-
PDF
- Split View
-
Views
-
Cite
Cite
Michael Heung, Luis M. Ortega, Lakhmir S. Chawla, Richard G. Wunderink, Wesley H. Self, Jay L. Koyner, Jing Shi, John A. Kellum, for the Sapphire and Topaz Investigators, Common chronic conditions do not affect performance of cell cycle arrest biomarkers for risk stratification of acute kidney injury, Nephrology Dialysis Transplantation, Volume 31, Issue 10, October 2016, Pages 1633–1640, https://doi.org/10.1093/ndt/gfw241
Close - Share Icon Share
Abstract
Identification of acute kidney injury (AKI) can be challenging in patients with underlying chronic disease, and biomarkers often perform poorly in this population. In this study we examined the performance characteristics of the novel biomarker panel of urinary tissue inhibitor of metalloproteinases-2 (TIMP2) and insulin-like growth factor-binding protein 7 ([IGFBP7]) in patients with a variety of comorbid conditions.
We analyzed data from two multicenter studies of critically ill patients in which [TIMP2]•[IGFBP7] was validated for prediction of Kidney Disease: Improving Global Outcomes (KDIGO) Stage 2 or 3 AKI within 12 h. We constructed receiver operating characteristic (ROC) curves for AKI prediction both overall and by comorbid conditions common among patients with AKI, including diabetes mellitus, congestive heart failure (CHF) and chronic kidney disease (CKD).
In the overall cohort of 1131 patients, 139 (12.3%) developed KDIGO Stage 2 or 3 AKI. [TIMP2]•[IGFBP7] was significantly higher in AKI versus non-AKI patients, both overall and within each comorbidity subgroup. The AUC for [TIMP2]•[IGFBP7] in predicting AKI was 0.81 overall. Higher AUC was noted in patients with versus without CHF (0.89 versus 0.79; P = 0.026) and CKD (0.91 versus 0.80; P = 0.024).
We observed no significant impairment in the performance of cell cycle arrest biomarkers due to the presence of chronic comorbid conditions.
INTRODUCTION
Acute kidney injury (AKI) occurs in up to 20% of hospitalized patients and is associated with high morbidity and mortality [1–4]. Although management of patients with AKI remains largely supportive, limiting further renal damage when injury occurs is an important goal [5], and it logically follows that early diagnosis and recognition of AKI may provide the best opportunity to attenuate ongoing injury. The ability to accurately identify patients at risk for AKI could inform clinical decision-making, such as through emphasizing resuscitation, more stringent avoidance of nephrotoxic exposures and more attention to appropriate medication dosing. Thus, an important goal of AKI research has been to develop biomarkers to accurately identify the onset of renal injury or predict AKI risk earlier than current methods allow. However, AKI biomarkers may have variable performance in the setting of comorbid disease. For example, a recent study demonstrated markedly inferior test characteristics for six biomarkers in AKI patients with pre-existing chronic kidney disease (CKD) [6]. This is particularly problematic, as CKD is a well-recognized risk factor for AKI [7].
We recently reported data from two multicenter studies of critically ill patients (the Sapphire and Topaz studies) where a panel consisting of urinary tissue inhibitor of metalloproteinases-2 (TIMP2) and insulin-like growth factor-binding protein 7 (IGFBP7), both markers associated with cell cycle arrest, was validated for risk stratification for moderate–severe AKI [8, 9]. Urinary [TIMP2]•[IGFBP7] >0.3 (ng/mL)2/1000 had >90% sensitivity in predicting the development of moderate–severe AKI within 12 h [9]. In contrast to other novel AKI biomarkers that reflect renal cell injury or decline in renal function, these markers are believed to reflect the renal tubular epithelial response to cellular stress from injury [10, 11]. [TIMP2]•[IGFBP7] was found to have superior performance characteristics for AKI risk stratification, as assessed by area under the receiver operating characteristics (ROC) curve (AUC), compared with other markers such as neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule 1 (KIM-1), cystatin C and interleukin-18 (IL-18) [8]. However, although patients with CKD and other chronic conditions were enrolled in these trials, we have not previously analyzed test performance when restricted to these patients.
Here we pool data from two trials of critically ill subjects at high risk for AKI [8, 9] and examine the impact of various comorbidities [e.g. CKD, congestive heart failure (CHF)] on the performance characteristics of cell cycle arrest markers for the prediction of moderate–severe [Kidney Disease: Improving Global Outcomes (KDIGO) Stage 2 or 3] AKI in critically ill patients.
MATERIALS AND METHODS
Patient population
We conducted a secondary analysis of data collected from two multicenter clinical trials used to validate [TIMP2]•[IGFBP7] in AKI, the Sapphire and Topaz studies [8, 9]. The Sapphire study enrolled 744 critically ill adult (>21 years) patients who were at risk for development of AKI. In the Topaz study, 420 critically ill adult patients were enrolled, with similar inclusion/exclusion criteria to the Sapphire study. Specifically, subjects in both studies were required to have evidence of significant pulmonary [respiratory Sequential Organ Failure Assessment (SOFA) score ≥2] or cardiovascular (cardiac SOFA score ≥1) dysfunction and could not yet have met criteria for moderate–severe AKI (KDIGO Stage 2 or 3). Patient recruitment occurred within 24 h of intensive care unit (ICU) admission. More detailed descriptions of both studies have previously been published [8, 9]. Both the Sapphire and Topaz studies were approved by the Western Institutional Review Board (Olympia, WA, USA) as well as individual site investigational review boards if required and written informed consent was obtained from all subjects (or their legally authorized representatives).
