-
PDF
- Split View
-
Views
-
Cite
Cite
Ditte Hansen, Jonas B. Olesen, Gunnar H. Gislason, Bo Abrahamsen, Kristine Hommel, Risk of fracture in adults on renal replacement therapy: a Danish national cohort study, Nephrology Dialysis Transplantation, Volume 31, Issue 10, October 2016, Pages 1654–1662, https://doi.org/10.1093/ndt/gfw073
Close - Share Icon Share
Abstract
Patients on dialysis treatment or living with a transplanted kidney have several risk factors for bone fracture, especially disturbances in mineral metabolism and immunosuppressive therapy. We describe the incidence of fracture in this retrospective national Danish cohort study and explore the influence of age, gender, comorbidity and prescribed medication.
By individual-level linkage between nationwide administrative registries, the risk of fracture was compared between the group of patients receiving chronic dialysis treatment and patients receiving their first renal transplant in the study period, using the Danish background population as reference group. All three groups were followed up until first fracture, emigration, death or end of study. Cox proportional hazard models with fracture as outcome were fitted to the data.
The hazard ratio (HR) for any fracture was 3.14 [95% confidence interval (95% CI):2.97–3.31] in the dialysis group and 1.94 (95% CI: 1.72–2.18) in the renal transplanted group. The HR remained increased, but was modified by adjustment for age, gender, comorbidity and prior fracture [dialysis group: 1.85 (95% CI: 1.75–1.95); renal transplanted group: 1.82 (95% CI: 1.62–2.06)]. Prescribed diuretics, lipid-modifying agents and proton pump inhibitors also modulated the fracture risk.
Patients on dialysis or living with a transplanted kidney have a significantly higher risk of fracture than the Danish background population. Differences in age, gender, drug use and comorbidity only partly explain this increased risk. Further studies are warranted to explore the reason for this increased fracture risk in patients on renal replacement therapy.
INTRODUCTION
Bone fracture in patients on renal replacement therapy is associated with increased morbidity and mortality [1, 2]. Therefore, it is important to describe the incidence of fracture and identify the risk factors associated with fracture—some of which may be modifiable—in dialysis and renal transplanted patients.
Disturbances in mineral metabolism develop as kidney function diminishes, and almost every dialysis patient has disturbances in mineral metabolism [3, 4]. Kidney transplanted patients continue to have disturbances in mineral metabolism after transplantation, although the pattern of the disturbances changes markedly when renal function is partly or completely restored [5–8]. Changes in mineral metabolism influence bone structure and strength, and renal osteodystrophy is common [9, 10]. Renal osteodystrophy encompasses a spectrum of changes in bone quality, from adynamic bone disease with low bone turnover to osteitis fibrosa cystica with high bone turnover [11]. Both induce an increased risk of fracture. Immunosuppressive therapy for primary kidney disease and post kidney transplantation also influences bone quality [6]. In particular, the use of steroids may increase the risk of fracture [12]. Therefore, patients on chronic renal replacement therapy, receiving dialysis treatment or living with a renal transplant carry several risk factors associated with bone fracture. Furthermore, patients on renal replacement therapy have an enormous pill burden as high as 12 ± 5 tablets daily [13], which emphasizes the importance of exploring the influence of concomitant medication on fracture risk.
The fracture risk in patients on renal replacement therapy has been reported to be 4.4- to 14-fold higher compared with the general population [14–16]. However, most studies were limited to the USA, chiefly included the elderly, and used data generated before 2000, when clinical management strategies differed from today’s on important points. Moreover, most studies focussed on the incidence of hip fracture [17–19].
In this study, we considered the risk of all types of fractures in a population-based study of adult Danish patients on chronic renal replacement therapy from 2000 to 2011. We examined the location of the fractures and explored the influence of several clinical factors such as age, gender, comorbidity and detailed information on prescriptions filled for concomitant medications.
MATERIALS AND METHODS
This was a national retrospective cohort study describing risk of fracture for dialysis patients and patients living with a renal transplant, compared with the Danish background population.
Participants and setting
Three groups were compared: patients on dialysis treatment (DG), patients living with a renal transplant (RTX) and the Danish background population. All were adults ≥18 years of age at the time of inclusion.
The DG consisted of patients receiving dialysis treatment on 1 January 2000, or starting dialysis treatment from 1 January 2000 to 31 December 2011 in Denmark.
The RTX consisted of patients receiving a renal transplant from 1 January 2000 to 31 December 2011. Patients entering RTX stayed in this group for the rest of the follow-up. Patients included in RTX were excluded from the DG.
The Danish population ≥18 years of age at 1 January 2000 was used as the reference group. Patients entering the DG or RTX group during the study period were excluded from the reference group.
The index date was defined as 1 January 2000, or the date of dialysis initiation or renal transplantation during the period from 1 January 2000 to 31 December 2011.
Participants were followed up until first fracture, emigration, death or end of study at 31 December 2011.
Data sources
Information on the date of chronic renal replacement therapy start, modality and renal diagnosis was obtained from the Danish Nephrology Registry [20], where all patients actively treated for end-stage chronic kidney disease in Denmark are registered.
The Danish civil registration system holds information including date of birth and death on the Danish population [21].
Fractures during the period 1 January 2000 to 31 December 2011 were identified in the National Patient Registry [22, 23]. This contains information on all hospital admissions in Denmark, including diagnoses, operations, dates, hospitals and departments since 1978, and from 1995 also information on out-patient treatment. Fractures were identified by ICD-10: S02.0–S02.9, S12.0–S12.9, S22.0–S22.9, S32.0–S32.8, S42.0–S42.9, S52.0–S52.9, S62.0–S62.9, S72.0–S72.9, S82.0–S82.9 and S92.0–S92.9. Location of major fractures was described by ICD-10 as hip fracture (ICD10: S72.0 and S72.1), forearm fracture (ICD10: S52.5 and S52.6), upper arm fracture (ICD-10: S42.2, S42.3 and S42.4) or spine fracture (ICD10: S12.0, S12.1, S12.2, S12.7, S12.9, S22.0, S22.1, S32.0, S32.7 and S32.8). The number of fractures was counted by identifying the occurrence of any first fracture in the population, counting persons with a given fracture type as opposed to number of fracture events, as the latter can be inflated by multiple hospital contacts. Major fractures (termed major osteoporotic fractures in studies of osteoporosis) were defined as occurrence of first hip, first forearm, first spine or first upper arm fracture in each participant.
