-
PDF
- Split View
-
Views
-
Cite
Cite
Ming Wu, Junhui Gu, Shuqin Mei, Dechao Xu, Ying Jing, Qing Yao, Meihan Chen, Ming Yang, Sixiu Chen, Bo Yang, Na Qi, Huimin Hu, Rudolf P. Wüthrich, Changlin Mei, Resveratrol delays polycystic kidney disease progression through attenuation of nuclear factor κB-induced inflammation, Nephrology Dialysis Transplantation, Volume 31, Issue 11, November 2016, Pages 1826–1834, https://doi.org/10.1093/ndt/gfw058
Close - Share Icon Share
Abstract
Inflammation plays an important role in polycystic kidney disease (PKD). The current study aimed to examine the efficacy of the anti-inflammatory compound resveratrol in PKD and to investigate its underlying mechanism of action.
Male Han:SPRD (Cy/+) rats with PKD were treated with 200 mg/kg/day resveratrol or vehicle by gavage for 5 weeks. Human autosomal dominant (AD) PKD cells, three-dimensional (3D) Madin-Darby canine kidney cells and zebrafish were treated with various concentrations of resveratrol or the nuclear factor κB (NF-κB) inhibitor QNZ.
Resveratrol treatment reduced blood urea nitrogen levels and creatinine levels by 20 and 24%, respectively, and decreased two-kidney/total body weight ratio by 15% and cyst volume density by 24% in Cy/+ rats. The proliferation index and the macrophage infiltration index were reduced by 40 and 43%, respectively, in resveratrol-treated cystic kidneys. Resveratrol reduced the levels of the pro-inflammatory factors monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor-α (TNF-α) and complement factor B (CFB) in Cy/+ rat kidneys in parallel with the decreased activity of NF-κB (p50/p65). The activation of NF-κB and its correlation with pro-inflammatory factor expression were confirmed in human ADPKD cells and kidney tissues. Resveratrol and QNZ inhibited the expression of MCP-1, TNF-α and CFB and reduced NF-κB activity in ADPKD cells. Moreover, NF-κB blockage minimized the inhibition of inflammatory factor production by resveratrol treatment. Furthermore, resveratrol or QNZ inhibited cyst formation in the 3D cyst and zebrafish models.
The NF-κB signaling pathway is activated and partly responsible for inflammation in polycystic kidney tissues. Targeting inflammation through resveratrol could be a new strategy for PKD treatment in the future.
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited polycystic kidney disease (PKD) [1]. The formation and growth of renal cysts in ADPKD patients are due to mutations in either the PKD1 or the PKD2 gene, which encode polycystin-1 and polycystin-2, respectively [2]. Effective therapeutic agents to delay the progression of ADPKD are being developed [3].
There is growing interest regarding the role played by inflammation in the pathogenesis of PKD [4]. Pro-inflammatory factors have been identified in urine samples and in renal cyst fluid from human ADPKD patients [4]. Some of these factors were found to play important roles in cyst formation, cyst growth and disease progression in PKD [5, 6]. Moreover, inflammatory cells such as macrophages accumulate in cystic kidneys and were shown to promote renal cyst growth [4, 7, 8]. Furthermore, the inhibition of the complement system was found to attenuate disease progression of PKD and to reduce macrophage infiltration in cystic kidneys [9–11].
Resveratrol is a polyphenolic substance that is found in grapes, peanuts, berries and their derivatives [12]. Beneficial effects of resveratrol have been shown in various physiological and pathological conditions including aging, hypertension, cancer and kidney diseases [12–14]. Resveratrol exerts its anti-inflammatory, antioxidant and anti-proliferative effects through different intracellular targets which include mammalian target of rapamycin (mTOR) and nuclear factor κB (NF-κB) [13, 14].
The direct targeting of mTOR was shown to inhibit renal epithelial cell proliferation and retard disease progression in animal models of PKD [3, 15, 16]. Resveratrol was also shown to inhibit cell proliferation through suppression of mTOR activity [13, 14]. NF-κB is a central regulator of inflammation that is inhibited by resveratrol [17]. In the canonical NF-κB pathway, p50 and p65 form a complex with the inhibitory component IκBα in the cytoplasm. Degradation of IκBα releases the p50/p65 dimer into the nucleus to regulate downstream inflammatory gene transcription [17].