Data collection
Clinical data collection included patient demographics, reason for ICU admission, Acute Physiology and Chronic Health Evaluation (APACHE) III score variables, hourly urine output and laboratory testing results. Comorbid conditions were determined based on review of available medical records and based on diagnostic codes. Estimated glomerular filtration rate (eGFR) was determined using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation; patients with missing or unknown race were considered not to be black. Each site extracted data from clinical sources and input the data into an online electronic case report form in a deidentified password-protected dataset. Study data were stored at an independent server site (Medidata Solutions, New York, NY, USA).
The primary outcome was moderate–severe AKI as defined by KDIGO criteria for Stage 2 or 3 AKI developing within 12 h of enrollment. In the Topaz study, final determination of AKI was adjudicated by an expert panel of three independent nephrologists who were blinded to biomarker results.
Laboratory testing
Urine and blood samples were collected and processed using standard methods. Sample supernatants were frozen within 2 h of collection and stored at −70°C before batch transport to a central lab for analysis. Samples were thawed immediately before analysis. Testing for [TIMP2]•[IGFBP7] was performed using a clinical immunoassay (NEPHROCHECK Test and ASTUTE140 Meter; Astute Medical, San Diego, CA, USA), and lab technicians performing the testing were blinded to patient outcomes. For subjects recruited in the Sapphire study, [TIMP2]•[IGFBP7] testing was performed at Astute Medical laboratories; in the Topaz study, testing was performed in triplicate at three independent laboratories (University of California at San Diego, San Diego, CA, USA; University of Louisville, Louisville, KY, USA; and ARUP Laboratories, Salt Lake City, UT, USA). Test results for [TIMP2]•[IGFBP7] are uniformly reported in units of (ng/mL)2/1000 throughout the text.
Statistical methods
Continuous variables were compared between AKI groups using the Wilcoxon rank sum test or t-test. Categorical variables were compared between AKI groups using the χ2 test. To assess the effect of comorbidity on levels of [TIMP2]•[IGFBP7], we performed multiple linear regression analysis for each comorbidity where the response variable is rank transformed [TIMP2]•[IGFBP7]; the explanatory variables are AKI status, comorbidity status and the interaction between them. AUC calculation and testing for differences in two AUCs were based on the Delong method, using the R package ‘pROC’ [12]. When necessary, P-values were adjusted for multiple testing by the Benjamini–Hochberg method. A multivariate logistic regression model was constructed to predict the primary outcome of AKI accounting for comorbid conditions. Backward selection was used to eliminate variables with P-values <0.10 and arrive at the final model. Statistical analyses were performed using R version 3.1.0. (R Foundation, http://www.r-project.org/) and SAS 9.3 (SAS Institute, Cary, NC, USA).
RESULTS
Patient characteristics
Figure 1 shows the flow of patient recruitment from both the Sapphire and Topaz studies. A total of 1164 patients were recruited; in the Sapphire study, 21 (2.8%) patients were excluded after enrollment, while 12 (2.9%) patients were excluded in the Topaz study. Patient characteristics were similar between the two studies, as reported previously [13]. The final cohort consisted of 1131 patients, of whom 139 (12.3%) developed moderate–severe AKI (KDIGO Stage 2 or 3) [5]. For simplicity, throughout the manuscript AKI refers to KDIGO Stage 2 or 3 AKI, while no AKI indicates either no AKI or Stage 1 AKI.
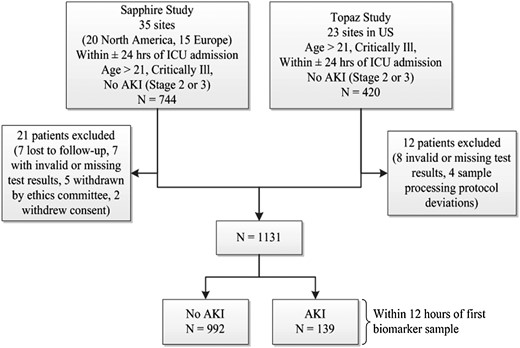
Diagram of subject recruitment and enrollment in the Sapphire and Topaz studies, and derivation of the final cohort for this study. AKI was defined as moderate to severe (KDIGO Stage 2–3) AKI.
Patient characteristics stratified by AKI status are shown in Table 1. A greater percentage of patients who developed AKI had preexisting diabetes and hypertension compared with patients without AKI. The percentage of patients with underlying CKD was similar between AKI groups, but median enrollment serum creatinine was higher in patients who developed AKI.