Fracture history up to 5 years prior to inclusion was identified by the above-described fracture definition. Comorbidity was defined by the Charlson Comorbidity Index, which has been validated in chronic kidney disease [24]. Information regarding hospitalizations within 5 years prior to inclusion was used.
Information on medical treatment was obtained from the Danish Register of Medicinal Product Statistics of the Danish Medicines Agency. The registry contains information on dispensed drugs from Danish pharmacies since 1995, excluding in-hospital drug use [25]. Medical treatment was defined as filled prescriptions 180 days before inclusion in the study.
Cross-linkage between registries was possible due to the unique personal identification number assigned to all Danish citizens from birth or immigration.
Ethical considerations
In Denmark, retrospective register studies do not require approval from the ethics committees. The Danish Data Protection Agency approved this study (ref. no.: 2007-58-0015/GEH-2014-018 I-Suite no.: 02736), and data were made available to us in an anonymized format such that specific individuals could not be identified.
Statistical analysis
Baseline characteristics of dialysis patients, renal transplanted patients and the Danish background population were compared using χ2 and Kruskal–Wallis tests where appropriate. Incidence rates were standardized according to gender and age using five age groups with the direct method and the Danish background population as reference. The age strata 18–39, 40–49, 50–59, 60–69 and +70 years of age were used. Due to the large age difference between groups, matching for age was not possible.
Test for interaction was performed, and if present, appropriate stratification was done.
There was a significant interaction between treatment group and age, gender, peripheral artery disease, chronic obstructive pulmonary disease, autoimmune disease, diabetes with complications, aspirin, proton pump inhibitors, digoxin, lipid-modifying agents and bisphosphonates. Only age appeared to influence the fracture risk, and accordingly, stratified analyses were made.
Cox proportional hazards models with fracture as outcome were fitted to the data. Stepwise adjustment was made for gender, age, comorbidity, time on dialysis, prior fracture and drug use. The proportional hazards assumption was checked graphically, confirming that the assumption was met.
Sensitivity analysis for time on dialysis prior to entering the study was performed.
Analyses were performed using SAS version 9.2 (SAS Institute Inc.).
RESULTS
In the dialysis group (DG), 7566 patients were included, in the transplanted group (RTX), 1504 patients were included and the Danish background population consisted of 4 091 776 individuals. The dialysis patients were older and had more comorbidity than the transplanted patients or the Danish background population. Both the dialysis and the transplanted patients were mostly men, and both groups had higher prevalence of prior fracture during the past 5 years, compared with the Danish background population. Participant characteristics are shown in Table 1. The total number of first fractures was 1273 (16.8%) in the DG, 265 (17.6%) in the RTX and 757 196 (18.5%) in the Danish background population. The median duration of follow-up was 750 [interquartile range (IQR): 317–1433] days in the DG, 1589 (IQR: 786–2702) days in the RTX and 4382 (IQR: 3339–4382) days in the Danish background population.
| . | Dialysis patients (n = 7566)a . | Transplanted patients (n = 1504)a . | Danish background population 2000 (n = 4 091 776) . | P-valueb . |
|---|---|---|---|---|
| Age, years (IQR) (range) | 69 (60–76)* (19–95) | 49 (38–58) (18–76) | 46 (33–60) (18–110) | <0.0001* |
| Women | 2791 (36.9)* | 580 (38.6)* | 2 095 958 (51.2) | <0.0001 |
| Time on dialysis | 0 (0–0)* | 1.7 (0.6–3.4)* | 0 | <0.0001* |
| Prior fracture 5 years before | 967 (12.8)* | 162 (10.8)** | 365 794 (8.9) | <0.0001*** |
| Charlson Comorbidity Index | 2 (0–3)* | 0 (0–2)* | 0 (0–0) | <0.0001* |
| Comorbidity for each parameter in Charlson score | ||||
| Myocardial infarction | 776 (10.3)* | 46 (3.1)* | 36 366 (0.9) | <0.0001* |
| Congestive heart failure | 1488 (19.7)* | 85 (5.7)* | 39 977 (1.0) | <0.0001* |
| Peripheral vascular disease | 1324 (17.5)* | 85 (5.7)* | 36 216 (0.9) | <0.0001* |
| Cerebrovascular disease | 377 (5.0)* | 29 (1.9)* | 23 391 (0.6) | <0.0001* |
| Dementia | 5 (0.1) | 0 (0.54) | 1038 (<0.1) | 0.07 |
| Chronic pulmonary disease | 713 (9.4)* | 26 (1.7) | 48 635 (1.2) | <0.0001* |
| Rheumatic disease | 240 (3.2)* | 24 (1.6)* | 23 798 (0.6) | <0.0001** |
| Peptic ulcer disease | 865 (11.4)* | 126 (8.4)* | 72 674 (1.8) | <0.0001** |
| Mild liver disease | 185 (2.5)* | 24 (1.6)* | 12 976 (0.3) | <0.0001 |
| Diabetes without chronic complications | 199 (2.6)* | 34 (2.3)* | 34 014 (0.8) | <0.0001 |
