months | No | M | OL | Portugal | 91 | 44/47 | 33/35 | 54.7 (14.5) | 6/23 | HD >3 months | <8.1 |
| Kakuta (2011) [31] | 0 weeks | 12 months | No | M | OL | Japan | 183 | 91/92 | 79/84 | 58.0 (12.0) | 23/19 | Stable HD | None |
| Koiwa (2005) [38] | 0 weeks | 4 weeks | No | M | OL | Japan | 56 | 29/27 | 16/20 | 57.1 (10.6) | 23 | HD >12 months | None |
| Lin (2014) [32] | 2 weeks | 48 weeks | No | M | OL | Taiwan | 75 | 36/39 | 23/27 | 58.2 (8.0) | NR | HD >3 months | ≥5.5 |
| Russo (2007) [36] | 0 weeks | 24 months | No | M | OL | Italy | 60 | 30/30 | 27/28 | 54.7 (12.7) | 0 | CKD stages 3–5 | None |
| Sadek (2003) [33] | 0 weeks | 5 months | No | S | OL | France | 42 | 21/21 | 15/16 | NR | NR | Chronic HD | NR |
| Shaheen (2004) [34] | 2 weeks | 8 weeks | Yes | S | OL | Saudi Arabia | 20 | 10/10 | 19/18 | 42.7 (9.9) | 20 | HD >3 months | ≥5.5 |
| Vlassara (2012) [35]b | 0 weeks | 8 weeks | Yes | S | OL | USA | 20 | 10/10 | 10/10 | 61.1 (11.5) | 100 | CKD stage 2–4 | NR |
| Sevelamer versus Ca-acetate | |||||||||||||
| Barreto (2008) [41] | 2 weeks | 12 months | No | M | OL | Brazil | 101 | 52/49 | 41/30 | 47 (13.3) | 15/13 | HD >3 months | ≥5.5 |
| Bleyer (1999) [39] | 2 weeks | 8 weeks | Yes | M | OL | USA | 83 | 83 | 80 | 54.5 (15) | 29 | Stable HD | ≥6 |
| Block (2012) [49]b | 0 weeks | 9 months | No | S | DBc | USA | 90 | 30/30 | 30/30 | 68 (11) | 53/57 | CKD < stage 5D | 10.8–18.6 |
| Caglar (2008) [40] | 2 weeks | 8 weeks | No | S | NR | Turkey | 50 | 25/25 | 25/25 | 40.4 (13.0) | 0 | CKD stage 4 | ≥5.5 |
| Evenepoel (2009) [42] | 2 weeks | 12 weeks | No | M | OL | Europe | 143 | 97/46 | 74/30 | 54.4 (15.7) | 20/26 | PD >6 months | ≥5.5 |
| Hervas (2003) [43] | 2 weeks | 34 weeks | No | NR | NR | Spain | 51 | 18/22 | 18/22 | 60.4 (15.1) | 15 | HD >3 months | ≥6 |
| Lin (2010) [44] | 2 weeks | 8 weeks | No | S | OL | Taiwan | 52 | 26/26 | 23/20 | 57.3 (12.0) | 42/27 | HD >3 months | ≥5.5 |
| Liu (2006) [71] | 2 weeks | 8 weeks | No | S | OL | Asian | 73 | 37/36 | 33/30 | 48.9 (11.5) | 8/15 | HD >3 months | ≥6 |
| Navarro-González (2011) [72] | 2–3 weeks | 12 weeks | No | S | OL | Spain | 65 | 33/32 | 30/29 | 61.2 (15.5) | 43/41 | HD >3 months | NR |
| Oliveira (2010) [45] | 0 weeks | 6 weeks | No | S | OL | Brazil | 40 | 21/19 | 21/17 | 50.38 (11.4) | 0 | CKD stage 3–4 | None |
| Qunibi (2008) [46] | 6 weeks | 12 months | No | M | OL | USA | 203 | 100/103 | 70/59 | 59.4 (12.5) | 57/57 | HD >3 months | ≥5.5 |
| Qunibi (2004) [47] | 1–3 weeks | 8 weeks | No | M | DB | USA | 100 | 50/48 | 45/46 | 53.1 (14.0) | NR | HD > 3 months | ≥6 |
| Yilmaz (2012) [48] | 2 weeks | 8 weeks | No | S | OL | Turkey | 100 | 47/53 | 47/53 | 46 (median) | 0 | CKD stage 4 | ≥6 |
| Sevelamer versus unspecified calcium-based binder | |||||||||||||
| Block (2005) [51] | 0 weeks | 18 months | No | M | OL | USA | 148 | 73/75 | 54/55 | 58.0 (15.0) | 63/56 | New to HD | None |
| Chertow (2002) [52] | 2 weeks | 12 months | No | M | OL | USA/Europe | 200 | 99/101 | 81/88 | 56.5 (15.0) | 32/33 | Stable HD | ≥5.5 |
| Suki (2007) [53] | 0 weeks | 36 months | No | M | OL | USA | 2013 | 1053/1068 | 551/517 | 60.0 (14.7) | 51/50 | HD >3 months | None |
| Sevelamer versus lanthanum | |||||||||||||
| Block (2012) [49]b | 0 weeks | 9 months | No | S | DBc | USA | 90 | 30/30 | 30/28 | 68 (11) | 53/57 | CKD < stage 5D | 10.8–18.6 |
| Kasai (2012) [54] | 9 weeks | 13 weeks | Yes | S | OL | Japan | 42 | 42 | 41 | 60.9 (11.9) | 31 | HD >3 months | NR |
| Sprague (2009) [55] | 3 weeks | 4 weeks | Yes | M | OL | Intl | 182 | 86/95 | 60/59 | 55.5 (13.1) | HD >2 months | ≥6 | |
| Sevelamer versus magnesium carbonate | |||||||||||||
| de Francisco (2010) [50]d | 2–3 weeks | 24 weeks | No | M | OL | Europe | 255 | 129/126 | 99/105 | 57.6 (12.9) | 20/25 | HD/HDF >3 months | ≥5.5 |
| Zwiech (2011) [56] | 2 weeks | 12 weeks | No | S | OL | Poland | 40 | 10/30 | 10/28 | 57.8 (13.6) | NR | HD >6 months | ≥5.5 |
| Sevalamer hydrochloride versus sevelamer carbonate | |||||||||||||
| Abraham (2012) [57] | 2 weeks | 6 weeks | No | M | OL | Indian | 97 | 48/49 | 44/44 | 47.7 (12.6) | 29/20 | Stable HD | ≥6 |
| Delmez (2007) [58] | 0 weeks | 8 weeks | Yes | M | DB | USA | 79 | 40/39 | 19/21 | 58.1 (12.3) | Chronic HD | None | |
| Fan (2009) [59] | 2 weeks | 4 weeks | Yes | M | OL | UK (London) | 31 | 14/17 | 24 | 59.2 (13.2) | 13 | HD >3 months | ≥5.5 |
| Sevalamer versus iron-based binder | |||||||||||||
| Chen (2011) [73] | 1–2 weeks | 12 weeks | No | M | OL | Japan/Taiwan | 203 | 68/135 | 54/119 | 58.6 (11.2) | 29 | HD >3 months | ≥6.0 |
| Floege (2014) [74]b | 2–4 weeks | 12 weeks | No | M | OL | Intl | 1059 | 349/710 | 293/515 | 56 (14) | 28 | HD/PD | ≥6.0 |
| Yokoyama (2014) [75] | 2 weeks | 12 weeks | No | M | OL | Japan | 230 | 114/116 | 97/102 | 60.8 (10.1) | 26 | HD >3 months | ≥6.1 |
| Lanthanum versus calcium-based binder | |||||||||||||
| D'Haese (2003) [60] | 8–12 d | 12 months | No | M | OL | Europe | 98 | 49/49 | 34/34 | 55 (14.3) | 26 | HD >3 months | None |
| Hutchison (2005) [67] | 1–3 weeks | 5 weeks | No | M | OL | Europe | 800 | 533/267 | 453/209 | 57.5 (14.0) | NR | HD > 3 months | >5.6 |
| Lee (2013) [68] | 0 weeks | 24 weeks | No | M | NR | S Korea | 72 | 35/35 | 20/30 | 50.4 (11.4) | PD >6 months | >5.6 | |
| Ohtake (2013) [64] | 0 weeks | 6 months | No | S | OL | Japan | 52 | 26/26 | 19/23 | 67.8 (6.3) | 43 | Stable HD | None |
| Scaria (2009) [70]e | 4 weeks | 4 weeks | Yes | S | OL | India | 26 | 13/13 | 10/10 | 49.9 | NR | CKD stage 4 | >5.5 |
| Shigematsu (2008) [62] | 2 weeks | 8 weeks | No | M | DB | Japan | 259 | 126/132 | 122/126 | 57.4 (11.1) | 20 | Stable HD | 5.6–11.0 |
| Soriano (2013) [65] | 0 weeks | 4 months | No | S | NR | Spain | 32 | 16/16 | 16/16 | ∼60 | 25/12.5 | CKD stages 3–5 | >4 |
| Spasovski (2006) [61] | 0 weeks | 12 months | No | S | NR | Macedonia | 24 | 12/12 | 10/10 | 56 (9.8) | 20 | New to HD | None |
| Toussaint (2011) [66] | 1 week | 6 months | No | S | OL | Australia | 45 | 22/23 | 17/13 | 57.4 (14.9) | 36/39 | HD > 3 months | >5 |
| Toida (2012) [69] | 2 weeks | 3 months | Yes | S | OL | Japan | 50 | 25/25 | 18/24 | 65.6 (11.5) | Stable HD | None | |
| Wada (2014) [63] | 2 weeks | 12 months | No | S | OL | Japan | 43 | 21/22 | 19/22 | 65.7 (9.26) | 100 | HD >6 months | None |
Sevelamer refers to sevelamer hydrochloride, unless otherwise specified. OL, open-label; DB, double-blind; SD, standard deviation; HD, hemodialysis; PD, peritoneal dialysis; HDF, hemodiafiltration; NDD-CKD, non-dialysis-dependent chronic kidney disease; NR, not reported.
aLength of time (weeks) prior to randomization that current phosphate binders were removed.
bIntervention is sevelamer carbonate.
cNo explanation of how this was successfully undertaken due to differences in taste and size as reported by others.
dComparator also includes calcium acetate.
eComparator is calcium acetate.