| . | No AKI or Stage 1 . | AKI Stage 2 or 3 . | P-value . |
|---|---|---|---|
| All patients | 992 | 139 | |
| Male | 589 (59%) | 74 (53%) | 0.169 |
| Age, years | 62 (16) | 65 (15) | 0.081 |
| Body mass index, kg/m2 | 27 (24–32) | 31 (26–38) | <0.001 |
| Race | 0.948 | ||
| Black | 127 (13%) | 16 (12%) | |
| White | 794 (80%) | 113 (81%) | |
| Other/unknown | 70 (7%) | 10 (7%) | |
| Medical history | |||
| Chronic kidney disease | 84 (8%) | 13 (9%) | 0.746 |
| Diabetes mellitus | 274 (28%) | 52 (37%) | 0.021 |
| Congestive heart failure | 174 (18%) | 32 (23%) | 0.127 |
| Coronary artery disease | 296 (30%) | 40 (29%) | 0.843 |
| Hypertension | 599 (60%) | 101 (73%) | 0.005 |
| Chronic obstructive pulmonary disease | 213 (21%) | 23 (17%) | 0.220 |
| Admitted to ICU from | 0.244 | ||
| ED | 380 (38%) | 62 (45%) | |
| Floor | 175 (18%) | 18 (13%) | |
| OR | 275 (28%) | 30 (22%) | |
| Other hospital | 140 (14%) | 25 (18%) | |
| Other ICU | 10 (1%) | 1 (1%) | |
| Unknown | 12 (1%) | 3 (2%) | |
| Reason for ICU admission | |||
| Respiratory | 447 (45%) | 67 (48%) | 0.525 |
| Surgery | 340 (34%) | 35 (25%) | 0.034 |
| Cardiovascular | 345 (35%) | 58 (42%) | 0.130 |
| Sepsis | 192 (19%) | 40 (29%) | 0.013 |
| Neurological | 112 (11%) | 10 (7%) | 0.188 |
| Trauma | 89 (9%) | 10 (7%) | 0.630 |
| Other | 207 (21%) | 40 (29%) | 0.038 |
| Time from ICU admission to biomarker sample collection, hours | 16 (7–20) | 16 (11–20) | 0.427 |
| Non-renal APACHE III | 57 (43–78) | 69 (50–87) | 0.001 |
| Enrollment eGFRa, mL/min/1.73m2 | 82 (55–101) | 52 (31–84) | <0.001 |
| Enrollment serum creatinineb, mg/dL | 0.9 (0.7–1.2) | 1.3 (0.9–1.8) | <0.001 |
| 6-h cumulative urine output at enrollmentc, mL | 424 (280–705) | 185 (115–303) | <0.001 |
| Radiocontrast agentsd | 354 (36%) | 47 (34%) | 0.706 |
| Blood transfusionse | |||
| PRBC | 283 (29%) | 32 (23%) | 0.190 |
| Platelets | 98 (10%) | 19 (14%) | 0.180 |
| Fresh frozen plasma | 139 (14%) | 25 (18%) | 0.246 |
| Albumin | 143 (14%) | 25 (18%) | 0.255 |
| Cryoprecipitate | 24 (2%) | 2 (1%) | 0.761 |
| . | No AKI or Stage 1 . | AKI Stage 2 or 3 . | P-value . |
|---|---|---|---|
| All patients | 992 | 139 | |
| Male | 589 (59%) | 74 (53%) | 0.169 |
| Age, years | 62 (16) | 65 (15) | 0.081 |
| Body mass index, kg/m2 | 27 (24–32) | 31 (26–38) | <0.001 |
| Race | 0.948 | ||
| Black | 127 (13%) | 16 (12%) | |
| White | 794 (80%) | 113 (81%) | |
| Other/unknown | 70 (7%) | 10 (7%) | |
| Medical history | |||
| Chronic kidney disease | 84 (8%) | 13 (9%) | 0.746 |
| Diabetes mellitus | 274 (28%) | 52 (37%) | 0.021 |
| Congestive heart failure | 174 (18%) | 32 (23%) | 0.127 |
| Coronary artery disease | 296 (30%) | 40 (29%) | 0.843 |
| Hypertension | 599 (60%) | 101 (73%) | 0.005 |
| Chronic obstructive pulmonary disease | 213 (21%) | 23 (17%) | 0.220 |
| Admitted to ICU from | 0.244 | ||
| ED | 380 (38%) | 62 (45%) | |
| Floor | 175 (18%) | 18 (13%) | |
| OR | 275 (28%) | 30 (22%) | |
| Other hospital | 140 (14%) | 25 (18%) | |
| Other ICU | 10 (1%) | 1 (1%) | |
| Unknown | 12 (1%) | 3 (2%) | |
| Reason for ICU admission | |||
| Respiratory | 447 (45%) | 67 (48%) | 0.525 |
| Surgery | 340 (34%) | 35 (25%) | 0.034 |
| Cardiovascular | 345 (35%) | 58 (42%) | 0.130 |
| Sepsis | 192 (19%) | 40 (29%) | 0.013 |
| Neurological | 112 (11%) | 10 (7%) | 0.188 |
| Trauma | 89 (9%) | 10 (7%) | 0.630 |
| Other | 207 (21%) | 40 (29%) | 0.038 |
| Time from ICU admission to biomarker sample collection, hours | 16 (7–20) | 16 (11–20) | 0.427 |
| Non-renal APACHE III | 57 (43–78) | 69 (50–87) | 0.001 |
| Enrollment eGFRa, mL/min/1.73m2 | 82 (55–101) | 52 (31–84) | <0.001 |
| Enrollment serum creatinineb, mg/dL | 0.9 (0.7–1.2) | 1.3 (0.9–1.8) | <0.001 |
| 6-h cumulative urine output at enrollmentc, mL | 424 (280–705) | 185 (115–303) | <0.001 |
| Radiocontrast agentsd | 354 (36%) | 47 (34%) | 0.706 |
| Blood transfusionse | |||
| PRBC | 283 (29%) | 32 (23%) | 0.190 |
| Platelets | 98 (10%) | 19 (14%) | 0.180 |
| Fresh frozen plasma | 139 (14%) | 25 (18%) | 0.246 |
| Albumin | 143 (14%) | 25 (18%) | 0.255 |
| Cryoprecipitate | 24 (2%) | 2 (1%) | 0.761 |
Categorical variables are shown as n (%) and numerical as mean (standard deviation) or median (interquartile range).