| Diabetes with chronic complications | 2274 (30.1)* | 288 (19.2)* | 29 887 (0.7) | <0.0001* |
| Hemi- or paraplegia | 33 (0.4)* | 4 (0.3)** | 3179 (0.1) | <0.0001 |
| Moderate to severe chronic kidney disease | 7566 (100)* | 1504 (100)* | 3096 (0.1) | <0.0001 |
| Solid malignant tumours | 872 (11.5)* | 47 (3.1)** | 91 596 (2.2) | <0.0001* |
| Leukaemia | 30 (0.4)* | 1 (0.1) | 3029 (0.1) | <0.0001 |
| Lymphoma | 245 (3.2)* | 6 (0.4)** | 5903 (0.1) | <0.0001* |
| Moderate or severe liver disease | 13 (0.2)* | 4 (0.3)* | 486 (<0.1) | <0.0001 |
| Metastatic solid tumour | 35 (0.5)* | 3 (0.2) | 2791 (0.1) | <0.0001 |
| AIDS/HIV | 14 (0.2)* | 3 (0.2)** | 1873 (0.1) | <0.0001 |
| Medication | ||||
| Vitamin K antagonists | 646 (8.5)* | 67 (4.5)* | 30 568 (0.8) | <0.0001* |
| Aspirin | 2737 (36.2)* | 227 (15.1)* | 192 531 (4.7) | <0.0001* |
| Clopidogrel | 268 (3.5)* | 14 (0.9)* | 1278 (<0.1) | <0.0001* |
| Proton pump inhibitors | 2446 (32.3)* | 503 (33.4)* | 114 787 (2.8) | <0.0001 |
| Diuretics | 5483 (72.5)* | 904 (60.1)* | 348 831 (8.5) | <0.0001* |
| β blockers | 3390 (44.8)* | 766 (50.9)* | 167 958 (4.1) | <0.0001* |
| Calcium antagonists | 3977 (52.6)* | 790 (52.5)* | 167 244 (4.1) | <0.0001 |
| Inhibitors of the renin–angiotensin system | 3457 (46.0)* | 964 (64.1)* | 183 876 (4.5) | <0.0001* |
| Digoxin | 561 (7.4)* | 21 (1.4) | 61 086 (1.5) | <0.0001* |
| Antiarrhythmic class I and III | 105 (1.4)* | 4 (0.3) | 6637 (0.2) | <0.0001* |
| Bisphosphonates | 74 (1.0)* | 4 (0.2) | 14 476 (0.4) | <0.0001*** |
| Lipid-modifying agents | 2146 (28.4)* | 3523 (23.4)* | 64 583 (1.6) | <0.0001* |
| . | Dialysis patients (n = 7566)a . | Transplanted patients (n = 1504)a . | Danish background population 2000 (n = 4 091 776) . | P-valueb . |
|---|---|---|---|---|
| Age, years (IQR) (range) | 69 (60–76)* (19–95) | 49 (38–58) (18–76) | 46 (33–60) (18–110) | <0.0001* |
| Women | 2791 (36.9)* | 580 (38.6)* | 2 095 958 (51.2) | <0.0001 |
| Time on dialysis | 0 (0–0)* | 1.7 (0.6–3.4)* | 0 | <0.0001* |
| Prior fracture 5 years before | 967 (12.8)* | 162 (10.8)** | 365 794 (8.9) | <0.0001*** |
| Charlson Comorbidity Index | 2 (0–3)* | 0 (0–2)* | 0 (0–0) | <0.0001* |
| Comorbidity for each parameter in Charlson score | ||||
| Myocardial infarction | 776 (10.3)* | 46 (3.1)* | 36 366 (0.9) | <0.0001* |
| Congestive heart failure | 1488 (19.7)* | 85 (5.7)* | 39 977 (1.0) | <0.0001* |
| Peripheral vascular disease | 1324 (17.5)* | 85 (5.7)* | 36 216 (0.9) | <0.0001* |
| Cerebrovascular disease | 377 (5.0)* | 29 (1.9)* | 23 391 (0.6) | <0.0001* |
| Dementia | 5 (0.1) | 0 (0.54) | 1038 (<0.1) | 0.07 |
| Chronic pulmonary disease | 713 (9.4)* | 26 (1.7) | 48 635 (1.2) | <0.0001* |
| Rheumatic disease | 240 (3.2)* | 24 (1.6)* | 23 798 (0.6) | <0.0001** |
| Peptic ulcer disease | 865 (11.4)* | 126 (8.4)* | 72 674 (1.8) | <0.0001** |
| Mild liver disease | 185 (2.5)* | 24 (1.6)* | 12 976 (0.3) | <0.0001 |
| Diabetes without chronic complications | 199 (2.6)* | 34 (2.3)* | 34 014 (0.8) | <0.0001 |
| Diabetes with chronic complications | 2274 (30.1)* | 288 (19.2)* | 29 887 (0.7) | <0.0001* |
| Hemi- or paraplegia | 33 (0.4)* | 4 (0.3)** | 3179 (0.1) | <0.0001 |
| Moderate to severe chronic kidney disease | 7566 (100)* | 1504 (100)* | 3096 (0.1) | <0.0001 |
| Solid malignant tumours | 872 (11.5)* | 47 (3.1)** | 91 596 (2.2) | <0.0001* |
| Leukaemia | 30 (0.4)* | 1 (0.1) | 3029 (0.1) | <0.0001 |
| Lymphoma | 245 (3.2)* | 6 (0.4)** | 5903 (0.1) | <0.0001* |
| Moderate or severe liver disease | 13 (0.2)* | 4 (0.3)* | 486 (<0.1) | <0.0001 |
| Metastatic solid tumour | 35 (0.5)* | 3 (0.2) | 2791 (0.1) | <0.0001 |
| AIDS/HIV | 14 (0.2)* | 3 (0.2)** | 1873 (0.1) | <0.0001 |
| Medication | ||||
| Vitamin K antagonists | 646 (8.5)* | 67 (4.5)* | 30 568 (0.8) | <0.0001* |
| Aspirin | 2737 (36.2)* | 227 (15.1)* | 192 531 (4.7) | <0.0001* |
| Clopidogrel | 268 (3.5)* | 14 (0.9)* | 1278 (<0.1) | <0.0001* |
| Proton pump inhibitors | 2446 (32.3)* | 503 (33.4)* | 114 787 (2.8) | <0.0001 |
| Diuretics | 5483 (72.5)* | 904 (60.1)* | 348 831 (8.5) | <0.0001* |
| β blockers | 3390 (44.8)* | 766 (50.9)* | 167 958 (4.1) | <0.0001* |
| Calcium antagonists | 3977 (52.6)* | 790 (52.5)* | 167 244 (4.1) | <0.0001 |
| Inhibitors of the renin–angiotensin system | 3457 (46.0)* | 964 (64.1)* | 183 876 (4.5) | <0.0001* |
| Digoxin | 561 (7.4)* | 21 (1.4) | 61 086 (1.5) | <0.0001* |
| Antiarrhythmic class I and III | 105 (1.4)* | 4 (0.3) | 6637 (0.2) | <0.0001* |
| Bisphosphonates | 74 (1.0)* | 4 (0.2) | 14 476 (0.4) | <0.0001*** |
| Lipid-modifying agents | 2146 (28.4)* | 3523 (23.4)* | 64 583 (1.6) | <0.0001* |
Baseline characteristics for dialysis patients, patients with renal transplants and the Danish background population year 2000. Data are presented as numbers (%) or medians (IQR). P-value for overall test for differences between groups.