| Reference . | Washouta . | Follow-up time . | Crossover . | Centres . | Blinding . | Ethnicity . | Random (n) . | Baseline (n) . | End-of-study (n) . | Age, years (SD) . | Percent diabetic . | Dialysis vintage . | Inclusion (phosphorus mg/dL) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sevelamer versus CaCO3 | |||||||||||||
| Braun (2004) [25] | 2 weeks | 2 years | No | M | OL | Europe | 114 | 55/59 | 42/40 | 56.5 (14.1) | 13/17 | Stable HD | ≥5.5 |
| Caravaca (2007) [37] | 2 weeks | 3 weeks | Yes | S | OL | Spain | 20 | 20 | 17 | 54 (17) | NR | CKD stages 3–4 | None |
| Chennasamudram (2013) [26]b | 2 weeks | 8 weeks | Yes | S | OL | USA | 15 | 7/8 | 7/8 | 54 (9) | 100 | Chronic PD | ≥5.5 |
| De Santo (2006) [27] | 2 weeks | 24 weeks | Yes | S | OL | Italian | 16 | 8/8 | 8/8 | 35–50 years | 0 | HD 6–10 months | ≥5.5 |
| Di Iorio (2012) [28] | None | 24 months | No | M | OL | Italian | 239 | 121/118 | 107/105 | 57.9 (12.2) | 27/29 | CKD stage 3–4 | None |
| Di Iorio (2013) [29] | None | 36 months | No | M | OL | Italian | 466 | 232/234 | 199/198 | 65.6 (14.8) | 30/29 | New to HD | None |
| Ferreira (2008) [30] | 0 weeks | 12 months | No | M | OL | Portugal | 91 | 44/47 | 33/35 | 54.7 (14.5) | 6/23 | HD >3 months | <8.1 |
| Kakuta (2011) [31] | 0 weeks | 12 months | No | M | OL | Japan | 183 | 91/92 | 79/84 | 58.0 (12.0) | 23/19 | Stable HD | None |
| Koiwa (2005) [38] | 0 weeks | 4 weeks | No | M | OL | Japan | 56 | 29/27 | 16/20 | 57.1 (10.6) | 23 | HD >12 months | None |
| Lin (2014) [32] | 2 weeks | 48 weeks | No | M | OL | Taiwan | 75 | 36/39 | 23/27 | 58.2 (8.0) | NR | HD >3 months | ≥5.5 |
| Russo (2007) [36] | 0 weeks | 24 months | No | M | OL | Italy | 60 | 30/30 | 27/28 | 54.7 (12.7) | 0 | CKD stages 3–5 | None |
| Sadek (2003) [33] | 0 weeks | 5 months | No | S | OL | France | 42 | 21/21 | 15/16 | NR | NR | Chronic HD | NR |
| Shaheen (2004) [34] | 2 weeks | 8 weeks | Yes | S | OL | Saudi Arabia | 20 | 10/10 | 19/18 | 42.7 (9.9) | 20 | HD >3 months | ≥5.5 |
| Vlassara (2012) [35]b | 0 weeks | 8 weeks | Yes | S | OL | USA | 20 | 10/10 | 10/10 | 61.1 (11.5) | 100 | CKD stage 2–4 | NR |
| Sevelamer versus Ca-acetate | |||||||||||||
| Barreto (2008) [41] | 2 weeks | 12 months | No | M | OL | Brazil | 101 | 52/49 | 41/30 | 47 (13.3) | 15/13 | HD >3 months | ≥5.5 |
| Bleyer (1999) [39] | 2 weeks | 8 weeks | Yes | M | OL | USA | 83 | 83 | 80 | 54.5 (15) | 29 | Stable HD | ≥6 |
| Block (2012) [49]b | 0 weeks | 9 months | No | S | DBc | USA | 90 | 30/30 | 30/30 | 68 (11) | 53/57 | CKD < stage 5D | 10.8–18.6 |
| Caglar (2008) [40] | 2 weeks | 8 weeks | No | S | NR | Turkey | 50 | 25/25 | 25/25 | 40.4 (13.0) | 0 | CKD stage 4 | ≥5.5 |
| Evenepoel (2009) [42] | 2 weeks | 12 weeks | No | M | OL | Europe | 143 | 97/46 | 74/30 | 54.4 (15.7) | 20/26 | PD >6 months | ≥5.5 |
| Hervas (2003) [43] | 2 weeks | 34 weeks | No | NR | NR | Spain | 51 | 18/22 | 18/22 | 60.4 (15.1) | 15 | HD >3 months | ≥6 |
| Lin (2010) [44] | 2 weeks | 8 weeks | No | S | OL | Taiwan | 52 | 26/26 | 23/20 | 57.3 (12.0) | 42/27 | HD >3 months | ≥5.5 |
| Liu (2006) [71] | 2 weeks | 8 weeks | No | S | OL | Asian | 73 | 37/36 | 33/30 | 48.9 (11.5) | 8/15 | HD >3 months | ≥6 |
| Navarro-González (2011) [72] | 2–3 weeks | 12 weeks | No | S | OL | Spain | 65 | 33/32 | 30/29 | 61.2 (15.5) | 43/41 | HD >3 months | NR |
| Oliveira (2010) [45] | 0 weeks | 6 weeks | No | S | OL | Brazil | 40 | 21/19 | 21/17 | 50.38 (11.4) | 0 | CKD stage 3–4 | None |
| Qunibi (2008) [46] | 6 weeks | 12 months | No | M | OL | USA | 203 | 100/103 | 70/59 | 59.4 (12.5) | 57/57 | HD >3 months | ≥5.5 |
| Qunibi (2004) [47] | 1–3 weeks | 8 weeks | No | M | DB | USA | 100 | 50/48 | 45/46 | 53.1 (14.0) | NR | HD > 3 months | ≥6 |
| Yilmaz (2012) [48] | 2 weeks | 8 weeks | No | S | OL | Turkey | 100 | 47/53 | 47/53 | 46 (median) | 0 | CKD stage 4 | ≥6 |
| Sevelamer versus unspecified calcium-based binder | |||||||||||||
| Block (2005) [51] | 0 weeks | 18 months | No | M | OL | USA | 148 | 73/75 | 54/55 | 58.0 (15.0) | 63/56 | New to HD | None |
| Chertow (2002) [52] | 2 weeks | 12 months | No | M | OL | USA/Europe | 200 | 99/101 | 81/88 | 56.5 (15.0) | 32/33 | Stable HD | ≥5.5 |
| Suki (2007) [53] | 0 weeks | 36 months | No | M | OL | USA | 2013 | 1053/1068 | 551/517 | 60.0 (14.7) | 51/50 | HD >3 months | None |
| Sevelamer versus lanthanum | |||||||||||||
| Block (2012) [49]b | 0 weeks | 9 months | No | S | DBc | USA | 90 | 30/30 | 30/28 | 68 (11) | 53/57 | CKD < stage 5D | 10.8–18.6 |
| Kasai (2012) [54] | 9 weeks | 13 weeks | Yes | S | OL | Japan | 42 | 42 | 41 | 60.9 (11.9) | 31 | HD >3 months | NR |
| Sprague (2009) [55] | 3 weeks | 4 weeks | Yes | M | OL | Intl | 182 | 86/95 | 60/59 | 55.5 (13.1) | HD >2 months | ≥6 | |
| Sevelamer versus magnesium carbonate | |||||||||||||
| de Francisco (2010) [50]d | 2–3 weeks | 24 weeks | No | M | OL | Europe | 255 | 129/126 | 99/105 | 57.6 (12.9) | 20/25 | HD/HDF >3 months | ≥5.5 |
| Zwiech (2011) [56] | 2 weeks | 12 weeks | No | S | OL | Poland | 40 | 10/30 | 10/28 | 57.8 (13.6) | NR | HD >6 months | ≥5.5 |
| Sevalamer hydrochloride versus sevelamer carbonate | |||||||||||||
| Abraham (2012) [57] | 2 weeks | 6 weeks | No | M | OL | Indian | 97 | 48/49 | 44/44 | 47.7 (12.6) | 29/20 | Stable HD | ≥6 |
| Delmez (2007) [58] | 0 weeks | 8 weeks | Yes | M | DB | USA | 79 | 40/39 | 19/21 | 58.1 (12.3) | Chronic HD | None | |
| Fan (2009) [59] | 2 weeks | 4 weeks | Yes | M | OL | UK (London) | 31 | 14/17 | 24 | 59.2 (13.2) | 13 | HD >3 months | ≥5.5 |
| Sevalamer versus iron-based binder | |||||||||||||
| Chen (2011) [73] | 1–2 weeks | 12 weeks | No | M | OL | Japan/Taiwan | 203 | 68/135 | 54/119 | 58.6 (11.2) | 29 | HD >3 months | ≥6.0 |
| Floege (2014) [74]b | 2–4 weeks | 12 weeks | No | M | OL | Intl | 1059 | 349/710 | 293/515 | 56 (14) | 28 | HD/PD | ≥6.0 |
| Yokoyama (2014) [75] | 2 weeks | 12 weeks | No | M | OL | Japan | 230 | 114/116 | 97/102 | 60.8 (10.1) | 26 | HD >3 months | ≥6.1 |
| Lanthanum versus calcium-based binder | |||||||||||||
| D'Haese (2003) [60] | 8–12 d | 12 months | No | M | OL | Europe | 98 | 49/49 | 34/34 | 55 (14.3) | 26 | HD >3 months | None |
| Hutchison (2005) [67] | 1–3 weeks | 5 weeks | No | M | OL | Europe | 800 | 533/267 | 453/209 | 57.5 (14.0) | NR | HD > 3 months | >5.6 |
| Lee (2013) [68] | 0 weeks | 24 weeks | No | M | NR | S Korea | 72 | 35/35 | 20/30 | 50.4 (11.4) | PD >6 months | >5.6 | |
| Ohtake (2013) [64] | 0 weeks | 6 months | No | S | OL | Japan | 52 | 26/26 | 19/23 | 67.8 (6.3) | 43 | Stable HD | None |
| Scaria (2009) [70]e | 4 weeks | 4 weeks | Yes | S | OL | India | 26 | 13/13 | 10/10 | 49.9 | NR | CKD stage 4 | >5.5 |
| Shigematsu (2008) [62] | 2 weeks | 8 weeks | No | M | DB | Japan | 259 | 126/132 | 122/126 | 57.4 (11.1) | 20 | Stable HD | 5.6–11.0 |
| Soriano (2013) [65] | 0 weeks | 4 months | No | S | NR | Spain | 32 | 16/16 | 16/16 | ∼60 | 25/12.5 | CKD stages 3–5 | >4 |
| Spasovski (2006) [61] | 0 weeks | 12 months | No | S | NR | Macedonia | 24 | 12/12 | 10/10 | 56 (9.8) | 20 | New to HD | None |
| Toussaint (2011) [66] | 1 week | 6 months | No | S | OL | Australia | 45 | 22/23 | 17/13 | 57.4 (14.9) | 36/39 | HD > 3 months | >5 |
| Toida (2012) [69] | 2 weeks | 3 months | Yes | S | OL | Japan | 50 | 25/25 | 18/24 | 65.6 (11.5) | Stable HD | None | |
| Wada (2014) [63] | 2 weeks | 12 months | No | S | OL | Japan | 43 | 21/22 | 19/22 | 65.7 (9.26) | 100 | HD >6 months | None |
| Reference . | Washouta . | Follow-up time . | Crossover . | Centres . | Blinding . | Ethnicity . | Random (n) . | Baseline (n) . | End-of-study (n) . | Age, years (SD) . | Percent diabetic . | Dialysis vintage . | Inclusion (phosphorus mg/dL) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sevelamer versus CaCO3 | |||||||||||||