The aim of this study was to test the hypothesis that inhibition of inflammation by the dual mTOR/NF-κB inhibitor resveratrol can prevent disease progression in the Han:SPRD rat model of PKD and to explore its mechanism of action in ADPKD cells.
MATERIALS AND METHODS
Animal and human samples
This study was conducted in male heterozygous cystic (Cy/+) and wild-type normal (+/+) rats. Han:SPRD rats were maintained according to local regulations and guidelines.
Tissue samples from anonymous ADPKD patients and normal controls were obtained from Shanghai Changzheng Hospital. Informed consent was obtained from all participants.
Reagents
Antibodies targeting P50/105, p-S6K and S6K were obtained from Cell Signaling Technology. Antibodies targeting 8-hydroxy-2′-deoxyguanosine (8-OHdG), β-actin and GAPDH were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies for p-p65, p65, monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor-α (TNF-α), superoxide dismutase-2 (SOD2) and H3 were purchased from Affinity Biosciences (Zhenjiang, China). The complement factor B (CFB) antibody was from Proteintech (Chicago, IL, USA). Nitrotyrosine, proliferating cell nuclear antigen (PCNA) and CD68 antibodies were from Abcam (Cambridge, UK). The NF-κB-specific inhibitor quinazoline (QNZ) was obtained from Selleckchem (Houston, TX, USA). Resveratrol was from Sigma (St Louis, MO, USA).
Experimental animal protocol
Male Cy/+ and +/+ rats were treated with 200 mg/kg/day resveratrol (Cy/+, n = 12; +/+, n = 12) or vehicle (0.5% sodium carboxymethyl cellulose/saline solution) (Cy/+, n = 15; +/+, n = 13) at 4 weeks of age by gavage. Resveratrol was administered once daily for 5 weeks (Day 0 to Day 35). Tail blood was obtained from rats on Days 0, 17 and 35. Blood urea nitrogen (BUN) and creatinine were determined after 5 weeks of treatment. Kidneys were excised and used for histologic examinations and protein analysis.
Zebrafish care and morpholino injection
Zebrafish were raised according to standard protocols in Ying Cao's laboratory at Shanghai Tongji University. A morpholino oligonucleotide that targets Pkd2 (5′-AGGACGAACGCGACTGGAGCTCATC-3′) was purchased from Genetools. The oligonucleotide (0.3–0.5 ng) was injected into embryos at the one- to two-cell stage, as described before [18].
Cell cultures
Human immortalized cystic (OX161) and normal (UCL93) renal epithelial cells were kindly provided by Prof. A.C. Ong (University of Sheffield, Sheffield, UK) [19]. Cells were cultured to 70–80% confluence and treated with various concentrations of resveratrol or QNZ for 48 h. In some experiments, cells were pretreated with QNZ for 2 h, followed by resveratrol treatment for 46 h.
MTT assay
OX161 cells were treated with various concentrations of resveratrol for 48 h. Then 20 µL per well of tetrazolium (MTT) (5 mg/mL) was added for 4 h and subsequently sub-cultured in the medium with 100 µL DMSO. The absorbance of each well was determined at 490 nm.
Histology and immunohistochemistry staining
Cyst volume density was assessed by morphometry, using the method of point counting as described before [16].
Immunohistochemical staining for PCNA, 8-OHdG, nitrotyrosine or CD68 was performed on 3-µm-thick tissue sections. For proliferation index analysis, PCNA-positive nuclei were counted among the 500–600 cells (×400 magnification) per three sections from each group. CD68 staining was used to determine the macrophage infiltration index, as described by Karihaloo et al. [8].
Positive areas for 8-OHdG or nitrotyrosine in the tubulointerstitium were quantified at a ×200 magnification in a blinded manner using NIH ImageJ (Bethesda, MD, USA). Five tubulointerstitial areas per section were randomly selected from each animal for quantification (n = 3 in each group).