aPost hoc analysis of baseline characteristics between groups: *P < 0.0001 or **P < 0.05 in treated group versus Danish background population.
bP-values: *P < 0.0001, **P < 0.001 or ***P < 0.05 in dialysis versus renal transplanted patients.
| . | Dialysis patients (n = 7566)a . | Transplanted patients (n = 1504)a . | Danish background population 2000 (n = 4 091 776) . | P-valueb . |
|---|---|---|---|---|
| Age, years (IQR) (range) | 69 (60–76)* (19–95) | 49 (38–58) (18–76) | 46 (33–60) (18–110) | <0.0001* |
| Women | 2791 (36.9)* | 580 (38.6)* | 2 095 958 (51.2) | <0.0001 |
| Time on dialysis | 0 (0–0)* | 1.7 (0.6–3.4)* | 0 | <0.0001* |
| Prior fracture 5 years before | 967 (12.8)* | 162 (10.8)** | 365 794 (8.9) | <0.0001*** |
| Charlson Comorbidity Index | 2 (0–3)* | 0 (0–2)* | 0 (0–0) | <0.0001* |
| Comorbidity for each parameter in Charlson score | ||||
| Myocardial infarction | 776 (10.3)* | 46 (3.1)* | 36 366 (0.9) | <0.0001* |
| Congestive heart failure | 1488 (19.7)* | 85 (5.7)* | 39 977 (1.0) | <0.0001* |
| Peripheral vascular disease | 1324 (17.5)* | 85 (5.7)* | 36 216 (0.9) | <0.0001* |
| Cerebrovascular disease | 377 (5.0)* | 29 (1.9)* | 23 391 (0.6) | <0.0001* |
| Dementia | 5 (0.1) | 0 (0.54) | 1038 (<0.1) | 0.07 |
| Chronic pulmonary disease | 713 (9.4)* | 26 (1.7) | 48 635 (1.2) | <0.0001* |
| Rheumatic disease | 240 (3.2)* | 24 (1.6)* | 23 798 (0.6) | <0.0001** |
| Peptic ulcer disease | 865 (11.4)* | 126 (8.4)* | 72 674 (1.8) | <0.0001** |
| Mild liver disease | 185 (2.5)* | 24 (1.6)* | 12 976 (0.3) | <0.0001 |
| Diabetes without chronic complications | 199 (2.6)* | 34 (2.3)* | 34 014 (0.8) | <0.0001 |
| Diabetes with chronic complications | 2274 (30.1)* | 288 (19.2)* | 29 887 (0.7) | <0.0001* |
| Hemi- or paraplegia | 33 (0.4)* | 4 (0.3)** | 3179 (0.1) | <0.0001 |
| Moderate to severe chronic kidney disease | 7566 (100)* | 1504 (100)* | 3096 (0.1) | <0.0001 |
| Solid malignant tumours | 872 (11.5)* | 47 (3.1)** | 91 596 (2.2) | <0.0001* |
| Leukaemia | 30 (0.4)* | 1 (0.1) | 3029 (0.1) | <0.0001 |
| Lymphoma | 245 (3.2)* | 6 (0.4)** | 5903 (0.1) | <0.0001* |
| Moderate or severe liver disease | 13 (0.2)* | 4 (0.3)* | 486 (<0.1) | <0.0001 |
| Metastatic solid tumour | 35 (0.5)* | 3 (0.2) | 2791 (0.1) | <0.0001 |
| AIDS/HIV | 14 (0.2)* | 3 (0.2)** | 1873 (0.1) | <0.0001 |
| Medication | ||||
| Vitamin K antagonists | 646 (8.5)* | 67 (4.5)* | 30 568 (0.8) | <0.0001* |
| Aspirin | 2737 (36.2)* | 227 (15.1)* | 192 531 (4.7) | <0.0001* |
| Clopidogrel | 268 (3.5)* | 14 (0.9)* | 1278 (<0.1) | <0.0001* |
| Proton pump inhibitors | 2446 (32.3)* | 503 (33.4)* | 114 787 (2.8) | <0.0001 |
| Diuretics | 5483 (72.5)* | 904 (60.1)* | 348 831 (8.5) | <0.0001* |
| β blockers | 3390 (44.8)* | 766 (50.9)* | 167 958 (4.1) | <0.0001* |
| Calcium antagonists | 3977 (52.6)* | 790 (52.5)* | 167 244 (4.1) | <0.0001 |
| Inhibitors of the renin–angiotensin system | 3457 (46.0)* | 964 (64.1)* | 183 876 (4.5) | <0.0001* |
| Digoxin | 561 (7.4)* | 21 (1.4) | 61 086 (1.5) | <0.0001* |
| Antiarrhythmic class I and III | 105 (1.4)* | 4 (0.3) | 6637 (0.2) | <0.0001* |
| Bisphosphonates | 74 (1.0)* | 4 (0.2) | 14 476 (0.4) | <0.0001*** |
| Lipid-modifying agents | 2146 (28.4)* | 3523 (23.4)* | 64 583 (1.6) | <0.0001* |
| . | Dialysis patients (n = 7566)a . | Transplanted patients (n = 1504)a . | Danish background population 2000 (n = 4 091 776) . | P-valueb . |