| Braun (2004) [25] | 2 weeks | 2 years | No | M | OL | Europe | 114 | 55/59 | 42/40 | 56.5 (14.1) | 13/17 | Stable HD | ≥5.5 |
| Caravaca (2007) [37] | 2 weeks | 3 weeks | Yes | S | OL | Spain | 20 | 20 | 17 | 54 (17) | NR | CKD stages 3–4 | None |
| Chennasamudram (2013) [26]b | 2 weeks | 8 weeks | Yes | S | OL | USA | 15 | 7/8 | 7/8 | 54 (9) | 100 | Chronic PD | ≥5.5 |
| De Santo (2006) [27] | 2 weeks | 24 weeks | Yes | S | OL | Italian | 16 | 8/8 | 8/8 | 35–50 years | 0 | HD 6–10 months | ≥5.5 |
| Di Iorio (2012) [28] | None | 24 months | No | M | OL | Italian | 239 | 121/118 | 107/105 | 57.9 (12.2) | 27/29 | CKD stage 3–4 | None |
| Di Iorio (2013) [29] | None | 36 months | No | M | OL | Italian | 466 | 232/234 | 199/198 | 65.6 (14.8) | 30/29 | New to HD | None |
| Ferreira (2008) [30] | 0 weeks | 12 months | No | M | OL | Portugal | 91 | 44/47 | 33/35 | 54.7 (14.5) | 6/23 | HD >3 months | <8.1 |
| Kakuta (2011) [31] | 0 weeks | 12 months | No | M | OL | Japan | 183 | 91/92 | 79/84 | 58.0 (12.0) | 23/19 | Stable HD | None |
| Koiwa (2005) [38] | 0 weeks | 4 weeks | No | M | OL | Japan | 56 | 29/27 | 16/20 | 57.1 (10.6) | 23 | HD >12 months | None |
| Lin (2014) [32] | 2 weeks | 48 weeks | No | M | OL | Taiwan | 75 | 36/39 | 23/27 | 58.2 (8.0) | NR | HD >3 months | ≥5.5 |
| Russo (2007) [36] | 0 weeks | 24 months | No | M | OL | Italy | 60 | 30/30 | 27/28 | 54.7 (12.7) | 0 | CKD stages 3–5 | None |
| Sadek (2003) [33] | 0 weeks | 5 months | No | S | OL | France | 42 | 21/21 | 15/16 | NR | NR | Chronic HD | NR |
| Shaheen (2004) [34] | 2 weeks | 8 weeks | Yes | S | OL | Saudi Arabia | 20 | 10/10 | 19/18 | 42.7 (9.9) | 20 | HD >3 months | ≥5.5 |
| Vlassara (2012) [35]b | 0 weeks | 8 weeks | Yes | S | OL | USA | 20 | 10/10 | 10/10 | 61.1 (11.5) | 100 | CKD stage 2–4 | NR |
| Sevelamer versus Ca-acetate | |||||||||||||
| Barreto (2008) [41] | 2 weeks | 12 months | No | M | OL | Brazil | 101 | 52/49 | 41/30 | 47 (13.3) | 15/13 | HD >3 months | ≥5.5 |
| Bleyer (1999) [39] | 2 weeks | 8 weeks | Yes | M | OL | USA | 83 | 83 | 80 | 54.5 (15) | 29 | Stable HD | ≥6 |
| Block (2012) [49]b | 0 weeks | 9 months | No | S | DBc | USA | 90 | 30/30 | 30/30 | 68 (11) | 53/57 | CKD < stage 5D | 10.8–18.6 |
| Caglar (2008) [40] | 2 weeks | 8 weeks | No | S | NR | Turkey | 50 | 25/25 | 25/25 | 40.4 (13.0) | 0 | CKD stage 4 | ≥5.5 |
| Evenepoel (2009) [42] | 2 weeks | 12 weeks | No | M | OL | Europe | 143 | 97/46 | 74/30 | 54.4 (15.7) | 20/26 | PD >6 months | ≥5.5 |
| Hervas (2003) [43] | 2 weeks | 34 weeks | No | NR | NR | Spain | 51 | 18/22 | 18/22 | 60.4 (15.1) | 15 | HD >3 months | ≥6 |
| Lin (2010) [44] | 2 weeks | 8 weeks | No | S | OL | Taiwan | 52 | 26/26 | 23/20 | 57.3 (12.0) | 42/27 | HD >3 months | ≥5.5 |
| Liu (2006) [71] | 2 weeks | 8 weeks | No | S | OL | Asian | 73 | 37/36 | 33/30 | 48.9 (11.5) | 8/15 | HD >3 months | ≥6 |
| Navarro-González (2011) [72] | 2–3 weeks | 12 weeks | No | S | OL | Spain | 65 | 33/32 | 30/29 | 61.2 (15.5) | 43/41 | HD >3 months | NR |
| Oliveira (2010) [45] | 0 weeks | 6 weeks | No | S | OL | Brazil | 40 | 21/19 | 21/17 | 50.38 (11.4) | 0 | CKD stage 3–4 | None |
| Qunibi (2008) [46] | 6 weeks | 12 months | No | M | OL | USA | 203 | 100/103 | 70/59 | 59.4 (12.5) | 57/57 | HD >3 months | ≥5.5 |
| Qunibi (2004) [47] | 1–3 weeks | 8 weeks | No | M | DB | USA | 100 | 50/48 | 45/46 | 53.1 (14.0) | NR | HD > 3 months | ≥6 |
| Yilmaz (2012) [48] | 2 weeks | 8 weeks | No | S | OL | Turkey | 100 | 47/53 | 47/53 | 46 (median) | 0 | CKD stage 4 | ≥6 |
| Sevelamer versus unspecified calcium-based binder | |||||||||||||
| Block (2005) [51] | 0 weeks | 18 months | No | M | OL | USA | 148 | 73/75 | 54/55 | 58.0 (15.0) | 63/56 | New to HD | None |
| Chertow (2002) [52] | 2 weeks | 12 months | No | M | OL | USA/Europe | 200 | 99/101 | 81/88 | 56.5 (15.0) | 32/33 | Stable HD | ≥5.5 |
| Suki (2007) [53] | 0 weeks | 36 months | No | M | OL | USA | 2013 | 1053/1068 | 551/517 | 60.0 (14.7) | 51/50 | HD >3 months | None |
| Sevelamer versus lanthanum | |||||||||||||
| Block (2012) [49]b | 0 weeks | 9 months | No | S | DBc | USA | 90 | 30/30 | 30/28 | 68 (11) | 53/57 | CKD < stage 5D | 10.8–18.6 |
| Kasai (2012) [54] | 9 weeks | 13 weeks | Yes | S | OL | Japan | 42 | 42 | 41 | 60.9 (11.9) | 31 | HD >3 months | NR |
| Sprague (2009) [55] | 3 weeks | 4 weeks | Yes | M | OL | Intl | 182 | 86/95 | 60/59 | 55.5 (13.1) | HD >2 months | ≥6 | |
| Sevelamer versus magnesium carbonate | |||||||||||||
| de Francisco (2010) [50]d | 2–3 weeks | 24 weeks | No | M | OL | Europe | 255 | 129/126 | 99/105 | 57.6 (12.9) | 20/25 | HD/HDF >3 months | ≥5.5 |
| Zwiech (2011) [56] | 2 weeks | 12 weeks | No | S | OL | Poland | 40 | 10/30 | 10/28 | 57.8 (13.6) | NR | HD >6 months | ≥5.5 |
| Sevalamer hydrochloride versus sevelamer carbonate | |||||||||||||
| Abraham (2012) [57] | 2 weeks | 6 weeks | No | M | OL | Indian | 97 | 48/49 | 44/44 | 47.7 (12.6) | 29/20 | Stable HD | ≥6 |
| Delmez (2007) [58] | 0 weeks | 8 weeks | Yes | M | DB | USA | 79 | 40/39 | 19/21 | 58.1 (12.3) | Chronic HD | None | |
| Fan (2009) [59] | 2 weeks | 4 weeks | Yes | M | OL | UK (London) | 31 | 14/17 | 24 | 59.2 (13.2) | 13 | HD >3 months | ≥5.5 |
| Sevalamer versus iron-based binder | |||||||||||||
| Chen (2011) [73] | 1–2 weeks | 12 weeks | No | M | OL | Japan/Taiwan | 203 | 68/135 | 54/119 | 58.6 (11.2) | 29 | HD >3 months | ≥6.0 |
| Floege (2014) [74]b | 2–4 weeks | 12 weeks | No | M | OL | Intl | 1059 | 349/710 | 293/515 | 56 (14) | 28 | HD/PD | ≥6.0 |
| Yokoyama (2014) [75] | 2 weeks | 12 weeks | No | M | OL | Japan | 230 | 114/116 | 97/102 | 60.8 (10.1) | 26 | HD >3 months | ≥6.1 |
| Lanthanum versus calcium-based binder | |||||||||||||
| D'Haese (2003) [60] | 8–12 d | 12 months | No | M | OL | Europe | 98 | 49/49 | 34/34 | 55 (14.3) | 26 | HD >3 months | None |
| Hutchison (2005) [67] | 1–3 weeks | 5 weeks | No | M | OL | Europe | 800 | 533/267 | 453/209 | 57.5 (14.0) | NR | HD > 3 months | >5.6 |
| Lee (2013) [68] | 0 weeks | 24 weeks | No | M | NR | S Korea | 72 | 35/35 | 20/30 | 50.4 (11.4) | PD >6 months | >5.6 | |
| Ohtake (2013) [64] | 0 weeks | 6 months | No | S | OL | Japan | 52 | 26/26 | 19/23 | 67.8 (6.3) | 43 | Stable HD | None |
| Scaria (2009) [70]e | 4 weeks | 4 weeks | Yes | S | OL | India | 26 | 13/13 | 10/10 | 49.9 | NR | CKD stage 4 | >5.5 |
| Shigematsu (2008) [62] | 2 weeks | 8 weeks | No | M | DB | Japan | 259 | 126/132 | 122/126 | 57.4 (11.1) | 20 | Stable HD | 5.6–11.0 |
| Soriano (2013) [65] | 0 weeks | 4 months | No | S | NR | Spain | 32 | 16/16 | 16/16 | ∼60 | 25/12.5 | CKD stages 3–5 | >4 |
| Spasovski (2006) [61] | 0 weeks | 12 months | No | S | NR | Macedonia | 24 | 12/12 | 10/10 | 56 (9.8) | 20 | New to HD | None |
| Toussaint (2011) [66] | 1 week | 6 months | No | S | OL | Australia | 45 | 22/23 | 17/13 | 57.4 (14.9) | 36/39 | HD > 3 months | >5 |
| Toida (2012) [69] | 2 weeks | 3 months | Yes | S | OL | Japan | 50 | 25/25 | 18/24 | 65.6 (11.5) | Stable HD | None | |
| Wada (2014) [63] | 2 weeks | 12 months | No | S | OL | Japan | 43 | 21/22 | 19/22 | 65.7 (9.26) | 100 | HD >6 months | None |
Sevelamer refers to sevelamer hydrochloride, unless otherwise specified. OL, open-label; DB, double-blind; SD, standard deviation; HD, hemodialysis; PD, peritoneal dialysis; HDF, hemodiafiltration; NDD-CKD, non-dialysis-dependent chronic kidney disease; NR, not reported.
aLength of time (weeks) prior to randomization that current phosphate binders were removed.
bIntervention is sevelamer carbonate.
cNo explanation of how this was successfully undertaken due to differences in taste and size as reported by others.
dComparator also includes calcium acetate.
eComparator is calcium acetate.