Protein extraction and western blot analysis
Protein extraction and western blot analysis were performed as described previously [15]. For nuclear extracts, fresh kidney tissues were lysed in NE-PER extraction reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's protocol.
ELISA
Cell culture supernatants were collected and centrifuged at 500 g for 5 min, then stored at −80°C. MCP-1 (BMS281, eBioscience, CA, USA), CFB (KA3816, Abnova, Taipei City, Taiwan) and TNF-α (BMS223/4, eBioscience) were analyzed using commercially available ELISA kits, according to the manufacturer's protocols.
Statistical analysis
Statistical analyses were performed by unpaired t-test or one-way ANOVA with the Newman–Keuls post hoc test using GraphPad Prism version 5.0 (GraphPad, San Diego, CA, USA). All data are expressed as means ± standard deviation, and P < 0.05 was considered as statistically significant.
RESULTS
Resveratrol retards disease progression in Cy/+ rats
Figure 1A shows that BUN and creatinine levels increased in Cy/+ Han:SPRD rats from Week 4 to Week 9, but not in wild type +/+ rats. The increase in BUN and creatinine levels was significantly reduced by resveratrol by 20 and 24%, respectively, in Cy/+ rats. Resveratrol treatment did not change body weight (data not shown) in +/+ and Cy/+ rats, but significantly decreased the two-kidney/total body weight (2K/TBW) ratio in Cy/+ rats by 15% (Figure 1B). Five weeks of resveratrol treatment also significantly reduced the cyst volume density by 24% (Figure 1C and D).
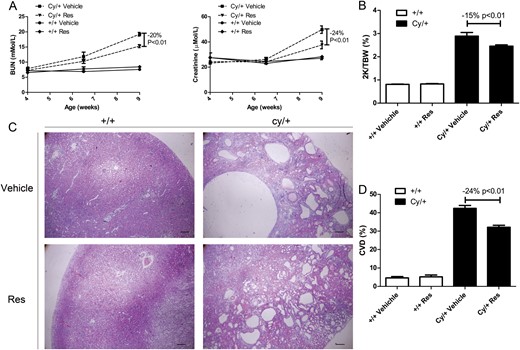
Effect of resveratrol on disease progression in a rat model of PKD. (A) BUN and creatinine levels in resveratrol (Res)- or vehicle-treated wild type (+/+) and cystic (Cy/+) Han:SPRD rats from Week 4 to Week 9. (B) 2K/TBW ratio of 9-week-old +/+ and Cy/+ rats. (C) Hematoxylin and eosin staining on kidney of 9-week-old +/+ and Cy/+ rats. Scale bar = 200 µm. (D) Quantification of cyst volume density (CVD).
PCNA staining revealed that resveratrol significantly reduced the proliferation index in Cy/+ kidneys by 40% (Figure 2A and B). The reduction of cell proliferation in resveratrol-treated Cy/+ kidneys was further confirmed by western blot analysis for PCNA (Figure 2C). The infiltration by macrophages was assessed by CD68 staining. Figure 2D and E shows that the infiltration by macrophages was markedly increased in Cy/+ kidneys, but significantly reduced by resveratrol in Cy/+ kidneys.
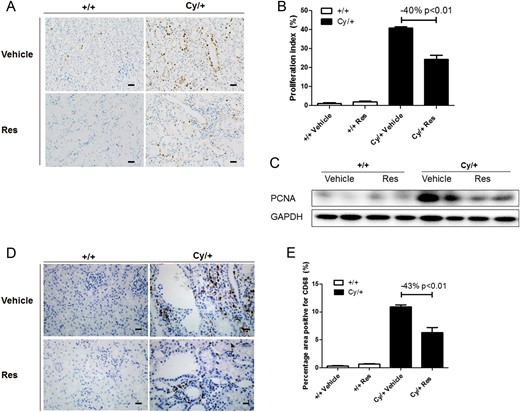
Effect of resveratrol (Res) on cell proliferation and macrophage infiltration in cystic kidneys. (A) Proliferation marker PCNA was stained in 9-week-old +/+ and Cy/+ kidneys. Scale bar = 50 µm. (B) Quantification of PCNA staining. (C) Western blot of PCNA for 9-week-old +/+ and Cy/+ rat kidneys. (D) Macrophage marker CD68 was stained in 9-week-old +/+ and Cy/+ kidneys. Scale bar = 20 µm. (E) Quantification of CD68 staining. Blots are representative of three independent experiments.