|---|---|---|---|---|
| Age, years (IQR) (range) | 69 (60–76)* (19–95) | 49 (38–58) (18–76) | 46 (33–60) (18–110) | <0.0001* |
| Women | 2791 (36.9)* | 580 (38.6)* | 2 095 958 (51.2) | <0.0001 |
| Time on dialysis | 0 (0–0)* | 1.7 (0.6–3.4)* | 0 | <0.0001* |
| Prior fracture 5 years before | 967 (12.8)* | 162 (10.8)** | 365 794 (8.9) | <0.0001*** |
| Charlson Comorbidity Index | 2 (0–3)* | 0 (0–2)* | 0 (0–0) | <0.0001* |
| Comorbidity for each parameter in Charlson score | ||||
| Myocardial infarction | 776 (10.3)* | 46 (3.1)* | 36 366 (0.9) | <0.0001* |
| Congestive heart failure | 1488 (19.7)* | 85 (5.7)* | 39 977 (1.0) | <0.0001* |
| Peripheral vascular disease | 1324 (17.5)* | 85 (5.7)* | 36 216 (0.9) | <0.0001* |
| Cerebrovascular disease | 377 (5.0)* | 29 (1.9)* | 23 391 (0.6) | <0.0001* |
| Dementia | 5 (0.1) | 0 (0.54) | 1038 (<0.1) | 0.07 |
| Chronic pulmonary disease | 713 (9.4)* | 26 (1.7) | 48 635 (1.2) | <0.0001* |
| Rheumatic disease | 240 (3.2)* | 24 (1.6)* | 23 798 (0.6) | <0.0001** |
| Peptic ulcer disease | 865 (11.4)* | 126 (8.4)* | 72 674 (1.8) | <0.0001** |
| Mild liver disease | 185 (2.5)* | 24 (1.6)* | 12 976 (0.3) | <0.0001 |
| Diabetes without chronic complications | 199 (2.6)* | 34 (2.3)* | 34 014 (0.8) | <0.0001 |
| Diabetes with chronic complications | 2274 (30.1)* | 288 (19.2)* | 29 887 (0.7) | <0.0001* |
| Hemi- or paraplegia | 33 (0.4)* | 4 (0.3)** | 3179 (0.1) | <0.0001 |
| Moderate to severe chronic kidney disease | 7566 (100)* | 1504 (100)* | 3096 (0.1) | <0.0001 |
| Solid malignant tumours | 872 (11.5)* | 47 (3.1)** | 91 596 (2.2) | <0.0001* |
| Leukaemia | 30 (0.4)* | 1 (0.1) | 3029 (0.1) | <0.0001 |
| Lymphoma | 245 (3.2)* | 6 (0.4)** | 5903 (0.1) | <0.0001* |
| Moderate or severe liver disease | 13 (0.2)* | 4 (0.3)* | 486 (<0.1) | <0.0001 |
| Metastatic solid tumour | 35 (0.5)* | 3 (0.2) | 2791 (0.1) | <0.0001 |
| AIDS/HIV | 14 (0.2)* | 3 (0.2)** | 1873 (0.1) | <0.0001 |
| Medication | ||||
| Vitamin K antagonists | 646 (8.5)* | 67 (4.5)* | 30 568 (0.8) | <0.0001* |
| Aspirin | 2737 (36.2)* | 227 (15.1)* | 192 531 (4.7) | <0.0001* |
| Clopidogrel | 268 (3.5)* | 14 (0.9)* | 1278 (<0.1) | <0.0001* |
| Proton pump inhibitors | 2446 (32.3)* | 503 (33.4)* | 114 787 (2.8) | <0.0001 |
| Diuretics | 5483 (72.5)* | 904 (60.1)* | 348 831 (8.5) | <0.0001* |
| β blockers | 3390 (44.8)* | 766 (50.9)* | 167 958 (4.1) | <0.0001* |
| Calcium antagonists | 3977 (52.6)* | 790 (52.5)* | 167 244 (4.1) | <0.0001 |
| Inhibitors of the renin–angiotensin system | 3457 (46.0)* | 964 (64.1)* | 183 876 (4.5) | <0.0001* |
| Digoxin | 561 (7.4)* | 21 (1.4) | 61 086 (1.5) | <0.0001* |
| Antiarrhythmic class I and III | 105 (1.4)* | 4 (0.3) | 6637 (0.2) | <0.0001* |
| Bisphosphonates | 74 (1.0)* | 4 (0.2) | 14 476 (0.4) | <0.0001*** |
| Lipid-modifying agents | 2146 (28.4)* | 3523 (23.4)* | 64 583 (1.6) | <0.0001* |
Baseline characteristics for dialysis patients, patients with renal transplants and the Danish background population year 2000. Data are presented as numbers (%) or medians (IQR). P-value for overall test for differences between groups.
aPost hoc analysis of baseline characteristics between groups: *P < 0.0001 or **P < 0.05 in treated group versus Danish background population.
bP-values: *P < 0.0001, **P < 0.001 or ***P < 0.05 in dialysis versus renal transplanted patients.