ED, emergency department; OR, operating room; ICU, intensive care unit; eGFR, estimated glomerular filtration rate.
aCalculated from enrollment serum creatinine using the CKD-EPI equation.
bValue from medical record closest to time of enrollment.
c15% of no AKI or Stage 1 and 12% of AKI Stage 2 or 3 did not have urine output data for 6 h prior to enrollment.
dNumber of patients receiving intravenous or intra-arterial contrast administered within 5 days prior to and including the day of enrollment.
eNumber of patients receiving blood products within 5 days prior to and including the day of enrollment.
| . | No AKI or Stage 1 . | AKI Stage 2 or 3 . | P-value . |
|---|---|---|---|
| All patients | 992 | 139 | |
| Male | 589 (59%) | 74 (53%) | 0.169 |
| Age, years | 62 (16) | 65 (15) | 0.081 |
| Body mass index, kg/m2 | 27 (24–32) | 31 (26–38) | <0.001 |
| Race | 0.948 | ||
| Black | 127 (13%) | 16 (12%) | |
| White | 794 (80%) | 113 (81%) | |
| Other/unknown | 70 (7%) | 10 (7%) | |
| Medical history | |||
| Chronic kidney disease | 84 (8%) | 13 (9%) | 0.746 |
| Diabetes mellitus | 274 (28%) | 52 (37%) | 0.021 |
| Congestive heart failure | 174 (18%) | 32 (23%) | 0.127 |
| Coronary artery disease | 296 (30%) | 40 (29%) | 0.843 |
| Hypertension | 599 (60%) | 101 (73%) | 0.005 |
| Chronic obstructive pulmonary disease | 213 (21%) | 23 (17%) | 0.220 |
| Admitted to ICU from | 0.244 | ||
| ED | 380 (38%) | 62 (45%) | |
| Floor | 175 (18%) | 18 (13%) | |
| OR | 275 (28%) | 30 (22%) | |
| Other hospital | 140 (14%) | 25 (18%) | |
| Other ICU | 10 (1%) | 1 (1%) | |
| Unknown | 12 (1%) | 3 (2%) | |
| Reason for ICU admission | |||
| Respiratory | 447 (45%) | 67 (48%) | 0.525 |
| Surgery | 340 (34%) | 35 (25%) | 0.034 |
| Cardiovascular | 345 (35%) | 58 (42%) | 0.130 |
| Sepsis | 192 (19%) | 40 (29%) | 0.013 |
| Neurological | 112 (11%) | 10 (7%) | 0.188 |
| Trauma | 89 (9%) | 10 (7%) | 0.630 |
| Other | 207 (21%) | 40 (29%) | 0.038 |
| Time from ICU admission to biomarker sample collection, hours | 16 (7–20) | 16 (11–20) | 0.427 |
| Non-renal APACHE III | 57 (43–78) | 69 (50–87) | 0.001 |
| Enrollment eGFRa, mL/min/1.73m2 | 82 (55–101) | 52 (31–84) | <0.001 |
| Enrollment serum creatinineb, mg/dL | 0.9 (0.7–1.2) | 1.3 (0.9–1.8) | <0.001 |
| 6-h cumulative urine output at enrollmentc, mL | 424 (280–705) | 185 (115–303) | <0.001 |
| Radiocontrast agentsd | 354 (36%) | 47 (34%) | 0.706 |
| Blood transfusionse | |||
| PRBC | 283 (29%) | 32 (23%) | 0.190 |
| Platelets | 98 (10%) | 19 (14%) | 0.180 |
| Fresh frozen plasma | 139 (14%) | 25 (18%) | 0.246 |
| Albumin | 143 (14%) | 25 (18%) | 0.255 |
| Cryoprecipitate | 24 (2%) | 2 (1%) | 0.761 |
| . | No AKI or Stage 1 . | AKI Stage 2 or 3 . | P-value . |
|---|---|---|---|
| All patients | 992 | 139 | |
| Male | 589 (59%) | 74 (53%) | 0.169 |
| Age, years | 62 (16) | 65 (15) | 0.081 |
| Body mass index, kg/m2 | 27 (24–32) | 31 (26–38) | <0.001 |
| Race | 0.948 | ||
| Black | 127 (13%) | 16 (12%) | |
| White | 794 (80%) | 113 (81%) | |
| Other/unknown | 70 (7%) | 10 (7%) | |
| Medical history | |||
| Chronic kidney disease | 84 (8%) | 13 (9%) | 0.746 |
| Diabetes mellitus | 274 (28%) | 52 (37%) | 0.021 |
| Congestive heart failure | 174 (18%) | 32 (23%) | 0.127 |
| Coronary artery disease | 296 (30%) | 40 (29%) | 0.843 |
| Hypertension | 599 (60%) | 101 (73%) | 0.005 |
| Chronic obstructive pulmonary disease | 213 (21%) | 23 (17%) | 0.220 |
| Admitted to ICU from | 0.244 | ||
| ED | 380 (38%) | 62 (45%) | |
| Floor | 175 (18%) | 18 (13%) | |
| OR | 275 (28%) | 30 (22%) | |
| Other hospital | 140 (14%) | 25 (18%) | |
| Other ICU | 10 (1%) | 1 (1%) | |
| Unknown | 12 (1%) | 3 (2%) | |
| Reason for ICU admission | |||
| Respiratory | 447 (45%) | 67 (48%) | 0.525 |
| Surgery | 340 (34%) | 35 (25%) | 0.034 |
| Cardiovascular | 345 (35%) | 58 (42%) | 0.130 |
| Sepsis | 192 (19%) | 40 (29%) | 0.013 |
| Neurological | 112 (11%) | 10 (7%) | 0.188 |
| Trauma | 89 (9%) | 10 (7%) | 0.630 |
| Other | 207 (21%) | 40 (29%) | 0.038 |
| Time from ICU admission to biomarker sample collection, hours | 16 (7–20) | 16 (11–20) | 0.427 |
| Non-renal APACHE III | 57 (43–78) | 69 (50–87) | 0.001 |
| Enrollment eGFRa, mL/min/1.73m2 | 82 (55–101) | 52 (31–84) | <0.001 |
| Enrollment serum creatinineb, mg/dL | 0.9 (0.7–1.2) | 1.3 (0.9–1.8) | <0.001 |
| 6-h cumulative urine output at enrollmentc, mL | 424 (280–705) | 185 (115–303) | <0.001 |
| Radiocontrast agentsd | 354 (36%) | 47 (34%) | 0.706 |
| Blood transfusionse | |||
| PRBC | 283 (29%) | 32 (23%) | 0.190 |
| Platelets | 98 (10%) | 19 (14%) | 0.180 |
| Fresh frozen plasma | 139 (14%) | 25 (18%) | 0.246 |
| Albumin | 143 (14%) | 25 (18%) | 0.255 |
| Cryoprecipitate | 24 (2%) | 2 (1%) | 0.761 |
Categorical variables are shown as n (%) and numerical as mean (standard deviation) or median (interquartile range).