Resveratrol suppresses the expression of inflammatory factors and the NF-κB and mTOR pathways in Cy/+ rats
Figure 3A demonstrates that protein levels of the pro-inflammatory factors MCP-1, TNF-α and CFB were up-regulated in cystic kidneys in Cy/+ rats, and that resveratrol treatment for 5 weeks reduced the levels of these factors.
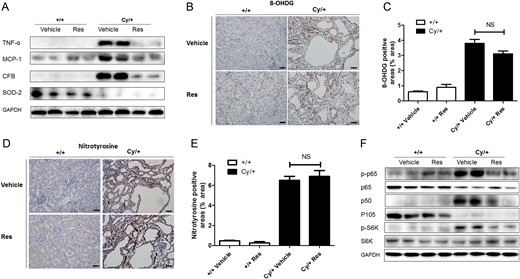
Pro-inflammatory factors, anti-oxidant enzyme and cell signaling pathways in resveratrol (Res)- or vehicle-treated cystic kidneys. (A) TNF-α, MCP-1, CFB and SOD2 were analyzed by western blot in 9-week-old +/+ and Cy/+ kidneys. (B–E) Immunohistochemical staining for oxidative stress markers 8-OHdG and nitrotyrosine in the tubulointerstitial area. Computer-assisted morphometry was used to quantify changes of 8-OHdG and nitrotyrosine in each group. Scale bar = 50 µm. (F) NF-κB pathway (p-p65, p65, p105 and p50) and mTOR pathway (p-S6K and total S6K) were analyzed by western blot in 9-week-old +/+ and Cy/+ kidneys. Blots are representative of three independent experiments.
Figure 3A also shows that the levels of SOD2 were reduced in cystic kidneys in Cy/+ rats compared with +/+ rats, but resveratrol treatment did not restore its expression. Moreover, immunohistological analysis showed that staining for the oxidative stress markers 8-OHdG or nitrotyrosine was significantly enhanced in polycystic kidneys, but that resveratrol did not inhibit 8-OHdG or nitrotyrosine expression (Figure 3B–E).
Next, the NF-κB (p50/p65) and mTOR pathways were analyzed in +/+ and Cy/+ rat kidneys (Figure 3F). Figure 3F shows that the phosphorylation of p65 was increased and that the total levels of p65 were reduced in Cy/+ kidneys as compared with +/+ kidneys. Resveratrol treatment reduced the phosphorylation of p65 without changing the levels of the p65 protein in Cy/+ kidneys. The expression of p105 was reduced in Cy/+ kidneys, whereas its active form p50 was markedly up-regulated in Cy/+ kidneys, but reduced by resveratrol treatment. Phosphorylation of S6K, a downstream target of the mTOR pathway, was up-regulated in cystic kidneys but also down-regulated by resveratrol.
Resveratrol inhibits the NF-κB and mTOR pathways and the expression of inflammatory factors in ADPKD cells
Figure 4A and B shows that resveratrol concentration-dependently reduced the expression of MCP-1, TNF-α and CFB in protein extracts of OX161 cells, a human ADPKD cell line, from 2 µM to 50 µM, which was correlated with the reduction of phosphorylated p65 and the levels of p65, p105 and p50. Supernatants were collected from OX161 cells to measure the secretion of these pro-inflammatory factors by ELISA. MCP-1 and CFB levels were significantly reduced by resveratrol in a dose-dependent manner from 2 µM to 50 µM (Figure 4C). TNF-α was not detectable in the supernatants (detection limit 7.8 pg/mL, data not shown).