The crude incidence rate of fracture per 10 000 person years (PY) was higher in the DG at 604 [95% confidence interval (95% CI): 571–637] and in the RTX at 357 (95% CI: 314–400), compared with the Danish background population, at 186 (95% CI: 186–186). These differences were reduced, but remained clinically significant, after age standardization to adjust for the difference in age between the groups (Figure 1). The age-standardized incidence rates of fracture per 10 000 PY among women were: DG 568 (95% CI: 477–658); RTX 603 (95% CI: 345–860); Danish background population 204 (95% CI: 203–204), whereas the age-standardized incidence rates of fracture per 10 000 PY in men were: DG 223 (95% CI: 169–277); RTX 76 (95% CI: 54–99); Danish background population: 46 (95% CI: 46–47). The cumulative incidence curves for fracture with death as competing risk is shown in Figure 2. As reflected in the cumulative incidence curves, the mortality was high in the DG at 2555 (95% CI: 2191–2319) compared with the RTX at 232 (95% CI: 197–266) and the Danish background population at 121 (95% CI: 121–122) per 10 000 PY.
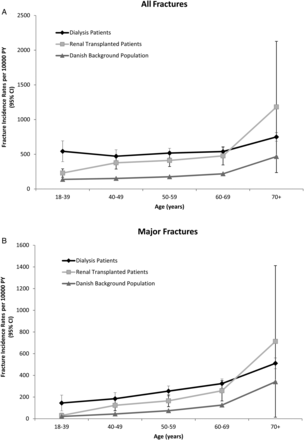
Fracture incidence rates per 10 000 PY. (A) Incidence rate per 10 000 PY of any first fracture. (B) Incidence rate per 10 000 PY of any first major fracture (spine, hip, forearm and upper arm fracture).
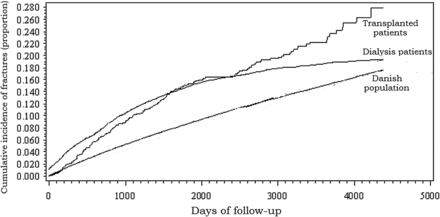
Cumulative incidence curves for fractures with death as competing end point.
There was a difference in crude incidence rates of fracture per 10 000 PY between dialysis patients on haemodialysis [639 (95% CI: 598–679)] and peritoneal dialysis [521 (95% CI: 464–578)]. However, this difference disappeared after age standardization [haemodialysis: 545 (95% CI: 461–628); peritoneal dialysis: 532 (95% CI: 417–646)].
The localization of any first fracture and first major fracture is described in Table 2.
| Fracture localization . | % of total number of fractures (% of first major fractures) . | ||
|---|---|---|---|
| Dialysis population . | Renal transplanted population . | Danish background population . | |
| Hip | 34 (49) | 10 (24) | 15 (30) |
| Forearm | 13 (19) | 16 (39) | 19 (38) |
| Proximal humerus | 13 (18) | 11 (27) | 10 (21) |
| Spine | 10 (14) | 4 (10) | 5 (11) |
| Other fractures | 30 | 59 | 51 |
| Fracture localization . | % of total number of fractures (% of first major fractures) . | ||
|---|---|---|---|
| Dialysis population . | Renal transplanted population . | Danish background population . | |
| Hip | 34 (49) | 10 (24) | 15 (30) |
| Forearm | 13 (19) | 16 (39) | 19 (38) |
| Proximal humerus | 13 (18) | 11 (27) | 10 (21) |
| Spine | 10 (14) | 4 (10) | 5 (11) |
| Other fractures | 30 | 59 | 51 |
| Fracture localization . | % of total number of fractures (% of first major fractures) . | ||
|---|---|---|---|
| Dialysis population . | Renal transplanted population . | Danish background population . | |
| Hip | 34 (49) | 10 (24) | 15 (30) |
| Forearm | 13 (19) | 16 (39) | 19 (38) |
| Proximal humerus | 13 (18) | 11 (27) | 10 (21) |
| Spine | 10 (14) | 4 (10) | 5 (11) |
| Other fractures | 30 | 59 | 51 |
| Fracture localization . | % of total number of fractures (% of first major fractures) . | ||
|---|---|---|---|
| Dialysis population . | Renal transplanted population . | Danish background population . | |
| Hip | 34 (49) | 10 (24) | 15 (30) |
| Forearm | 13 (19) | 16 (39) | 19 (38) |
| Proximal humerus | 13 (18) | 11 (27) | 10 (21) |
| Spine | 10 (14) | 4 (10) | 5 (11) |
| Other fractures | 30 | 59 | 51 |
The crude incidence rate of hip fracture as first fracture per 10 000 PY was 189 (95% CI: 171–207) in the DG, 32 (95% CI: 20–44) in the RTX and 25 (95% CI: 25–26) in the Danish background population. The age-standardized incidence rate of hip fracture per 10 000 PY was: DG 92 (95% CI: 76–109); RTX 24 (95% CI: 15–34); Danish background population 25 (95% CI: 25–26).
The risk of fracture increased with age. However, in the young dialysis patients, an increased fracture incidence was also observed (Figure 1A). The increased risk in young dialysis patients was not found when only considering major fractures (Figure 1B). The increased risk was caused by other types of fractures than major fractures. Lower leg fractures represented 36% of the non-major fractures in the DG, as opposed to 29% in the RTX and 15% in the Danish background population.
The incidence of fracture did not change in any of the groups over time, when the observed time was separated into three time periods (Figure 3).