ED, emergency department; OR, operating room; ICU, intensive care unit; eGFR, estimated glomerular filtration rate.
aCalculated from enrollment serum creatinine using the CKD-EPI equation.
bValue from medical record closest to time of enrollment.
c15% of no AKI or Stage 1 and 12% of AKI Stage 2 or 3 did not have urine output data for 6 h prior to enrollment.
dNumber of patients receiving intravenous or intra-arterial contrast administered within 5 days prior to and including the day of enrollment.
eNumber of patients receiving blood products within 5 days prior to and including the day of enrollment.
[TIMP2]•[IGFBP7] performance
In the overall cohort, median [TIMP2]•[IGFBP7] was significantly higher in AKI patients compared with non-AKI patients {1.5 [interquartile range (IQR) 0.6–2.8] versus 0.3 [IQR 0.1–0.7]; P < 0.001}. These values were consistent across a variety of comorbid states and remained statistically different between AKI and non-AKI patients (Figure 2).
![Boxplots of [TIMP2]•[IGFBP7] values across different comorbidity subpopulations by acute kidney injury status. Boxes and whiskers show interquartile ranges (IQRs) and total observed ranges, censored by 1.5 times the IQR. Horizontal lines within the boxes show the medians. Dotted lines refer to the 0.3 and 2.0 cutoffs, for illustrative purposes. Gray and white shading of the boxes indicate the absence or presence of AKI within 12 h. [TIMP2]•[IGFBP7] values were significantly higher in AKI patients than in non-AKI patients for all comparisons shown (P < 0.001 for all). CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ndt/31/10/10.1093_ndt_gfw241/2/m_gfw24102.jpeg?Expires=1747861146&Signature=W9HEiIwDvHkqoFfbbAYYSOPQl4bjzORlpm1r~88JS7iZkZGxKyudhON7JCX2QM4TtM9M8~DkmaxXR5gbJ5-bGnF5~itV6KuxK3KiS4TfDkVAgSWELOeFhQH2Qnt6BXd4G2e4N9YNx2TZMoYK4wyDE94qHH6q3VcFUi~zbFlSWgEKA8fYYk82ganEap-kKaFsFGTvV7exgKLPe9zzruPHkitwVo51gUcEcy~5PwQbKOr0yqRMAwvDv9rNeMFLm5zaZd81i9OPQMr2o3TLQFHjIk1uzT593QhFqpZBidjAUcQNrQ1nb1lR6UCqvrRv40p5v5hMfpnAVqqlRUijMKRxGA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Boxplots of [TIMP2]•[IGFBP7] values across different comorbidity subpopulations by acute kidney injury status. Boxes and whiskers show interquartile ranges (IQRs) and total observed ranges, censored by 1.5 times the IQR. Horizontal lines within the boxes show the medians. Dotted lines refer to the 0.3 and 2.0 cutoffs, for illustrative purposes. Gray and white shading of the boxes indicate the absence or presence of AKI within 12 h. [TIMP2]•[IGFBP7] values were significantly higher in AKI patients than in non-AKI patients for all comparisons shown (P < 0.001 for all). CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease.
In the cohort of 97 patients with CKD, the AUC for [TIMP2]•[IGFBP7] prediction of moderate–severe AKI was 0.91 (95% CI 0.85–0.97) (Figure 3). The relative risk for AKI with a [TIMP2]•[IGFBP7] value above the previously validated cutoff of 0.3 was 32.4 (95% CI 3.7–284.8). There were 326 patients with diabetes mellitus in the overall study cohort. [TIMP2]•[IGFBP7] testing yielded an AUC of 0.83 (95% CI 0.77–0.89) (Figure 4), and a value >0.3 was associated with a relative risk for moderate–severe AKI of 12.8 (95% CI 4.1–40.1). In recognition of the overlap between the CKD and diabetic patient populations, a sensitivity analysis was performed focusing on patients with either CKD or diabetes alone. For patients with diabetes and not CKD (n = 273), the AUC was 0.82 (95% CI 0.75–0.88). In those with CKD but not diabetes (n = 47), the AUC was 0.95 (95% CI 0.86–1.00).
![ROC curve of [TIMP2]•[IGFBP7] for the prediction of moderate–severe AKI in patients with CKD.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ndt/31/10/10.1093_ndt_gfw241/2/m_gfw24103.jpeg?Expires=1747861146&Signature=1DWqHR2QdXM0TVj80evzJv5fGCQF~I0vsQIsf3rGInhzF5SFrIJgPoDgTEA2SasyMhYciaJ2hiExhWYAqSukR3cKON4X9z-RWLJClfYEhRQM1OW8~dS~KEjDH-H0kZzATECNzfO5EexdtjeLj-KNyNxjA46Z3wFbZZ-dzPDQt-fNCDZsekW~v6ThGNP5VECPe8UiacWVfePA278qKrBmelMKbSUkKiHrikKH2Z3Zd93D8N4dC~qf3MlEI8hE7ugIahCtqq4mLkK8CZD1nI4TkrGpVENl~OgHv06fAvQ2vw0NMZIXMDYDXL~xodkbJZTUX4m6zOWBNGi0w7S4GT-K7g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
ROC curve of [TIMP2]•[IGFBP7] for the prediction of moderate–severe AKI in patients with CKD.