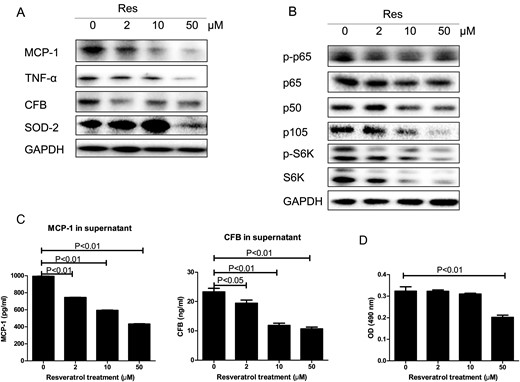
Effect of resveratrol (Res) in ADPKD cells. ADPKD cells (OX161) were exposed to 2, 10 and 50 µM resveratrol for 48 h. (A) TNF-α, MCP-1, CFB and SOD2 were analyzed by western blot in resveratrol-treated ADPKD cells. (B) The NF-κB (p-p65, p65, p105 and p50) and mTOR pathways (p-S6K and total S6K) in resveratrol-treated ADPKD cells were analyzed by western blot. (C) The supernatants MCP-1 or CFB from resveratrol-treated ADPKD cells were measured by ELISA. (D) Cell proliferation was evaluated using the MTT assay. One representative of three independent experiments is shown.
Resveratrol concentration-dependently reduced the expression of p-S6K and total S6K in OX161 cells from 2 µM to 50 µM, whereas the reduction of cell proliferation was only detected in 50 µM resveratrol-treated cells (Figure 4B and D). The antioxidant enzyme SOD2 was increased by resveratrol from 2 µM to 10 µM, but decreased by 50 µM resveratrol treatment (Figure 4A).
NF-κB pathway is activated in rat PKD and human ADPKD
Nuclear proteins were extracted from fresh kidney tissues of +/+ and Cy/+ rats to further confirm the activation of the NF-κB pathway in rat PKD. Figure 5A shows that the expression of p50 and phosphorylated p65 were increased whereas the expression of total p65 was decreased in nuclear extracts of Cy/+ compared with +/+.
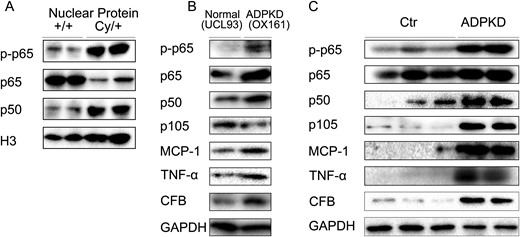
NF-κB pathway is activated in rat PKD and human ADPKD. (A) Expression of p-p65, p65 and p50 in nuclear extracts from fresh rat kidney tissues. (B) Expression of p-p65, p65, p105, p50, TNF-α, MCP-1 and CFB in immortalized human kidney cells (UCL93) and ADPKD cells (OX161). (C) Expression of p-p65, p65, p105, p50, TNF-α, MCP-1 and CFB in control (Ctr) and ADPKD kidney tissues. Blots are representative of three independent experiments.
Figure 5B shows that p-p65, p65 and p50 were up-regulated in OX161 ADPKD cells as compared with UCL93 control cells. The up-regulation of p50/p65 NF-κB in OX161 cells correlated with increased expression of MCP-1, TNF-α and CFB (Figure 5B). In addition there was an up-regulation of p-p65, p50 and p105 in human ADPKD kidneys as compared with control tissues (Figure 5C). The activation of the p50/p65 NF-κB pathway correlated with the increased expression of MCP-1, TNF-α and CFB in human ADPKD kidney tissues (Figure 5C).
NF-κB is required for inhibition of MCP-1, TNF-α and CFB expression by resveratrol treatment
Figure 6A shows that the NF-κB-specific inhibitor QNZ dose-dependently reduced the expression of MCP-1, TNF-α and CFB in ADPKD cells from 5 to 500 nM, which correlated with down-regulation of p-p65, total p65, p50 and p105.