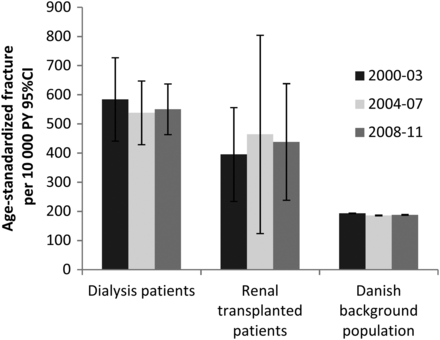
Fracture incidence during three time periods. The risk of fracture did not change during the three time periods 2000–03, 2004–07 and 2008–11 in the dialysis patients, the renal transplanted patients or the Danish background population.
The hazard ratio (HR) for any fracture with the Danish background population as reference group was 3.14 (95% CI: 2.97–3.31) in DG and 1.94 (95% CI: 1.72–2.18) in RTX (Figure 4). The HR adjusted for age, sex, Charlson Comorbidity Index and prior fracture were 1.85 (95% CI: 1.75–1.95) in DG and 1.82 (95% CI: 1.62–2.06) in RTX. Further adjustment for any single medication did not change the HR significantly. However, a tendency towards a lower estimate after adjustment for the use of proton pump inhibitors and diuretics was observed, as was a tendency towards an increased fracture risk after adjustment for lipid-modifying agents (Figure 4).
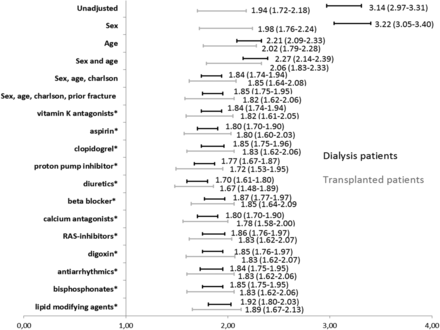
HR (95% CI) for any first fracture as a function of treatment group (dialysis or transplanted patients) with the Danish background population as reference. The first row shows the unadjusted HR associated with membership of each of the two treatment groups. The next rows show adjusted models where the effect of group membership is stepwise adjusted for sex, age, Charlson Comorbidity Index score, fracture during prior 5 years, and *adjusted for sex, age, Charlson Comorbidity Index score, fracture during prior 5 years and each group of medication, one at a time.
The risk modification for fracture due to proton pump inhibitors was further explored by Cox analysis, comparing patients treated versus untreated with proton pump inhibitors in the DG, RTX and Danish background population, respectively. The analysis was adjusted for age, gender, Charlson Comorbidity Index score, time in dialysis and prior fracture. The risk of fracture in the proton pump inhibitor-treated patients was increased in both DG [HR 1.31 (95% CI: 1.16–1.47)] and the Danish background population [HR 1.22 (95% CI: 1.20–1.29)], although no significant difference was observed in the RTX [HR 1.02 (95% CI: 0.78–1.33)].
In the same way, the risk of fracture for patients treated versus untreated with loop diuretics or lipid-modifying drugs was explored by adjusted Cox analysis. No significant difference was observed in the DG [HR 1.04 (95% CI: 0.92–1.17)] or the RTX [HR 1.01 (95% CI: 0.78–1.32)], whereas the risk was increased in the Danish background population [HR 1.37 (95% CI: 1.36–1.39)] by loop diuretics. Lipid-modifying drugs did not affect the HR in either the DG [HR 0.97 (95% CI: 0.86–1.11)] or the RTX [HR 1.16 (95% CI: 0.87–1.56)], or the Danish background population [HR 0.83 (95% CI: 0.82–0.85)].
Sensitivity analysis for time on dialysis prior to entering the study did not make any major changes to the risk of fracture in any of the groups (Supplementary data, Figure S1).
DISCUSSION
In a nationwide Danish cohort of patients on renal replacement therapy, we demonstrated increased risk of fracture in adult Danish patients on renal replacement therapy, compared with the general adult Danish population. Dialysis patients had a 3-fold higher risk of fracture and renal transplanted patients had a 2-fold higher risk of fracture in unadjusted analysis. Although the risk remained elevated during follow-up, the cumulative incidence curve demonstrated a reduced risk over time in the dialysis population. The high risk of mortality in the dialysis group is well known, and is confirmed in the present study.
As expected, the increased risk was reduced after adjustment for age, gender, comorbidity, prior fracture and time on dialysis. However, it was still significantly elevated compared with the general adult Danish population. There was no difference in fracture risk between patients on haemodialysis and peritoneal dialysis after adjustment for age differences between the groups.
The risk increased with increasing age, except for the young (18–39 years) dialysis patients who demonstrate a risk of fracture comparable to the middle-aged population (50–59 years). In observational studies only including major fractures such as hip and vertebral fractures, the incidence steadily increased with age [14, 26]. The high risk in the younger patients in the present study was driven by a higher frequency of other types of fractures. The non-major fractures were mostly lower leg fractures in the dialysis patients. This is in accordance with the lower leg being mostly cortical bone, which is highly influenced by parathyroid hormone. A higher risk of non-major fractures was also found in the young general adult Danish population, but not in the renal transplanted patients. This is surprising as the transplanted group would be expected to have a lifestyle at least as active as the other groups have.
Older studies before 2000 have described a 4.4–14-fold elevated risk of fracture, compared with the general population [14–16]. We did not find the same high risk in the present study, and the risk may have improved in this millennium. However, we did not find any change in the incidence of fractures from 2000 to 2011.
Recent data from the Dialysis Outcomes and Practice Patterns Study (DOPPS) [2] described the incidence of hip fracture among dialysis patients from 12 countries around the world. The findings in the present study are similar to the reported data from our neighbouring country Sweden (incidence of hip fracture reported as 200 per 10 000 PY). This incidence is high compared with the incidences reported by the other countries participating in the DOPPS (i.e. Japan 30 per 10 000 PY). Whether these differences are due to differences in age, race, comorbidity or treatment regimens remains to be explored. Arneson et al. [27] described a decreased incidence of hip fracture in the US Medicare haemodialysis patients from 2004 to 2010, and speculate that the decreased fracture risk could be due to cinacalcet and lanthanum. In Denmark, cinacalcet and lanthanum were introduced during the observed period in 2004 and 2005, and were increasingly used during the observational period. Our data do not support any dramatic changes in fracture incidence due to new treatment modalities during the observed period.