![ROC curve of [TIMP2]•[IGFBP7] for the prediction of moderate–severe AKI in patients with diabetes mellitus.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ndt/31/10/10.1093_ndt_gfw241/2/m_gfw24104.jpeg?Expires=1747861146&Signature=GLKkhuB519mVSvHxVmGOl0tLXTfPsAmYZssyABPaTrDPHncFT40TJ3-vlHO1D5Uo9PEoMSPmWUbm8RdFO3KtxB51179juX-c5TbyoMDCX4bfIT9XzJsryP1tWkRQZgJ8KWW794qLplN328AD2ZN3nx7ZkyCRcYuFiBl0w24NZIPf3D84raA4Ts5l~FYgEOZKkFZuuprasCiJX1sAYom1GU~UuP-iA9~i8zZMz518pGM~B08mHJSWz9xvcNkuIuohNUsNEAFHhegosTiZcF~lS0sGGrqh21pMOx1ILfi3UPBJSX-AjIZdXeXUKmJLKGEx2NPn6aqFKZePIXZ9~YTkMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
ROC curve of [TIMP2]•[IGFBP7] for the prediction of moderate–severe AKI in patients with diabetes mellitus.
To further assess the impact of comorbidity on [TIMP2]•[IGFBP7], multiple linear regression models were performed with [TIMP2]•[IGFBP7] as a function of comorbidities, adjusted for AKI status. CKD was found to have a statistically significant effect on [TIMP2]•[IGFBP7] in non-AKI patients (P = 0.009) but not in AKI patients. Notably, in non-AKI patients, the direction of effect was such that the presence of CKD was associated with lower [TIMP2]•[IGFBP7]. The interactions between each comorbidity and AKI status were not significant.
To examine the predictive ability of [TIMP2]•[IGFBP7] in different comorbid states, a multivariate logistic regression model for AKI was developed that included all comorbid states and their interaction terms with [TIMP2]•[IGFBP7] (as well as age, race, sex, body mass index, enrollment creatinine and non-renal SOFA score). Overall, [TIMP2]•[IGFBP7] remained a significant predictor for AKI at 12 h [OR 5.0 (95% CI 2.8–8.8) per unit increase in log10([TIMP-2]•[IGFBP7])]. ROC curves were created for subpopulations stratified by each comorbid condition, and the resultant AUC values are shown in Figure 5. The majority of AUC values remained >0.80 and did not differ statistically between patients with and without coronary artery disease (CAD), chronic obstructive pulmonary disease (COPD), diabetes or hypertension. [TIMP2]•[IGFBP7] in patients with CHF had a significantly higher AUC compared with those without CHF (0.89 versus 0.79; P = 0.026), and a similar relationship was seen with [TIMP2]•[IGFBP7] in CKD versus non-CKD patients (0.91 versus 0.80; P = 0.024).
![AUC assessing [TIMP2]•[IGFBP7] for the prediction of Stage 2 or 3 AKI within 12 h, stratified by comorbid conditions. Error bars show the 95% CI of the AUC values. Horizontal dashed line shows an AUC of 0.80 for illustrative purposes.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ndt/31/10/10.1093_ndt_gfw241/2/m_gfw24105.jpeg?Expires=1747861146&Signature=K9EPIXNs~u86yFweLAV8uwESah8TfaHQczUaZ5Vm-pCxeL2Ggau26xIVse7r3YNseR4-vfg0xOw5oDx5JR1ULQ~F8fP10gO8w2ocAD~-vppxXQAl24dI9AmRKWmFIrzgULiO19KDAY1fPPdUnbHOXIdsXtb-JBkAZs79omEky0UMrFXJuaPL72D5a2xuK9n9e9v8bHEJjWvJ7cOXlT2BVHp5VpkaDSVMkNRdArJ-i2x4DdL20jU-N~CI~YhA4xPa8p~PLT6sa7nNv6baSSCpuCl~XcxRgYW5yPcDnrghIbz-NWdCnmy-P4MWDbSJj5n7MtMS1wBtxqudOvafAV71vA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
AUC assessing [TIMP2]•[IGFBP7] for the prediction of Stage 2 or 3 AKI within 12 h, stratified by comorbid conditions. Error bars show the 95% CI of the AUC values. Horizontal dashed line shows an AUC of 0.80 for illustrative purposes.
The sensitivity and specificity of [TIMP2]•[IGFBP7] at both the 0.3 and 2.0 cutoffs were examined by comorbidity subgroup, and values are presented in Table 2. There was no significant difference in sensitivity or specificity across all subgroups for either cutoff.