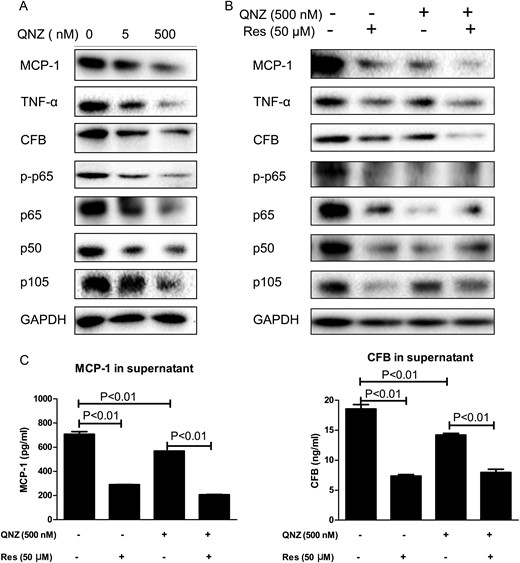
NF-κB partly mediated resveratrol (Res) inhibition on PKD inflammation. (A) Expression of p-p65, p65, p105, p50, TNF-α, MCP-1 and CFB in QNZ-treated OX161 cells. (B) OX161 cells were pretreated with QNZ for 2 h and followed by the treatment of resveratrol for 46 h. The expression of p-p65, p65, p105, p50, TNF-α, MCP-1 and CFB were evaluated by western blot. (C) MCP-1 or CFB of QNZ-and/or resveratrol-treated OX161 cell supernatants were measured by ELISA. One representative of three independent experiments is shown.
Resveratrol or QNZ treatment decreased the expression of MCP-1, TNF-α and CFB, and resveratrol further reduced the expression of these factors in the QNZ-pretreated group (Figure 6B), whereas resveratrol did not further reduce the expression of p-p65, total p65 and p50 in the QNZ-pretreated group, indicating that NF-κB activity was completely blocked by QNZ pretreatment (Figure 6B).
Furthermore, supernatants from resveratrol and/or QNZ-treated OX161 cells were collected for ELISA analysis. Figure 6C shows that resveratrol or QNZ treatment decreased the secretion of MCP-1 by 417 pg/mL or 140 pg/mL, respectively, in comparison with vehicle, and resveratrol further reduced the secretion of MCP-1 in QNZ-pretreated ADPKD cells by 360 pg/mL. Supernatant CFB was reduced by resveratrol or QNZ treatment by 11.2 ng/mL and 4.4 ng/mL, respectively, in comparison with control cells, and resveratrol further reduced CFB secretion in QNZ-pretreated ADPKD cells by 6.3 ng/mL (Figure 6C). TNF-α was again not detected in the supernatant in all groups (data not shown).
Resveratrol inhibits cyst growth partly through NF-κB
To determine whether NF-κB mediates the anti-cystic effect of resveratrol, canine renal epithelial cells (MDCK cells) were grown in three-dimensional (3D) matrigel collagen I gels that were treated either with resveratrol or QNZ or their combination. We found that resveratrol or QNZ significantly inhibited cyst numbers, and combination treatment further inhibited cyst numbers (Supplementary data, Figure S1A). Moreover, resveratrol or QNZ inhibited cyst size significantly and combination treatment tended to further reduce cyst size (Supplementary data, Figure S1B).
By morpholino knockdown of Pkd2 expression in zebrafish, the injected larvae exhibited pronephric cysts and body curvature on Day 3 post-fertilization. Resveratrol significantly inhibited cyst formation at 150 µM, but not at 50 µM (Figure 7A). QNZ significantly inhibited renal cyst formation at 1 and 5 µM (Figure 7B). However, both wild-type and mutant larvae were not able to tolerate the combination treatment of resveratrol and QNZ. CFB, TNF-α and MCP-1 expression in Pkd2 knockdowns were down-regulated by resveratrol or QNZ (Figure 7C). The activity of NF-κB in zebrafish could not be detected by commercially available antibodies (data not shown).