Additionally, in the renal transplanted patients, we did not find changes in the risk of fracture from 2000 to 2011. Sukumaran et al. [28] demonstrated a decrease in risk of fracture in renal transplanted patients from 1997 to 2010, which decreased when adjusting for baseline immunosuppressive therapy. It could be speculated that a decrease in fracture risk due to increased use of bone-sparing immunosuppressive therapy is outweighed by expanded criteria for kidney recipients. Unfortunately, our data are too limited to explore this issue.
In Denmark, vitamin D analogues, phosphorus binders and calcimimetics are provided to the patient by the treating department. Therefore, the Register of Medicinal Product Statistics does not contain information to help us explore the influence of medications for treatment of disturbances in mineral metabolism on the risk of fracture.
We explored the influence of cardiovascular medication, antithrombotic medication, bisphosphonates and lipid-modifying agents on risk of fracture. We did not find any significant changes in risk when adjusting for any single medication. This may be due to the sample size in the dialysis and renal transplanted groups being too small to uncover any significant differences. Conversely, the trend towards a lower risk after adjusting for use of diuretics and proton pump inhibitors, and a higher risk when adjusting for lipid-modifying agents, may be due to residual cofounding. Therefore, these trends were further explored.
Loop diuretics increase renal calcium excretion and have been found to be associated with reduced bone mineral density and an increased risk of fracture [29, 30]. Thiazides and spironolactone are sparsely used in patients on renal replacement therapy, and have not been further explored. Loop diuretics were associated with a 27% increased risk of fracture in the general population. However, although the risk of fracture increased in the dialysis and renal transplanted patients treated with diuretics, this was not significant, which may be due to the small sample size.
Proton pump inhibitors have been associated with an increased risk of fracture in former observational studies [31]. The increased risk may be due to increased risk of falling, as bone quality appears unaffected by treatment with proton pump inhibitors in women [32]. The present data support an increased risk of fracture both in the general population and in patients on renal replacement therapy treated with proton pump inhibitors. Proton pump inhibitors are often used as a standard precaution in prednisolone-treated patients to prevent gastrointestinal bleeding. Proton pump inhibitor treatment may be a marker of prednisolone treatment, with a consequently increased risk of fracture. Unfortunately, we are unable to explore this in the present study. Furthermore, proton pump inhibitors decrease the level of s-magnesium in both the healthy population and patients with reduced renal function [33, 34]. Magnesium deficiency may in turn increase the risk of bone fracture, due to decreased bone strength or increased risk of fall [35, 36]. Statins have been associated with lower fracture risk in former epidemiologic studies [37]. However, secondary analysis of interventional trials did not support any bone protective effect [38]. Whereas treatment with lipid-modifying drugs was associated with a reduced risk of fracture in the general adult Danish population, we could not confirm any significant influence on fracture risk in dialysis-treated or renal transplanted patients.
This is a registry-based cohort study. The prevalence of vertebral fracture not diagnosed by X-ray, and therefore not entered into the registries, may cause severe underestimation of the incidence of vertebral fractures in all groups. Indeed, a prevalence of vertebral fractures of above 50% has been reported in dialysis patients [39]. As immunosuppressive therapies are provided to patients directly by the treating hospital departments, the influence of these could not be explored from prescription data.
The mortality in the total Danish dialysis population has previously been reported to be 20% [20]. We found a mortality risk of 25% in the dialysis group in the current study. This is probably accounted for by dialysis patients who receive a renal transplant during the observational period being reported as a part of the renal transplanted group. These transplanted patients have better survival prospects than the patients remaining on dialysis.
The increased fracture risk in the dialysis and renal transplanted patients in the present study may be associated with an increased risk of cardiovascular disease and mortality. The association between disturbances in mineral metabolism, bone abnormalities, extraskeletal calcification and in turn an increased risk of fracture, cardiovascular disease and mortality is well established as chronic kidney disease mineral and bone disorder [40]. Recently, Tentori et al. [2] found that dialysis patients having a fracture have an increased risk of death. Therefore, it is of utmost importance to describe groups at risk of fracture and explore possible modifiable risk factors, as we did in the present study.
In conclusion, we demonstrated an increased risk of fracture in Danish patients on renal replacement therapy, a risk that was most pronounced in the dialysis population, even after adjusting for age. However, over time the fracture incidence was highest in the transplanted group, due to the high competing risk of death in the dialysis patients. Despite improved standards of care, fracture incidence had not changed during the last decade. The apparent association between proton pump inhibitors, lipid-modifying agents, diuretics and the risk of fracture remains to be further explored.
CONFLICT OF INTEREST STATEMENT
D.H. has received speaker fees from Fresenius, Baxter and a research grant from Abbott Laboratories A/S. J.B.O. has received speaker fees from Bristol-Myers Squibb and Boehringer Ingelheim, and funding for research from the Lundbeck Foundation, Bristol-Myers Squibb and The Capital Region of Denmark, Foundation for Health Research. B.A. has Institutional research contracts with Novartis and UCB. G.H.G. has received research grants from Pfizer/Bristol Meyers Squibb, Bayer, Boehringer Ingelheim and AstraZeneca, and speaker fees from AstraZeneca, Pfizer and Sanofi Aventis. K.H. has no conflicts to declare. The results presented in this paper have not been published previously in whole or part, except in abstract format. (See related article by Messa. Skeletal fractures in patients on renal replacement therapy: how large still is the knowledge gap? Nephrol Dial Transplant 2016; 31: 1554–1556)
REFERENCES





Comments