Mortality
![Forest plot comparing all-cause mortality over study duration between patients treated with sevelamer or lanthanum and CBPBs. (1) Two deaths were not specified to which arm, but pooled estimate was not sensitive to whether both deaths were assigned to either sevelamer [RR 0.64 (95% CI 0.37–1.11)] or CBPBs [RR 0.59 (95% CI 0.34–1.02)]. (2) Abstracted from Navaneethan et al. [16]. (3) Eleven deaths occurred, but not specified to which arm. (4) Deaths not reported.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ndt/32/1/10.1093_ndt_gfw312/2/m_gfw312f2.jpeg?Expires=1750206024&Signature=Q5sLyGtx6BcOUMlevJeqEqG3d~L60kMG780fhTgvINXfe~yOp8lhRNwXTaplZNIs3DOWhFfTx5BrOukzaS7emIq6~mf-NTJWgBUGeHzXZF2l7L8zoFKNxkuABQxJ3jjSHTBlV2dbHtMz~GlznTRgEHY3Un2G45lNabzOTuh87yJsqgKavetyUuBimjiFbFBjOVRa32GPiryF6xd7LxB6NNgOz8RUWrrAEjGYPmxFD-IZqs-lLoUWiryRo2xvgSMrS~TK~KOPtZNNi2j2uh48J1sE6L8MKbacbQsbOzX9lEmy7bJoT1842y6huR-f-8bmu5m5TZPYTX9VgGKQp9JmPA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Forest plot comparing all-cause mortality over study duration between patients treated with sevelamer or lanthanum and CBPBs. (1) Two deaths were not specified to which arm, but pooled estimate was not sensitive to whether both deaths were assigned to either sevelamer [RR 0.64 (95% CI 0.37–1.11)] or CBPBs [RR 0.59 (95% CI 0.34–1.02)]. (2) Abstracted from Navaneethan et al. [16]. (3) Eleven deaths occurred, but not specified to which arm. (4) Deaths not reported.
| Discrete outcomes . | Sevelamer versus CBPBs . | Lanthanum versus CBPBs . | P-valuea . | Figure . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N . | nsev . | nCa . | RR (95% CI) . | P-value . | N . | nlan . | nCa . | RR (95% CI) . | P-value . | |||
| All-cause mortality | 12 | 325/1870 | 426/1899 | 0.62 (0.35–1.08) | 0.09 | 4b | 3/81 | 4/83 | 0.73 (0.18–3.00) | 0.66 | 0.86 | Figure 2 |
| Cardiovascular deaths | 3 | 152/1337 | 232/1351 | 0.29 (0.05–1.82) | 0.19 | 0 | – | – | – | – | – | – |
| Cardiovascular eventsc | 2 | 7/91 | 8/98 | – | – | 4 | 11/592 | 8/328 | – | – | – | – |
| Bone-related events | 1 | 1/1053 | 0/1068 | – | – | 1 | 0/22 | 0/23 | – | – | – | – |
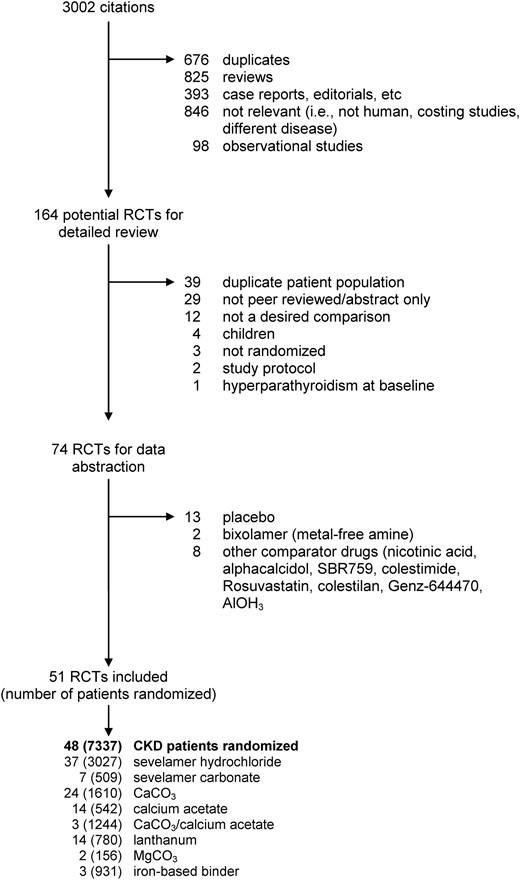
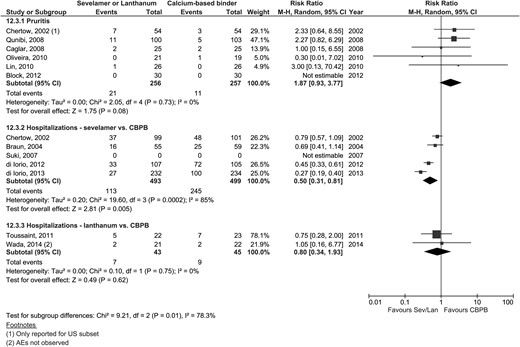
| Hospitalization rates | 4 | 113/493 | 245/499 | 0.50 (0.31–0.81) | 0.005 | 2 | 7/43 | 9/45 | 0.80 (0.34–1.93) | 0.62 | 0.36 | Figure 5B |
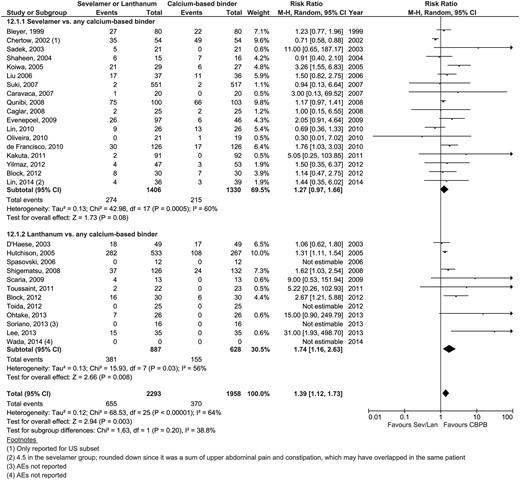
| Gastrointestinal events | 18 | 274/1406 | 215/1330 | 1.27 (0.97–1.66) | 0.08 | 8 | 381/834 | 155/575 | 1.74 (1.16–2.63) | 0.008 | 0.20 | Figure 3 |
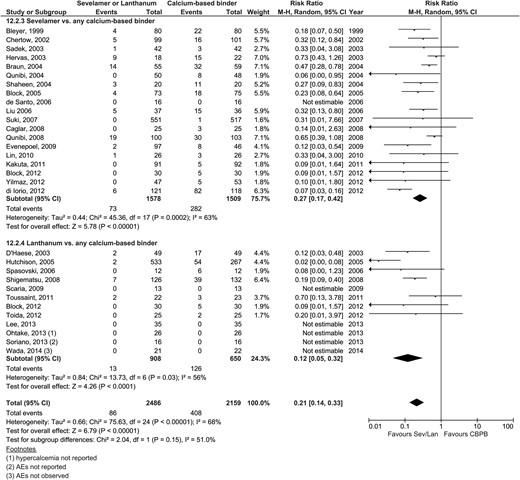
| Hypercalcemia | 18 | 73/1562 | 282/1493 | 0.27 (0.17–0.42) | <0.0001 | 7 | 13/797 | 126/38 | 0.12 (0.05–0.32) | <0.0001 | 0.15 | Figure 4 |
| Pruritis | 4 | 21/226 | 11/227 | 1.87 (0.93–3.77) | 0.08 | 0 | – | – | – | – | – | Figure 5A |
| Calciphylaxis | 1 | 0/1053 | 3/1068 | – | – | 0 | – | – | – | – | – | – |
| Hyperchloremic acidosis | 1 | 0/30 | 1/30 | 1 | 1/28 | 1/30 | – | – | – | – | ||
| Participant attrition | 23 | 736/2594 | 804/2572 | 0.91 (0.85–0.99) | 0.02 | 11 | 142/892 | 103/634 | 1.19 (0.75–1.88) | 0.46 | 0.27 | Supplement |
| Continuous outcomes . | N . | nsev . | nCa . | MD (95% CI) . | P-value . | N . | nlan . | nCa . | MD (95% CI) . | P-value . | P-valuea . | Figure . |
| Phosphorus (mg/dL) | 30 | 2178 | 2133 | −0.01 (−0.16–0.14) | 0.92 | 12 | 581 | 500 | 0.18 (0.10–0.27) | <0.0001 | 0.03 | Supplement |
| Calcium (mg/dL) | 28 | 2078 | 2055 | −0.35 (−0.49 to −0.22) | <0.0001 | 12 | 579 | 499 | −0.26 (−0.46 to −0.07) | 0.009 | 0.47 | Supplement |
| LDL (mg/dL) | 18 | 974 | 979 | −20.9 (−23.3 to −18.6) | <0.0001 | 2 | 47 | 53 | −2.20 (−11.19–6.79) | 0.63 | <0.0001 | Supplement |
| iPTH (pg/mL) | 17 | 634 | 634 | 39.0 (7.74–70.3) | 0.01 | 8 | 276 | 294 | 63.3 (11.5–115) | 0.02 | 0.43 | Supplement |
| CAC score | 8 | 412 | 383 | −101 (−160 to −41.7) | 0.0008 | 1 | 19 | 23 | −56.5 (−1308–1195) | 0.93 | – | Supplement |
| Discrete outcomes . | Sevelamer versus CBPBs . | Lanthanum versus CBPBs . | P-valuea . | Figure . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N . | nsev . | nCa . | RR (95% CI) . | P-value . | N . | nlan . | nCa . | RR (95% CI) . | P-value . | |||
| All-cause mortality | 12 | 325/1870 | 426/1899 | 0.62 (0.35–1.08) | 0.09 | 4b | 3/81 | 4/83 | 0.73 (0.18–3.00) | 0.66 | 0.86 | Figure 2 |
| Cardiovascular deaths | 3 | 152/1337 | 232/1351 | 0.29 (0.05–1.82) | 0.19 | 0 | – | – | – | – | – | – |
| Cardiovascular eventsc | 2 | 7/91 | 8/98 | – | – | 4 | 11/592 | 8/328 | – | – | – | – |
| Bone-related events | 1 | 1/1053 | 0/1068 | – | – | 1 | 0/22 | 0/23 | – | – | – | – |
| Hospitalization rates | 4 | 113/493 | 245/499 | 0.50 (0.31–0.81) | 0.005 | 2 | 7/43 | 9/45 | 0.80 (0.34–1.93) | 0.62 | 0.36 | Figure 5B |
| Gastrointestinal events | 18 | 274/1406 | 215/1330 | 1.27 (0.97–1.66) | 0.08 | 8 | 381/834 | 155/575 | 1.74 (1.16–2.63) | 0.008 | 0.20 | Figure 3 |
| Hypercalcemia | 18 | 73/1562 | 282/1493 | 0.27 (0.17–0.42) | <0.0001 | 7 | 13/797 | 126/38 | 0.12 (0.05–0.32) | <0.0001 | 0.15 | Figure 4 |
| Pruritis | 4 | 21/226 | 11/227 | 1.87 (0.93–3.77) | 0.08 | 0 | – | – | – | – | – | Figure 5A |
| Calciphylaxis | 1 | 0/1053 | 3/1068 | – | – | 0 | – | – | – | – | – | – |
| Hyperchloremic acidosis | 1 | 0/30 | 1/30 | 1 | 1/28 | 1/30 | – | – | – | – | ||
| Participant attrition | 23 | 736/2594 | 804/2572 | 0.91 (0.85–0.99) | 0.02 | 11 | 142/892 | 103/634 | 1.19 (0.75–1.88) | 0.46 | 0.27 | Supplement |
| Continuous outcomes . | N . | nsev . | nCa . | MD (95% CI) . | P-value . | N . | nlan . | nCa . | MD (95% CI) . | P-value . | P-valuea . | Figure . |
| Phosphorus (mg/dL) | 30 | 2178 | 2133 | −0.01 (−0.16–0.14) | 0.92 | 12 | 581 | 500 | 0.18 (0.10–0.27) | <0.0001 | 0.03 | Supplement |
| Calcium (mg/dL) | 28 | 2078 | 2055 | −0.35 (−0.49 to −0.22) | <0.0001 | 12 | 579 | 499 | −0.26 (−0.46 to −0.07) | 0.009 | 0.47 | Supplement |
| LDL (mg/dL) | 18 | 974 | 979 | −20.9 (−23.3 to −18.6) | <0.0001 | 2 | 47 | 53 | −2.20 (−11.19–6.79) | 0.63 | <0.0001 | Supplement |
| iPTH (pg/mL) | 17 | 634 | 634 | 39.0 (7.74–70.3) | 0.01 | 8 | 276 | 294 | 63.3 (11.5–115) | 0.02 | 0.43 | Supplement |
| CAC score | 8 | 412 | 383 | −101 (−160 to −41.7) | 0.0008 | 1 | 19 | 23 | −56.5 (−1308–1195) | 0.93 | – | Supplement |
CBPB, calcium-based phosphate binder; RR, risk ratio; MD, mean difference; CI, confidence interval; LDL, low-density lipoprotein; iPTH, intact parathyroid hormone; CAC, coronary artery calcification; n, number of events/total number for dichotomous outcomes and number of measurements for continuous outcomes among participants treated with sevelamer (nsev), CBPB (nCa) and lanthanum carbonate (nlan); N, number of studies.