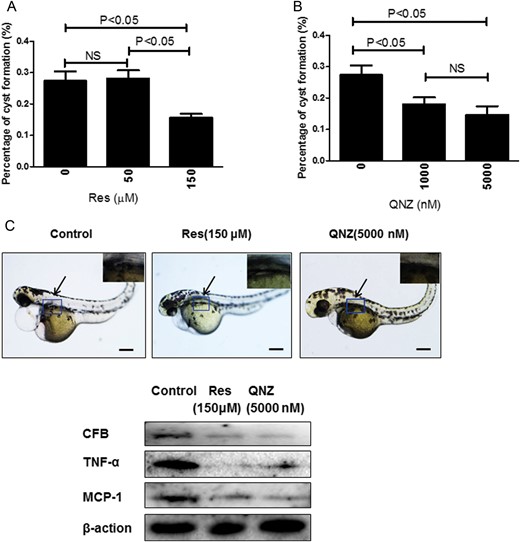
Suppression of Pkd2 morphants by resveratrol (Res) and QNZ. (A) Cyst formation was analyzed in Pkd2 morphants on Day 3 post-fertilization by treatment of resveratrol at 50 or 150 µM or by QNZ (B) at 1 or 5 µM (1000 or 5000 nM) from the shield stage. The average percentage of embryos with kidney cysts from three independent experiments is shown. Twenty-five embryos were examined in each experiment. (C) Embryos treated with 150 µM resveratrol or 5 µM (5000 nM) QNZ. The arrows point to the cyst. Insets show kidney cyst in control group, and lack of cyst in resveratrol- or QNZ-treated groups. Scale bar = 200 µm. The expression of CFB, TNF-α and MCP-1 in zebrafish were analyzed by western blot. Blots are representative of three independent experiments.
DISCUSSION
The inflammatory component of PKD has attracted significant attention in recent years, as a variety of pro-inflammatory factors and inflammatory cells have been found to play important roles in cytogenesis and PKD progression [4, 5, 7–9, 11, 20]. Therefore, we hypothesized that the inhibition of PKD inflammation might retard PKD progression. In the current study, we demonstrate that the well-known anti-inflammatory substance resveratrol delayed PKD progression by attenuating inflammation in cystic kidneys, as shown by reduced production of MCP-1, CFB and TNF-α, and decreased macrophage infiltration in cystic kidneys.
The transcription factor NF-κB is a master regulator of a myriad of inflammatory genes [21, 22]. NF-κB is implicated in the pathologies of acute and chronic renal diseases, but its activation and function in PKD is poorly understood [17, 21]. In our study we demonstrate that the classic NF-κB family members p50 and p-p65 were over-expressed in rat PKD kidney nuclei and tissues. The activation of p50/p65 in rat cystic kidney tissues was correlated with over-production of MCP-1, TNF-α and CFB, and associated with enhanced macrophage infiltration in cystic kidneys. Moreover, inhibition of p50/p65 in vivo by resveratrol was associated with delayed PKD progression and attenuation of PKD inflammation. Thus, our data suggest that NF-κB (p50/p65) may play an important role in PKD progression by promoting PKD inflammation. However, further work should be performed in PKD animal models by specific inhibition or knock-out of NF-κB to confirm its role in PKD progression.
A limitation of our study is that the Cy/+ Han:SPRD rat is not an orthologous model of ADPKD, thus NF-κB might not be a target in human ADPKD [23]. However, our study showed that NF-κB activation was correlated with over-production of MCP-1, TNF-α and CFB in human ADPKD cells and kidney tissues. Moreover, inhibition of p50/p65 by the NF-κB-specific inhibitor QNZ reduced the expression of MCP-1, TNF-α and CFB in ADPKD cells. It has been reported that an unspecific NF-κB protein is up-regulated in Pkd-2 transgenic kidneys and phosphorylation of p65 is increased in Pkd-1 knock-out cells [24, 25]. Thus, these data are in support of the idea that NF-κB could be targeted to ameliorate the aberrant inflammation in ADPKD.