Sensitivity and specificity of [TIMP2]•[IGFBP7] for moderate–severe AKI at both the 0.3 and 2.0 cutoffs, stratified by comorbidity subgroups
| Comorbidity . | Sensitivity or specificity . | 0.3 Cutoff . | 2.0 Cutoff . | ||||
|---|---|---|---|---|---|---|---|
| Without comorbidity (%) . | With comorbidity (%) . | P-value . | Without comorbidity (%) . | With comorbidity (%) . | P-value . | ||
| Coronary artery disease (n = 336) | Sensitivity | 90 | 88 | 0.92 | 41 | 33 | 0.661 |
| Specificity | 49 | 52 | 0.533 | 94 | 95 | 0.784 | |
| Congestive heart failure (n = 206) | Sensitivity | 88 | 97 | 0.561 | 37 | 50 | 0.648 |
| Specificity | 49 | 55 | 0.467 | 94 | 95 | 0.784 | |
| Chronic kidney disease (n = 97) | Sensitivity | 88 | 100 | 0.698 | 40 | 31 | 0.678 |
| Specificity | 49 | 63 | 0.097 | 94 | 98 | 0.671 | |
| Chronic obstructive pulmonary disease (n = 236) | Sensitivity | 88 | 96 | 0.698 | 41 | 30 | 0.661 |
| Specificity | 49 | 53 | 0.531 | 93 | 97 | 0.194 | |
| Diabetes mellitus (n = 326) | Sensitivity | 86 | 94 | 0.561 | 40 | 38 | 0.86 |
| Specificity | 50 | 51 | 0.723 | 94 | 95 | 0.784 | |
| Hypertension (n = 700) | Sensitivity | 89 | 89 | 1 | 50 | 36 | 0.648 |
| Specificity | 49 | 51 | 0.531 | 94 | 94 | 0.784 | |
| Comorbidity . | Sensitivity or specificity . | 0.3 Cutoff . | 2.0 Cutoff . | ||||
|---|---|---|---|---|---|---|---|
| Without comorbidity (%) . | With comorbidity (%) . | P-value . | Without comorbidity (%) . | With comorbidity (%) . | P-value . | ||
| Coronary artery disease (n = 336) | Sensitivity | 90 | 88 | 0.92 | 41 | 33 | 0.661 |
| Specificity | 49 | 52 | 0.533 | 94 | 95 | 0.784 | |
| Congestive heart failure (n = 206) | Sensitivity | 88 | 97 | 0.561 | 37 | 50 | 0.648 |
| Specificity | 49 | 55 | 0.467 | 94 | 95 | 0.784 | |
| Chronic kidney disease (n = 97) | Sensitivity | 88 | 100 | 0.698 | 40 | 31 | 0.678 |
| Specificity | 49 | 63 | 0.097 | 94 | 98 | 0.671 | |
| Chronic obstructive pulmonary disease (n = 236) | Sensitivity | 88 | 96 | 0.698 | 41 | 30 | 0.661 |
| Specificity | 49 | 53 | 0.531 | 93 | 97 | 0.194 | |
| Diabetes mellitus (n = 326) | Sensitivity | 86 | 94 | 0.561 | 40 | 38 | 0.86 |
| Specificity | 50 | 51 | 0.723 | 94 | 95 | 0.784 | |
| Hypertension (n = 700) | Sensitivity | 89 | 89 | 1 | 50 | 36 | 0.648 |
| Specificity | 49 | 51 | 0.531 | 94 | 94 | 0.784 | |
Sensitivity and specificity of [TIMP2]•[IGFBP7] for moderate–severe AKI at both the 0.3 and 2.0 cutoffs, stratified by comorbidity subgroups
| Comorbidity . | Sensitivity or specificity . | 0.3 Cutoff . | 2.0 Cutoff . | ||||
|---|---|---|---|---|---|---|---|
| Without comorbidity (%) . | With comorbidity (%) . | P-value . | Without comorbidity (%) . | With comorbidity (%) . | P-value . | ||
| Coronary artery disease (n = 336) | Sensitivity | 90 | 88 | 0.92 | 41 | 33 | 0.661 |
| Specificity | 49 | 52 | 0.533 | 94 | 95 | 0.784 | |
| Congestive heart failure (n = 206) | Sensitivity | 88 | 97 | 0.561 | 37 | 50 | 0.648 |
| Specificity | 49 | 55 | 0.467 | 94 | 95 | 0.784 | |
| Chronic kidney disease (n = 97) | Sensitivity | 88 | 100 | 0.698 | 40 | 31 | 0.678 |
| Specificity | 49 | 63 | 0.097 | 94 | 98 | 0.671 | |
| Chronic obstructive pulmonary disease (n = 236) | Sensitivity | 88 | 96 | 0.698 | 41 | 30 | 0.661 |
| Specificity | 49 | 53 | 0.531 | 93 | 97 | 0.194 | |
| Diabetes mellitus (n = 326) | Sensitivity | 86 | 94 | 0.561 | 40 | 38 | 0.86 |
| Specificity | 50 | 51 | 0.723 | 94 | 95 | 0.784 | |
| Hypertension (n = 700) | Sensitivity | 89 | 89 | 1 | 50 | 36 | 0.648 |
| Specificity | 49 | 51 | 0.531 | 94 | 94 | 0.784 | |
| Comorbidity . | Sensitivity or specificity . | 0.3 Cutoff . | 2.0 Cutoff . | ||||
|---|---|---|---|---|---|---|---|
| Without comorbidity (%) . | With comorbidity (%) . | P-value . | Without comorbidity (%) . | With comorbidity (%) . | P-value . | ||
| Coronary artery disease (n = 336) | Sensitivity | 90 | 88 | 0.92 | 41 | 33 | 0.661 |
| Specificity | 49 | 52 | 0.533 | 94 | 95 | 0.784 | |
| Congestive heart failure (n = 206) | Sensitivity | 88 | 97 | 0.561 | 37 | 50 | 0.648 |
| Specificity | 49 | 55 | 0.467 | 94 | 95 | 0.784 | |
| Chronic kidney disease (n = 97) | Sensitivity | 88 | 100 | 0.698 | 40 | 31 | 0.678 |
| Specificity | 49 | 63 | 0.097 | 94 | 98 | 0.671 | |
| Chronic obstructive pulmonary disease (n = 236) | Sensitivity | 88 | 96 | 0.698 | 41 | 30 | 0.661 |
| Specificity | 49 | 53 | 0.531 | 93 | 97 | 0.194 | |
| Diabetes mellitus (n = 326) | Sensitivity | 86 | 94 | 0.561 | 40 | 38 | 0.86 |
| Specificity | 50 | 51 | 0.723 | 94 | 95 | 0.784 | |
| Hypertension (n = 700) | Sensitivity | 89 | 89 | 1 | 50 | 36 | 0.648 |
| Specificity | 49 | 51 | 0.531 | 94 | 94 | 0.784 | |
DISCUSSION
Cell cycle arrest markers have recently emerged as important tools to predict moderate–severe AKI. In this study, we explored the impact of comorbidity on the performance characteristics of these biomarkers. Overall, the biomarkers performed equally well in distinguishing AKI from non-AKI in the presence or absence of a variety of comorbid conditions, including in patients with diabetes and CKD, who are at increased risk for AKI. Although the subpopulations with each comorbid condition were relatively small, these preliminary findings lend support for broad applicability of cell cycle arrest markers in patients with a variety of comorbidities.