aP-value for heterogeneity for subgroups of sevelamer and lanthanum trials.
bOne study reported 11 deaths out of 98 randomized patients but did not specify which arm.
cNature of event not specified or differed between studies.
| Discrete outcomes . | Sevelamer versus CBPBs . | Lanthanum versus CBPBs . | P-valuea . | Figure . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N . | nsev . | nCa . | RR (95% CI) . | P-value . | N . | nlan . | nCa . | RR (95% CI) . | P-value . | |||
| All-cause mortality | 12 | 325/1870 | 426/1899 | 0.62 (0.35–1.08) | 0.09 | 4b | 3/81 | 4/83 | 0.73 (0.18–3.00) | 0.66 | 0.86 | Figure 2 |
| Cardiovascular deaths | 3 | 152/1337 | 232/1351 | 0.29 (0.05–1.82) | 0.19 | 0 | – | – | – | – | – | – |
| Cardiovascular eventsc | 2 | 7/91 | 8/98 | – | – | 4 | 11/592 | 8/328 | – | – | – | – |
| Bone-related events | 1 | 1/1053 | 0/1068 | – | – | 1 | 0/22 | 0/23 | – | – | – | – |
| Hospitalization rates | 4 | 113/493 | 245/499 | 0.50 (0.31–0.81) | 0.005 | 2 | 7/43 | 9/45 | 0.80 (0.34–1.93) | 0.62 | 0.36 | Figure 5B |
| Gastrointestinal events | 18 | 274/1406 | 215/1330 | 1.27 (0.97–1.66) | 0.08 | 8 | 381/834 | 155/575 | 1.74 (1.16–2.63) | 0.008 | 0.20 | Figure 3 |
| Hypercalcemia | 18 | 73/1562 | 282/1493 | 0.27 (0.17–0.42) | <0.0001 | 7 | 13/797 | 126/38 | 0.12 (0.05–0.32) | <0.0001 | 0.15 | Figure 4 |
| Pruritis | 4 | 21/226 | 11/227 | 1.87 (0.93–3.77) | 0.08 | 0 | – | – | – | – | – | Figure 5A |
| Calciphylaxis | 1 | 0/1053 | 3/1068 | – | – | 0 | – | – | – | – | – | – |
| Hyperchloremic acidosis | 1 | 0/30 | 1/30 | 1 | 1/28 | 1/30 | – | – | – | – | ||
| Participant attrition | 23 | 736/2594 | 804/2572 | 0.91 (0.85–0.99) | 0.02 | 11 | 142/892 | 103/634 | 1.19 (0.75–1.88) | 0.46 | 0.27 | Supplement |
| Continuous outcomes . | N . | nsev . | nCa . | MD (95% CI) . | P-value . | N . | nlan . | nCa . | MD (95% CI) . | P-value . | P-valuea . | Figure . |
| Phosphorus (mg/dL) | 30 | 2178 | 2133 | −0.01 (−0.16–0.14) | 0.92 | 12 | 581 | 500 | 0.18 (0.10–0.27) | <0.0001 | 0.03 | Supplement |
| Calcium (mg/dL) | 28 | 2078 | 2055 | −0.35 (−0.49 to −0.22) | <0.0001 | 12 | 579 | 499 | −0.26 (−0.46 to −0.07) | 0.009 | 0.47 | Supplement |
| LDL (mg/dL) | 18 | 974 | 979 | −20.9 (−23.3 to −18.6) | <0.0001 | 2 | 47 | 53 | −2.20 (−11.19–6.79) | 0.63 | <0.0001 | Supplement |
| iPTH (pg/mL) | 17 | 634 | 634 | 39.0 (7.74–70.3) | 0.01 | 8 | 276 | 294 | 63.3 (11.5–115) | 0.02 | 0.43 | Supplement |
| CAC score | 8 | 412 | 383 | −101 (−160 to −41.7) | 0.0008 | 1 | 19 | 23 | −56.5 (−1308–1195) | 0.93 | – | Supplement |
| Discrete outcomes . | Sevelamer versus CBPBs . | Lanthanum versus CBPBs . | P-valuea . | Figure . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N . | nsev . | nCa . | RR (95% CI) . | P-value . | N . | nlan . | nCa . | RR (95% CI) . | P-value . | |||
| All-cause mortality | 12 | 325/1870 | 426/1899 | 0.62 (0.35–1.08) | 0.09 | 4b | 3/81 | 4/83 | 0.73 (0.18–3.00) | 0.66 | 0.86 | Figure 2 |
| Cardiovascular deaths | 3 | 152/1337 | 232/1351 | 0.29 (0.05–1.82) | 0.19 | 0 | – | – | – | – | – | – |
| Cardiovascular eventsc | 2 | 7/91 | 8/98 | – | – | 4 | 11/592 | 8/328 | – | – | – | – |
| Bone-related events | 1 | 1/1053 | 0/1068 | – | – | 1 | 0/22 | 0/23 | – | – | – | – |
| Hospitalization rates | 4 | 113/493 | 245/499 | 0.50 (0.31–0.81) | 0.005 | 2 | 7/43 | 9/45 | 0.80 (0.34–1.93) | 0.62 | 0.36 | Figure 5B |
| Gastrointestinal events | 18 | 274/1406 | 215/1330 | 1.27 (0.97–1.66) | 0.08 | 8 | 381/834 | 155/575 | 1.74 (1.16–2.63) | 0.008 | 0.20 | Figure 3 |
| Hypercalcemia | 18 | 73/1562 | 282/1493 | 0.27 (0.17–0.42) | <0.0001 | 7 | 13/797 | 126/38 | 0.12 (0.05–0.32) | <0.0001 | 0.15 | Figure 4 |
| Pruritis | 4 | 21/226 | 11/227 | 1.87 (0.93–3.77) | 0.08 | 0 | – | – | – | – | – | Figure 5A |
| Calciphylaxis | 1 | 0/1053 | 3/1068 | – | – | 0 | – | – | – | – | – | – |
| Hyperchloremic acidosis | 1 | 0/30 | 1/30 | 1 | 1/28 | 1/30 | – | – | – | – | ||
| Participant attrition | 23 | 736/2594 | 804/2572 | 0.91 (0.85–0.99) | 0.02 | 11 | 142/892 | 103/634 | 1.19 (0.75–1.88) | 0.46 | 0.27 | Supplement |
| Continuous outcomes . | N . | nsev . | nCa . | MD (95% CI) . | P-value . | N . | nlan . | nCa . | MD (95% CI) . | P-value . | P-valuea . | Figure . |
| Phosphorus (mg/dL) | 30 | 2178 | 2133 | −0.01 (−0.16–0.14) | 0.92 | 12 | 581 | 500 | 0.18 (0.10–0.27) | <0.0001 | 0.03 | Supplement |
| Calcium (mg/dL) | 28 | 2078 | 2055 | −0.35 (−0.49 to −0.22) | <0.0001 | 12 | 579 | 499 | −0.26 (−0.46 to −0.07) | 0.009 | 0.47 | Supplement |
| LDL (mg/dL) | 18 | 974 | 979 | −20.9 (−23.3 to −18.6) | <0.0001 | 2 | 47 | 53 | −2.20 (−11.19–6.79) | 0.63 | <0.0001 | Supplement |
| iPTH (pg/mL) | 17 | 634 | 634 | 39.0 (7.74–70.3) | 0.01 | 8 | 276 | 294 | 63.3 (11.5–115) | 0.02 | 0.43 | Supplement |
| CAC score | 8 | 412 | 383 | −101 (−160 to −41.7) | 0.0008 | 1 | 19 | 23 | −56.5 (−1308–1195) | 0.93 | – | Supplement |
CBPB, calcium-based phosphate binder; RR, risk ratio; MD, mean difference; CI, confidence interval; LDL, low-density lipoprotein; iPTH, intact parathyroid hormone; CAC, coronary artery calcification; n, number of events/total number for dichotomous outcomes and number of measurements for continuous outcomes among participants treated with sevelamer (nsev), CBPB (nCa) and lanthanum carbonate (nlan); N, number of studies.
aP-value for heterogeneity for subgroups of sevelamer and lanthanum trials.
bOne study reported 11 deaths out of 98 randomized patients but did not specify which arm.
cNature of event not specified or differed between studies.