It has been reported that resveratrol ameliorates inflammation through multiple downstream pathways including NF-κB [26]. In the current study, we show that resveratrol treatment reduced the expression of p50 and p-p65 in vivo and in vitro in parallel with a reduction of MCP-1, TNF-α and CFB expression and macrophage infiltration in cystic kidneys. Moreover, p50/p65 blockage by the NF-κB-specific inhibitor QNZ minimized the reduction of pro-inflammatory factor production by resveratrol in ADPKD cells. Our data suggest that resveratrol inhibits PKD inflammation at least partially through the NF-κB (p50/p65) signaling pathway. Nevertheless, NF-κB blockage did not completely abolish the inhibition of inflammatory factor production by resveratrol in vitro, suggesting that additional signaling pathways could be involved. Moreover, the inhibition of a single pathway may not be sufficient to improve PKD inflammation. Janus kinase (JAK)-signal transducer and activator of transcription (STAT) and mTOR pathways could also play a role in the anti-inflammatory effect of resveratrol in PKD, because both are activated in PKD and are involved in inflammation in multiple other diseases [4, 26].
To test whether resveratrol delays PKD progression specifically through the NF-κB pathway, we assessed the efficacy of resveratrol, QNZ or their combination on cyst growth in 3D cultures and in the zebrafish model. We observed that resveratrol or QNZ alone significantly inhibited cyst growth in the 3D cyst model to a similar level, and combination treatment further inhibited cyst growth. In the zebrafish model, QNZ and high dosage of resveratrol inhibited cyst formation and down-regulated inflammatory factor expression. These data suggest that the NF-κB pathway may play an important role in resveratrol-mediated inhibition of cyst growth.
Resveratrol is known to act at several levels, which include inhibition of cell proliferation, and suppression of inflammation and oxidative stress [27]. Here we show that resveratrol exerted its renal protective effects in PKD mainly through its anti-inflammatory effects. PKD is characterized by abnormal proliferation of renal tubular epithelial cells [3], and targeting the mTOR signaling pathway was shown to inhibit cell proliferation to retard PKD progression in animal models, although the effect of mTOR inhibition in human ADPKD was not evident [3, 15, 16, 28]. It has been shown that resveratrol can inhibit cell proliferation through mTOR inhibition [29]. Here we show that resveratrol down-regulated p-S6K, a marker of mTOR activity, which was correlated with the reduction of cell proliferation in resveratrol-treated cystic kidneys. However, our in vitro study showed that resveratrol down-regulated p-S6K, but this did not tightly correlate with the reduction of cell proliferation. Thus, proliferation inhibition by resveratrol is unlikely to occur through direct mTOR inhibition in cystic cells. It has been shown that macrophages promoted renal epithelial cell proliferation and cyst growth [7]. Therefore, there is a possibility that the reduction of cell proliferation in treated cystic kidneys is a secondary effect of the reduced infiltration of macrophages in cystic kidneys by resveratrol treatment.
Oxidative stress is evident in animal and human PKD, as shown by up-regulated markers of oxidative stress in urine, kidney and plasma samples of PKD [30–32]. In our study, we found that the decrease of SOD2 and the increase of 8-OHdG and nitrotyrosine in cystic kidneys were not altered by resveratrol treatment, suggesting that the protective effect of resveratrol in cystic kidneys was not occurring through its antioxidant property.
PKD is a ciliopathy, which is initiated from primary cilia dysfunction [33]. Recent studies have shown that activation of the NF-κB pathway impairs ciliogenesis in astrocytes and mesenchymal stromal cells [34, 35]. However, the underlying mechanisms for NF-κB-induced cilia dysregulation are not known, although it was suggested that ROCK or Lcn2 proteins are putative mediators of NF-κB-induced cilia dysregulation. Future studies should address whether NF-κB activation impairs ciliogenesis in renal epithelial cells, and explore the underlying mechanisms.
Taken together, our study shows for the first time beneficial effects of resveratrol on PKD progression by inhibiting inflammation in polycystic kidneys. Moreover, our study suggests that NF-κB is upstream of PKD-associated inflammation, and that targeting NF-κB could be a new strategy for PKD therapy.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
None declared.
ACKNOWLEDGEMENTS
This work was supported by NSFC General Projects (31371172), Shanghai International Science and Technology Cooperation Fund Project (0954070200) to C.M., and National Science & Technology Pillar Program (ID: 2013BAI09B04). We thank Prof. Ying Cao from Shanghai Tongji University for supervising zebrafish experiments.
REFERENCES
Author notes
These authors contributed equally to this work.





Comments