Selected subgroup analyses for end-of-study serum phosphorus and intact parathyroid hormone
| Subset (sevelamer only) . | Number ofstudies . | Number of patients . | Heterogeneity (I2, P-value) . | RR or MD (95% CI) . | P-value . | Test for interactiona (I2, P-value) . | |
|---|---|---|---|---|---|---|---|
| Sevelamer . | Calcium-based binder . | ||||||
| All-cause mortality | |||||||
| All studies | 12 | 325/1870 | 426/1899 | 75%, <0.0001 | 0.62 (0.35–1.08) | 0.09 | – |
| Comparator | 79%, 0.009 | ||||||
| CaCO3 | 6 | 45/475 | 128/481 | 32%, 0.20 | 0.44 (0.25–0.76) | 0.004 | |
| Calcium acetate | 3 | 6/170 | 17/174 | 30%, 0.24 | 0.43 (0.13–1.38) | 0.15 | |
| Any CBPB | 3 | 274/1225 | 281/1244 | 0%, 0.93 | 0.99 (0.86–1.14) | 0.87 | |
| Dialysis status | 94%, <0.0001 | ||||||
| Chronic HD | 9 | 284/1444 | 303/1472 | 0%, 0.43 | 0.96 (0.82–1.13) | 0.66 | |
| Incident HD | 2 | 29/305 | 101/309 | 0%, 0.36 | 0.29 (0.20–0.42) | <0.0001 | |
| Chronic PD | 0 | – | – | – | – | – | |
| NDD-CKD | 1 | 12/314 | 22/315 | – | 0.53 (0.28–1.03) | 0.06 | |
| Study follow-up | 0%, 0.67 | ||||||
| <6 months | 2 | 1/71 | 3/69 | 0%, 1.00 | 0.33 (0.06–1.98) | 0.23 | |
| 6 to <12 months | 2 | 4/54 | 3/61 | 0%, 0.71 | 1.52 (0.35–6.55) | 0.57 | |
| 12 to <24 months | 4 | 11/324 | 21/328 | 32%, 0.22 | 0.56 (0.21–1.52) | 0.26 | |
| ≥24 months | 4 | 309/1461 | 398/1479 | 92%, <0.0001 | 0.61 (0.26–1.43) | 0.25 | |
| Phosphorus, mg/dL | |||||||
| Dialysis status | 83%, 0.0005 | ||||||
| Chronic HD | 2618 | 20 141 517 | 19 271 523 | 3145%, 0.02 | 0.21 (−0.60–0.84) | 0.01 | |
| Incident HD | 2 | 253 | 253 | 82%, 0.02 | −0.43 (−0.72 to −0.15) | 0.002 | |
| Chronic PD | 2 | 110 | 59 | 82%, 0.02 | −0.28 (−1.06–0.49) | 0.47 | |
| NDD-CKD | 108 | 334 298 | 334 298 | 8273%, 0.0006 | −0.22 (−0.54–0.10) | 0.17 | |
| Ethnicity | 0%, 0.79 | ||||||
| White | 220 | 211 843 | 20 071 800 | 8381%, <0.0001 | 0.04 (−0.14–0.22) | 0.69 | |
| Asian | 15 | 419 177 | 450 187 | 339%, 0.13 | −0.08 (−0.42–0.27) | 0.67 | |
| Other | 5 | 158 | 146 | 86%, <0.0001 | −0.11 (−0.69–0.48) | 0.72 | |
| Dosing modality | 0%, 0.33 | ||||||
| Fixed | 3525 | 262 089 | 24 692 049 | 80%, <0.0001 | 0.03 (−0.11–0.16) | 0.73 | |
| Variable | 5 | 129 109 | 134 114 | 0%, 0.93 | 0.14 (−0.14–0.16) | 0.0.26 | |
| Intact parathyroid hormone, pg/mL | |||||||
| Dialysis status | 48%, 0.12 | ||||||
| Chronic HD | 1612 | 666 466 | 665 461 | 6574%, <0.0001 | 51.9 (6.67–97.0) | 0.02 | |
| Incident HD | 1 | 54 | 55 | – | 54.3 (0.68–108) | ||
| Chronic PD | 1 | 15 | 15 | – | 58.6 (33.9–83.3) | – | |
| NDD-CKD | 43 | 11 599 | 119 103 | 5164%, 0.06 | −5.43 (−52.4–41.5) | 0.82 | |
| Ethnicity | 0%, 0.56 | ||||||
| White | 1512 | 481 426 | 480 429 | 881%, <0.0001 | 40.4 (2.28–78.5) | 0.04 | |
| Asian | 62 | 304 134 | 325 134 | 190%, 0.51 | 55.6 7.29 (−50.2–64.8) | 0.8 | |
| Other | 3 | 107 | 101 | 79%, 0.008 | 57.9 (−38.6–154) | 0.24 | |
| Subset (sevelamer only) . | Number ofstudies . | Number of patients . | Heterogeneity (I2, P-value) . | RR or MD (95% CI) . | P-value . | Test for interactiona (I2, P-value) . | |
|---|---|---|---|---|---|---|---|
| Sevelamer . | Calcium-based binder . | ||||||
| All-cause mortality | |||||||
| All studies | 12 | 325/1870 | 426/1899 | 75%, <0.0001 | 0.62 (0.35–1.08) | 0.09 | – |
| Comparator | 79%, 0.009 | ||||||
| CaCO3 | 6 | 45/475 | 128/481 | 32%, 0.20 | 0.44 (0.25–0.76) | 0.004 | |
| Calcium acetate | 3 | 6/170 | 17/174 | 30%, 0.24 | 0.43 (0.13–1.38) | 0.15 | |
| Any CBPB | 3 | 274/1225 | 281/1244 | 0%, 0.93 | 0.99 (0.86–1.14) | 0.87 | |
| Dialysis status | 94%, <0.0001 | ||||||
| Chronic HD | 9 | 284/1444 | 303/1472 | 0%, 0.43 | 0.96 (0.82–1.13) | 0.66 | |
| Incident HD | 2 | 29/305 | 101/309 | 0%, 0.36 | 0.29 (0.20–0.42) | <0.0001 | |
| Chronic PD | 0 | – | – | – | – | – | |
| NDD-CKD | 1 | 12/314 | 22/315 | – | 0.53 (0.28–1.03) | 0.06 | |
| Study follow-up | 0%, 0.67 | ||||||
| <6 months | 2 | 1/71 | 3/69 | 0%, 1.00 | 0.33 (0.06–1.98) | 0.23 | |
| 6 to <12 months | 2 | 4/54 | 3/61 | 0%, 0.71 | 1.52 (0.35–6.55) | 0.57 | |
| 12 to <24 months | 4 | 11/324 | 21/328 | 32%, 0.22 | 0.56 (0.21–1.52) | 0.26 | |
| ≥24 months | 4 | 309/1461 | 398/1479 | 92%, <0.0001 | 0.61 (0.26–1.43) | 0.25 | |
| Phosphorus, mg/dL | |||||||
| Dialysis status | 83%, 0.0005 | ||||||
| Chronic HD | 2618 | 20 141 517 | 19 271 523 | 3145%, 0.02 | 0.21 (−0.60–0.84) | 0.01 | |
| Incident HD | 2 | 253 | 253 | 82%, 0.02 | −0.43 (−0.72 to −0.15) | 0.002 | |
| Chronic PD | 2 | 110 | 59 | 82%, 0.02 | −0.28 (−1.06–0.49) | 0.47 | |
| NDD-CKD | 108 | 334 298 | 334 298 | 8273%, 0.0006 | −0.22 (−0.54–0.10) | 0.17 | |
| Ethnicity | 0%, 0.79 | ||||||
| White | 220 | 211 843 | 20 071 800 | 8381%, <0.0001 | 0.04 (−0.14–0.22) | 0.69 | |
| Asian | 15 | 419 177 | 450 187 | 339%, 0.13 | −0.08 (−0.42–0.27) | 0.67 | |
| Other | 5 | 158 | 146 | 86%, <0.0001 | −0.11 (−0.69–0.48) | 0.72 | |
| Dosing modality | 0%, 0.33 | ||||||
| Fixed | 3525 | 262 089 | 24 692 049 | 80%, <0.0001 | 0.03 (−0.11–0.16) | 0.73 | |
| Variable | 5 | 129 109 | 134 114 | 0%, 0.93 | 0.14 (−0.14–0.16) | 0.0.26 | |
| Intact parathyroid hormone, pg/mL | |||||||
| Dialysis status | 48%, 0.12 | ||||||
| Chronic HD | 1612 | 666 466 | 665 461 | 6574%, <0.0001 | 51.9 (6.67–97.0) | 0.02 | |
| Incident HD | 1 | 54 | 55 | – | 54.3 (0.68–108) | ||
| Chronic PD | 1 | 15 | 15 | – | 58.6 (33.9–83.3) | – | |
| NDD-CKD | 43 | 11 599 | 119 103 | 5164%, 0.06 | −5.43 (−52.4–41.5) | 0.82 | |
| Ethnicity | 0%, 0.56 | ||||||
| White | 1512 | 481 426 | 480 429 | 881%, <0.0001 | 40.4 (2.28–78.5) | 0.04 | |
| Asian | 62 | 304 134 | 325 134 | 190%, 0.51 | 55.6 7.29 (−50.2–64.8) | 0.8 | |
| Other | 3 | 107 | 101 | 79%, 0.008 | 57.9 (−38.6–154) | 0.24 | |
MD, mean difference; CI, confidence interval; iPTH, intact parathyroid hormone; hemodialysis (HD) and peritoneal dialysis (PD) studies were restricted to >2 months of dialysis; NDD-CKD, non-dialysis-dependent chronic kidney disease; N, number of studies with events or poolable data; CBPB, calcium-based phosphate binder.
aTest for subgroup differences using Higgin's I2 and Cochrane's Q (P-value).
Selected subgroup analyses for end-of-study serum phosphorus and intact parathyroid hormone
| Subset (sevelamer only) . | Number ofstudies . | Number of patients . | Heterogeneity (I2, P-value) . | RR or MD (95% CI) . | P-value . | Test for interactiona (I2, P-value) . | |
|---|---|---|---|---|---|---|---|
| Sevelamer . | Calcium-based binder . | ||||||
| All-cause mortality | |||||||
| All studies | 12 | 325/1870 | 426/1899 | 75%, <0.0001 | 0.62 (0.35–1.08) | 0.09 | – |
| Comparator | 79%, 0.009 | ||||||
| CaCO3 | 6 | 45/475 | 128/481 | 32%, 0.20 | 0.44 (0.25–0.76) | 0.004 | |
| Calcium acetate | 3 | 6/170 | 17/174 | 30%, 0.24 | 0.43 (0.13–1.38) | 0.15 | |
| Any CBPB | 3 | 274/1225 | 281/1244 | 0%, 0.93 | 0.99 (0.86–1.14) | 0.87 | |
| Dialysis status | 94%, <0.0001 | ||||||
| Chronic HD | 9 | 284/1444 | 303/1472 | 0%, 0.43 | 0.96 (0.82–1.13) | 0.66 | |
| Incident HD | 2 | 29/305 | 101/309 | 0%, 0.36 | 0.29 (0.20–0.42) | <0.0001 | |
| Chronic PD | 0 | – | – | – | – | – | |
| NDD-CKD | 1 | 12/314 | 22/315 | – | 0.53 (0.28–1.03) | 0.06 | |
| Study follow-up | 0%, 0.67 | ||||||
| <6 months | 2 | 1/71 | 3/69 | 0%, 1.00 | 0.33 (0.06–1.98) | 0.23 | |
| 6 to <12 months | 2 | 4/54 | 3/61 | 0%, 0.71 | 1.52 (0.35–6.55) | 0.57 | |
| 12 to <24 months | 4 | 11/324 | 21/328 | 32%, 0.22 | 0.56 (0.21–1.52) | 0.26 | |
| ≥24 months | 4 | 309/1461 | 398/1479 | 92%, <0.0001 | 0.61 (0.26–1.43) | 0.25 | |
| Phosphorus, mg/dL | |||||||
| Dialysis status | 83%, 0.0005 | ||||||
| Chronic HD | 2618 | 20 141 517 | 19 271 523 | 3145%, 0.02 | 0.21 (−0.60–0.84) | 0.01 | |
| Incident HD | 2 | 253 | 253 | 82%, 0.02 | −0.43 (−0.72 to −0.15) | 0.002 | |
| Chronic PD | 2 | 110 | 59 | 82%, 0.02 | −0.28 (−1.06–0.49) | 0.47 | |
| NDD-CKD | 108 | 334 298 | 334 298 | 8273%, 0.0006 | −0.22 (−0.54–0.10) | 0.17 | |
| Ethnicity | 0%, 0.79 | ||||||
| White | 220 | 211 843 | 20 071 800 | 8381%, <0.0001 | 0.04 (−0.14–0.22) | 0.69 | |
| Asian | 15 | 419 177 | 450 187 | 339%, 0.13 | −0.08 (−0.42–0.27) | 0.67 | |
| Other | 5 | 158 | 146 | 86%, <0.0001 | −0.11 (−0.69–0.48) | 0.72 | |
| Dosing modality | 0%, 0.33 | ||||||
| Fixed | 3525 | 262 089 | 24 692 049 | 80%, <0.0001 | 0.03 (−0.11–0.16) | 0.73 | |
| Variable | 5 | 129 109 | 134 114 | 0%, 0.93 | 0.14 (−0.14–0.16) | 0.0.26 | |
| Intact parathyroid hormone, pg/mL | |||||||
| Dialysis status | 48%, 0.12 | ||||||
| Chronic HD | 1612 | 666 466 | 665 461 | 6574%, <0.0001 | 51.9 (6.67–97.0) | 0.02 | |
| Incident HD | 1 | 54 | 55 | – | 54.3 (0.68–108) | ||
| Chronic PD | 1 | 15 | 15 | – | 58.6 (33.9–83.3) | – | |
| NDD-CKD | 43 | 11 599 | 119 103 | 5164%, 0.06 | −5.43 (−52.4–41.5) | 0.82 | |
| Ethnicity | 0%, 0.56 | ||||||
| White | 1512 | 481 426 | 480 429 | 881%, <0.0001 | 40.4 (2.28–78.5) | 0.04 | |
| Asian | 62 | 304 134 | 325 134 | 190%, 0.51 | 55.6 7.29 (−50.2–64.8) | 0.8 | |
| Other | 3 | 107 | 101 | 79%, 0.008 | 57.9 (−38.6–154) | 0.24 | |
| Subset (sevelamer only) . | Number ofstudies . | Number of patients . | Heterogeneity (I2, P-value) . | RR or MD (95% CI) . | P-value . | Test for interactiona (I2, P-value) . | |
|---|---|---|---|---|---|---|---|
| Sevelamer . | Calcium-based binder . | ||||||
| All-cause mortality | |||||||
| All studies | 12 | 325/1870 | 426/1899 | 75%, <0.0001 | 0.62 (0.35–1.08) | 0.09 | – |
| Comparator | 79%, 0.009 | ||||||
| CaCO3 | 6 | 45/475 | 128/481 | 32%, 0.20 | 0.44 (0.25–0.76) | 0.004 | |
| Calcium acetate | 3 | 6/170 | 17/174 | 30%, 0.24 | 0.43 (0.13–1.38) | 0.15 | |
| Any CBPB | 3 | 274/1225 | 281/1244 | 0%, 0.93 | 0.99 (0.86–1.14) | 0.87 | |
| Dialysis status | 94%, <0.0001 | ||||||
| Chronic HD | 9 | 284/1444 | 303/1472 | 0%, 0.43 | 0.96 (0.82–1.13) | 0.66 | |
| Incident HD | 2 | 29/305 | 101/309 | 0%, 0.36 | 0.29 (0.20–0.42) | <0.0001 | |
| Chronic PD | 0 | – | – | – | – | – | |
| NDD-CKD | 1 | 12/314 | 22/315 | – | 0.53 (0.28–1.03) | 0.06 | |
| Study follow-up | 0%, 0.67 | ||||||
| <6 months | 2 | 1/71 | 3/69 | 0%, 1.00 | 0.33 (0.06–1.98) | 0.23 | |
| 6 to <12 months | 2 | 4/54 | 3/61 | 0%, 0.71 | 1.52 (0.35–6.55) | 0.57 | |
| 12 to <24 months | 4 | 11/324 | 21/328 | 32%, 0.22 | 0.56 (0.21–1.52) | 0.26 | |
| ≥24 months | 4 | 309/1461 | 398/1479 | 92%, <0.0001 | 0.61 (0.26–1.43) | 0.25 | |
| Phosphorus, mg/dL | |||||||
| Dialysis status | 83%, 0.0005 | ||||||
| Chronic HD | 2618 | 20 141 517 | 19 271 523 | 3145%, 0.02 | 0.21 (−0.60–0.84) | 0.01 | |
| Incident HD | 2 | 253 | 253 | 82%, 0.02 | −0.43 (−0.72 to −0.15) | 0.002 | |
| Chronic PD | 2 | 110 | 59 | 82%, 0.02 | −0.28 (−1.06–0.49) | 0.47 | |
| NDD-CKD | 108 | 334 298 | 334 298 | 8273%, 0.0006 | −0.22 (−0.54–0.10) | 0.17 | |
| Ethnicity | 0%, 0.79 | ||||||
| White | 220 | 211 843 | 20 071 800 | 8381%, <0.0001 | 0.04 (−0.14–0.22) | 0.69 | |
| Asian | 15 | 419 177 | 450 187 | 339%, 0.13 | −0.08 (−0.42–0.27) | 0.67 | |
| Other | 5 | 158 | 146 | 86%, <0.0001 | −0.11 (−0.69–0.48) | 0.72 | |
| Dosing modality | 0%, 0.33 | ||||||
| Fixed | 3525 | 262 089 | 24 692 049 | 80%, <0.0001 | 0.03 (−0.11–0.16) | 0.73 | |
| Variable | 5 | 129 109 | 134 114 | 0%, 0.93 | 0.14 (−0.14–0.16) | 0.0.26 | |
| Intact parathyroid hormone, pg/mL | |||||||
| Dialysis status | 48%, 0.12 | ||||||
| Chronic HD | 1612 | 666 466 | 665 461 | 6574%, <0.0001 | 51.9 (6.67–97.0) | 0.02 | |
| Incident HD | 1 | 54 | 55 | – | 54.3 (0.68–108) | ||
| Chronic PD | 1 | 15 | 15 | – | 58.6 (33.9–83.3) | – | |
| NDD-CKD | 43 | 11 599 | 119 103 | 5164%, 0.06 | −5.43 (−52.4–41.5) | 0.82 | |
| Ethnicity | 0%, 0.56 | ||||||
| White | 1512 | 481 426 | 480 429 | 881%, <0.0001 | 40.4 (2.28–78.5) | 0.04 | |
| Asian | 62 | 304 134 | 325 134 | 190%, 0.51 | 55.6 7.29 (−50.2–64.8) | 0.8 | |
| Other | 3 | 107 | 101 | 79%, 0.008 | 57.9 (−38.6–154) | 0.24 | |
MD, mean difference; CI, confidence interval; iPTH, intact parathyroid hormone; hemodialysis (HD) and peritoneal dialysis (PD) studies were restricted to >2 months of dialysis; NDD-CKD, non-dialysis-dependent chronic kidney disease; N, number of studies with events or poolable data; CBPB, calcium-based phosphate binder.
aTest for subgroup differences using Higgin's I2 and Cochrane's Q (P-value).
Lanthanum versus CBPBs: Lanthanum versus CBPBs did not significantly reduce the risk of all-cause mortality [RR 0.73 (95% CI 0.18–3.00)] based on 3/81 deaths (lanthanum) and 4/83 deaths (calcium binders) in four studies. However, two of the larger studies were considered to have a high risk of bias due to selective reporting. Hutchison et al. [67] randomized 800 patients who were followed for 5 weeks and 138 participants (17%) were lost during this period. Participants were selected to remain in the study for another 20 weeks (if their serum phosphorus was well controlled). While there were no deaths reported in this study, there remains concern that deaths may have been missed in the patients lost to follow-up. In the trial of D'Haese et al. [60], 11/98 participants died, but the number of deaths in each arm was not stated, and hence could not be included in the meta-analysis for death. Subgroup analysis by type of CBPB did not change the results.
Evidence of publication bias was not found [P = 0.51 for Egger's test (Supplementary data, Figure S2A)]. The risk of death using the beta-binomial method was RR = 0.83 (95% CI 0.38–1.82) for sevelamer, RR = 0.68 (95% CI 0.12–3.98) for lanthanum and RR = 0.81 (95% CI 0.39–1.66) combined.
Other clinically relevant outcomes (cardiac events, bone-related events)
Reporting on other important clinical outcomes was sparse (Table 2) and no significant differences were reported; however, the number of studies reporting outcomes provided insufficient power to yield definitive conclusions. Cardiovascular events were reported in six sevelamer trials: three reported cardiovascular mortality [RR 0.29 (95% CI 0.05–1.82); 152/1337 sevelamer, 232/1351 CBPBs] [29, 41, 53], two were unspecified [25, 32] and one was only qualitative [50] (an additional study reported a sudden death in a patient with a dilated cardiomyopathy [33]). Four lanthanum trials reported cardiovascular events, but these were also heterogeneous: one specified angina [67], two were unspecified [63, 66] and one reported any event inclusive of angina, heart failure, myocardial infarction, stroke or peripheral artery disease [65].
Bone-related adverse events were rarely documented (osteoporosis reported in one sevelamer patient [53], absence of fractures reported in one lanthanum trial [66]). This sparse reporting did not support meta-analysis (Table 2).
Hospitalization
Hospitalization was reported in five sevelamer trials, four of which provided data amenable to meta-analysis (Figure 5B) [25, 52, 53, 80, 81]. Sevelamer was associated with a significantly lower risk of hospitalization (113/493 events) compared with CPBPs (245/499 events) [RR 0.50 (95% CI 0.31–0.81)]. The study that could not be pooled reported a hospitalization rate of 2.1 (SD 4.4) and 2.3 (SD 4.9) hospitalizations/patient-year among sevelamer and CBPBs, respectively (P = 0.06) [53]. The NNT to prevent hospitalization was 4 (95% CI 2–50) for 2 years and 4 (95% CI 3–5) for 3 years, suggesting that four patients would need to be treated with sevelamer instead of CBPBs to prevent one additional hospitalization. Two studies reported longer length of stay among patients treated with CBPBs [52, 53]. Only two trials reported hospitalization rates for lanthanum (7/43 events) compared with CBPBs (9/45 events); a significant difference was not found [RR 0.80 (95% CI 0.34–1.93)] (Table 2).
Adverse events (gastrointestinal events, hypercalcemia, pruritis, calciphylaxis)
Forest plot comparing gastrointestinal adverse event rates over the study duration between patients treated with sevelamer or lanthanum (Sev/Lan) and calcium-based phosphate binders (CBPB). AE - adverse event.
Forest plot comparing hypercalcemia event rates over the study duration between patients treated with sevelamer or lanthanum (Sev/Lan) and calcium-based phosphate binders (CBPB). AE - adverse event.
Forest plot comparing hospitalization events and pruritis events over the study duration between patients treated with sevelamer or lanthanum (Sev/Lan) and calcium-based phosphate binders (CBPB). AE - adverse event; US - United States.
Loss to follow-up
Fewer patients receiving sevelamer than CBPBs were lost to follow-up (736/2594 versus 804/2572) [RR 0.91 (95% CI 0.85–0.99)] but not lanthanum (142/908) versus CBPBs (103/650) [RR 1.19 (95% CI 0.75–1.88)] (Supplementary data, Figure S3). Using beta-binomial methods, the risk of attrition was RR = 0.95 (95% CI 0.61–1.47) for sevelamer, RR = 1.41 (95% CI 0.74–2.69) for lanthanum and RR = 1.07 (95% CI 0.75–1.54) combined.
Serum phosphorus
Meta-analyses of end-of-study biochemical parameters are presented in the Supplementary figures and summarized in Table 2. Sevelamer reduced serum phosphorus (n = 2178) to a similar extent to CBPBs (n = 2133) [MD −0.01 (95% CI −0.16–0.14)], irrespective of the type of CBPB used (Supplementary data, Figure S3). Lanthanum (n = 581) provided slightly less effective phosphate reduction than CBPBs (n = 500) [MD 0.18 (95% CI 0.10–0.27)]. No evidence of publication bias was found [Egger's P = 0.15 (Supplementary data, Figure S2D)].
The heterogeneity observed among sevelamer trials was not explained by the type of CBPB used as the comparator (P = 0.85), ethnicity (P = 0.79) or dosage strategy (P = 0.33) (Table 3). A significant difference was found in subgroup analysis by dialysis modality (P = 0.0005), whereby sevelamer was less effective than CBPBs in chronic HD patients.
Serum calcium
Lower end-of-study serum calcium was observed with sevelamer (n = 2078) versus CBPBs (n = 2055) [MD −0.35 (95% CI −0.50 to −0.21)] and lanthanum (n = 579) [MD −0.26 (95% CI −0.46 to −0.07)] versus CBPBs (n = 499). Despite significant heterogeneity between studies (I2= 88%), results were consistently in the same direction across all studies (Supplementary data, Figure S4).
Low-density lipoprotein
Sevelamer use (n = 974) was associated with significantly lower LDL levels by 20.9 (95% CI 18.6–23.3) mg/dL compared with CBPBs (n = 979) (Supplementary data, Figure S5). Although there was significant heterogeneity between studies (I2= 69%), all point estimates were in favor of sevelamer, except one non-significant report [36]. Similar reductions were not observed with lanthanum (n = 47) versus CBPBs (n = 53), although only two studies provided data on LDL (Table 2).
Intact parathyroid hormone
Sevelamer (n = 634) and lanthanum (n = 276) were both associated with significantly higher iPTH levels: MD 43.5 (95% CI 11.1–75.9) pg/mL, n = 634 and MD 63.3 (95% CI 11.5–115) pg/mL, n = 294, respectively (Supplementary data, Figure S6). Differences were not observed in subgroup analyses (Table 3). Three studies that measured end-of-study iPTH levels in NDD-CKD patients could not be pooled since results were presented as medians, but all three trials reported lower end-of-study iPTH with sevelamer. We did not observe subgroup differences by the type of CBPB used as a comparator, ethnicity or dosing regimen.
Coronary artery calcification
By the end of the study, CAC was significantly lower among sevelamer-treated patients (n = 412) compared with CBPBs (n = 383) [MD −101 (95% CI −160 to −41.7)]. Heterogeneity between studies was observed (I2= 74%), but all estimates were in the same direction. Among the two studies whose data could not be pooled, the increase in CAC was also higher among CBPB-treated patients [29, 49]. Only one study reported CAC following lanthanum treatment, but conclusions were drawn from a subgroup analysis (n = 21) [63].
Head-to-head comparisons for noncalcium binders
Sevelamer hydrochloride was compared with sevelamer carbonate in three head-to-head trials (n = 207): no differences were observed for end-of-study serum phosphorus, serum calcium or LDL, but no study reported on hyperchloremic acidosis (primary motivator for introducing sevelamer carbonate) [57–59]. Two trials (n = 295) comparing sevelamer hydrochloride with magnesium carbonate obtained conflicting results on end-of-study phosphorus levels, although no difference in serum calcium was observed [50, 56]. Three studies compared sevelamer directly with lanthanum carbonate (n = 314 patients): similar end-of-study phosphorus and calcium levels were observed, but sevelamer was associated with lower LDL [MD −20.9 (95% CI −29.9 to −11.9) mg/dL] [49, 54, 55].
Sevelamer was compared with iron-based binders in three studies (n = 1492) (Supplementary data, Figure S9) [73–75]. All-cause mortality [(RR 1.07 (95% CI 0.38–2.99), I2= 0%], patient attrition [RR 1.03 (95% CI 0.49–2.13), I2= 83%] and incidence of gastrointestinal adverse events [RR 1.30 (95% CI 0.61–2.78), I2= 96%] were similar. Similar end-of-study phosphate [MD 0.07 (95% CI −0.42–0.56) mg/dL, n = 1206], calcium [MD −0.03 (95% CI −0.12–0.05) mg/dL, n = 398] and iPTH (only medians reported) were observed. Hypercalcemic events and hospitalization rates were not reported.
Meta-regression of relationship between biochemical parameters and mortality risk
The RR of mortality across studies was not associated with trial duration (P = 0.52) or the proportion of patients lost to follow-up in the intervention arm (P = 0.18) or CBPB arm (P = 0.26). A greater reduction in mortality risk was observed among studies with a greater reduction in end-of-study calcium (P < 0.0001), but not phosphorus (P = 0.27), LDL (P = 0.51) or CAC (P = 0.10) (Supplementary data, Figure S8).
DISCUSSION
When all available randomized evidence is considered, very few clinically relevant advantages have been proven for any particular phosphate binder. Confidence in any significant differences found is eroded by the shortcomings in the existing evidence base (lack of reporting clinically important outcomes, lack of blinding, selective reporting, publication bias and significant loss to follow-up). Despite >51 randomized trials of phosphate binders, there are few definitive answers, largely because the majority of the studies were focused on surrogate (biochemical) outcomes and not designed to study clinically relevant outcomes. In fact, few of the studies reported on the very reason that phosphate binders are given to patients with CKD: to prevent clinically important adverse events that (theoretically) may be due to hyperphosphatemia, such as bone events (bone deformity, fractures), cardiac events and ultimately all-cause mortality and overall quality of life.
The most contentious finding is whether sevelamer reduces the risk of all-cause mortality compared with CBPBs. In our meta-analysis, we found that the RR for all-cause mortality for sevelamer versus CBPBs was 0.62 (95% CI 0.35–1.08). The CIs show results that are compatible with both a 65% reduction and an 8% increase in the risk of death. As a result, the conclusions cannot be definitive about whether sevelamer reduces, has no impact or increases the risk of death. Our conclusions regarding mortality agree with some recent meta-analyses [82] and contrast with others that purport to show that sevelamer significantly reduces the risk of all-cause mortality [13, 16, 17]. We explore these reasons next.
We used imputation and digitization to include data from more trials than previous meta-analyses [13, 16, 17]. The most recent systematic review [14] obtained a risk for all-cause mortality of RR = 0.54 (95% CI 0.32–0.93) from 13 studies, which we believe is optimistic. Deaths were not reported in 4 of these studies [30, 38, 39, 47] (so only 9/13 studies contributed to the RR estimate). Moreover, we identified three additional studies [25, 32, 34] that were not included in previous reviews [14, 83]. By including more trial data, our numerical results are less biased and more representative of the evidence base than other recent reviews [13, 14, 83]. Differences in how the treatment of observational studies (or observational periods postrandomization) was considered may explain some of the numerical differences between meta-analyses. For example, the mortality risk of RR = 0.53 (95% CI 0.28–1.03) in favor of sevelamer from the study by Block et al. [51, 84] was based on continued observation of patients who were no longer on assigned treatment for up to 3 years, a period we omitted [84]. We also conduct a sensitivity analysis to exclude the sevelamer trial whose loss to follow-up renders the comparability of the groups questionable [52]. Finally, a recent network meta-analysis [83] incidentally included a non-randomized trial (Takei, 2008) [85].
Numerical differences may also be due to data abstraction decisions based on intenion to treat: Di Lorio et al. [28] randomized 239 patients, but 212 were used as the denominator by both prior reviews [13, 14]. To further account for potential bias, secondary analyses trials with double-zero counts were included because data from trials reporting zero deaths are not uninformative (they suggest that mortality is infrequent and is similar between treatments) [23]. Excluding double-zero trials may overestimate treatment effects. Also, pooling of sparse-event studies using this methodology negates concern about the continuity correction. Since fewer lanthanum trials reported mortality and trials were generally small, there was a large proportion of trials with very few deaths, leading to spuriously high RR estimates due to the continuity correction [i.e. RR 5.23 (95% CI 0.27–103) for Wada and Wada [63] in Figure 3]. Finally, our meta-analysis was investigator driven, which may provide less bias than perspectives from industry-sponsored syntheses [86].
Much of the apparent ‘controversy’ between meta-analyses can be resolved through a more rational understanding of the numbers rather than overinterpretation of P-values as bluntly indicating ‘significant’ versus not significant at the magical threshold of P = 0.05. In fact, interpreting the effect size and the CIs should be the focus rather than the P-value. The most clinically useful interpretation is likely through NNT. When we consider the absolute difference in mortality between sevelamer and CBPBs with the RR = 0.65 (nntonline.net), NNT = 16, suggesting that on average a total 16 patients would need to be treated chronically with sevelamer instead of CBPBs for up to 3 years in order to prevent one death. This is likely an underestimate since NNT=35 if RR=0.85 (beta-binomial estimate) is used. This NNT estimate would be similar across all meta-analyses, but the CIs around NNT would differ (ranging from benefit to harm in our analysis). Thus, we can conclude that sevelamer might provide a reduced risk of death, though at best this difference applies to an average of only 1/16 patients treated with the drug. The other 15 patients would have similar survival regardless of which phosphate binder they used. Furthermore, the confidence in this effect estimate for sevelamer on mortality is sensitive to inclusion of the largest trial (yet most biased due to large loss to follow-up). Finally, it is important to consider that only three studies drive most of this potential difference in mortality. As a result of these limitations, the evidence base does not allow us to be more definitive than this; the loss to follow-up across the most pivotal trials makes any attempt at definitive conclusions suspect.
Patients receiving sevelamer (but not lanthanum) were less likely to drop out by the trials' end date. The reasons for differential attrition are likely mixed (i.e. due to the open-label nature of most studies, the effect of adverse events or side effects, pill burden), making conclusions difficult to draw. Other outcomes that were significantly improved with sevelamer versus CBPBs included fewer hospitalizations and hypercalcemic events. However, other important outcomes (bone fractures, cardiac events, calciphylaxis, surgeries and overall quality of life) remain largely unstudied. Sevelamer and lanthanum were associated with a higher risk of gastrointestinal events but lower risk of hypercalcemia (and lower serum calcium) compared with CBPBs. Another limitation is the paucity of reporting on hyperchloremic acidosis in head-to-head trials of sevelamer hydrochloride with sevelamer carbonate, lanthanum or iron-based binders—the very basis for the attempt to supplant sevelamer hydrochloride. If calcium-free phosphate binders are indeed the future of phosphate binder treatment [87], trials should focus on the relevant outcomes attributable to the specific binders to determine whether their balance of benefits and risks is worthy of supplanting the cheaper CBPBs. If randomized trials are unable to provide data that require long-term follow-up (particularly for rare outcomes like calciphylaxis [88] or long-term effects of lanthanum storage in the body [11]), methodologically sound large-scale observational studies may help fill this gap.
Sevelamer was as effective as CBPBs at reducing serum phosphorus, while lanthanum was less effective. Sevelamer also had significantly greater reductions in LDL, serum calcium, and CAC than CBPBs, and increased iPTH. However, the clinical relevance of these differences is unknown. Lanthanum generally did not have significant effects on these biochemical parameters. Results from observational studies suggest that mortality is elevated with higher serum phosphorus, LDL and calcification scores in a dose-dependent manner [89–92]. However, as is often the case with surrogate outcomes, these effects may not translate to better clinical outcomes [90, 93–96]. Although the relationship between lower serum calcium and survival is supported by our meta-regression, this is hypothesis-generating only and needs to be the focus of clinical trials designed to test the relationship prospectively. Furthermore, studies provided short-term follow-up (maximum 3 years), which reduces our confidence in adequately studying relationships between biochemical parameters and risk of death. Our meta-regressions did not show a relationship with other biochemical parameters, including phosphate, LDL and CAC, and risk of mortality.
Most trials employed treat-to-target methodology, whereby the dose of the phosphate binder could be adjusted throughout the study. Although the recommended phosphate target of 3.5–5.5 mg/dL established by the Kidney Disease: Improving Global Outcomes guideline [97] was often used, some studies aimed as low as 2.5 mg/dL [34, 39] or as high as 6.5 mg/dL [51]. Given such differences in methodology, a random effects model was used to calculate pooled estimates. One limitation to pooling data may arise due to differences in how results are presented. Phosphorus control may be more appropriately measured as a time-weighted average to reflect the differences between groups over the entire course of follow-up. End-of-study phosphorus levels may not be representative of the general trends, as was the case with Lin et al. [44]. The extent to which these different measurement strategies impact conclusions depends on the temporal variation in phosphorus control throughout the study.
In conclusion, in this comprehensive update on the efficacy and safety of calcium-free binders compared with cheaper alternatives (i.e. CBPBs), sevelamer was associated with lower hospitalization rates, lower rates of hypercalcemia and a nonsignificant reduction in mortality. However, differences in some of the most important outcomes (cardiac events, fractures, calciphylaxis, hyperchloremic acidosis and health-related quality of life) remain unstudied. While sevelamer resulted in favorable biochemical outcomes, the importance of these surrogate outcomes remains unknown due to a lack of follow-up for associated clinically relevant outcomes. Future randomized trials should be of adequate power and duration to measure clinically important outcomes (the reason why phosphate binders are prescribed in the first place). Future studies that fail to address these outcomes will be wasteful.
ETHICS APPROVAL
This is a secondary analysis of publicly available data. No ethical approval was required.
ACKNOWLEDGEMENTS
Dr Amit X Garg was supported by the Dr Adam Linton Chair in Kidney Health Analytics. S.H., S.P. and R.A. were supported by the Lilibeth Caberto Kidney Clinical Research Unit.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to disclose.
(See related article by Elder. Calcium-based phosphate binders; down, but not out. Nephrol Dial Transplant 2017; 32: 5–8)
REFERENCES
- parathyroid hormones
- low-density lipoproteins
- iron
- hypercalcemia
- kidney failure, chronic
- calcium
- cardiac event
- fractures
- hyperphosphatemia
- metabolic acidosis, nag, acidifying salts
- calciphylaxis
- lanthanum
- safety
- mortality
- calcium test, serum
- sevelamer
- phosphate binding agents
- coronary artery calcium





Comments