We chose to examine the influence of comorbidities on biomarker performance for a number of reasons. First, patients with various comorbidities (e.g. CKD, diabetes, CHF) are among the highest risk for developing AKI, and therefore demonstration of the utility of a biomarker in these populations is important. Second, previous AKI biomarker studies have reported variable performance in different clinical settings, and some of that variation may be attributable to patient comorbidity characteristics. Cell cycle arrest markers could theoretically be elevated due to a variety of stress states and not specifically in response to AKI. Understanding the limitations of biomarkers in different patient populations is critical to their appropriate application to the clinical setting. Indeed, a recent editorial concluded that ‘unscrutinized use of these [novel AKI] biomarkers may distract from adequate clinical evaluation and carries the risk of worse instead of better patient outcome’ [14].
Prior studies of other novel AKI biomarkers have specifically noted the influence of baseline renal impairment on diagnostic characteristics. Endre et al. [6] examined several biomarkers, including NGAL, IL-18 and KIM-1, in a prospective study of 529 ICU patients. They found that biomarker performance depended on baseline renal function as well as the amount of time after the renal insult, with AUC values for AKI diagnosis at 6–12 h ranging from 0.69–0.72 in patients without CKD (eGFR ≥60 mL/min) to only 0.39–0.61 in patients with CKD [6]. Koyner et al. [15] also reported differences in AKI biomarker performance when stratifying by the presence of CKD, concluding that urinary NGAL and KIM-1 ‘did not display the ability to predict future development of AKI in those with an eGFR <60 mL/min’. Other studies have shown that NGAL levels rise with decreasing eGFR in the absence of AKI [16, 17]. Similarly, KIM-1 levels are elevated in many different types of kidney disease other than AKI and appear correlated to chronic inflammation and fibrosis [18]. While initially identified as AKI biomarkers, both NGAL and KIM-1 are now also being explored as markers for CKD and CKD progression [17, 19, 20]. Not surprisingly, a workgroup from the Acute Dialysis Quality Initiative has identified biomarker performance in patients with normal versus abnormal renal function as an important area for research needed to bring biomarkers closer to clinical utility [21].
In this study, the AUC of [TIMP2]•[IGFBP7] was significantly greater in patients with CKD compared with those without CKD. While these results are encouraging, it should be noted that there were a limited number of CKD patients in the overall cohort, and only 13 CKD patients developed AKI. Nevertheless, several theoretical explanations exist for why cell cycle arrest markers for AKI may have better diagnostic performance in CKD patients compared with other biomarkers. Patients with CKD have less renal reserve and may be more susceptible to insults and/or more likely to overtly manifest injury compared with patients with intact renal function, where subclinical injury can occur. Indeed, recent studies have established the concept of subclinical AKI whereby renal injury has occurred (biomarker positive), but in the absence of a significant increase in creatinine (creatinine criteria negative) [22], CKD patients may be less likely to fall into this gray zone and have ‘false positive’ results with biomarkers. Another consideration is that cell cycle arrest markers may be a more specific response to renal injury than the release of other biomarkers, which may reflect more chronic processes such as inflammation and fibrosis. Part of the clinical challenge with respect to AKI is that a renal signal follows a variety of discordant insults to the kidney, including sepsis, toxicity and muscle damage to name but a few. Thus the suggestion that the expression of markers of cell cycle arrest represents a uniform response to a heterogeneous insult is enticing.
Diabetes mellitus is another important risk factor for AKI and therefore another important population in which to evaluate AKI biomarkers. As with CKD, previous AKI biomarker studies have had variable results in diabetic patients. Ahmed and Hamed [23] recently evaluated urinary KIM-1 levels in diabetic patients without AKI. Compared with healthy controls, male diabetics had greater than five times the levels of KIM-1; in females, the difference was >10-fold. Sabbisetti et al. [24] note that KIM-1 levels were elevated in post-cardiac surgery diabetic patients without AKI compared with controls, although not to the same degree as patients that did develop AKI. In our study, [TIMP2]•[IGFBP7] performance was fairly constant in the diabetic versus non-diabetic populations, with AUC values ranging from 0.80 to 0.83. Our findings suggest that the performance of cell cycle arrest markers is not compromised in patients with diabetes.
Some limitations of this study are worth noting. As a post hoc analysis, the original study data were not powered to examine biomarker performance in subpopulations. Despite pooling, the subpopulations of interest remained relatively small (e.g. 97 patients with CKD), and these findings are not definitive evidence of equivalent performance, although they represent the largest cohort currently available for study. Our results would be strengthened by confirmation in larger, prospective studies. In addition, we did not have albuminuria data for the majority of patients and therefore cannot speculate as to whether biomarker performance might be affected by the presence of albuminuria.
In summary, we examined the performance of cell cycle arrest biomarkers for moderate to severe AKI in patients with various common comorbid conditions. In patients with diabetes, who are at elevated risk for AKI, we found no significant decrease in the ability of urinary [TIMP2]•[IGFBP7] to predict AKI risk. In a small cohort of CKD patients, another high-risk group for AKI, the biomarker panel showed superior AUCs compared with non-CKD patients. Thus, our results suggest no significant impairment in the performance of cell cycle arrest biomarkers due to chronic comorbid conditions.
AUTHORS' CONTRIBUTIONS
J.S. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. J.A.K. and L.S.C. designed and conducted the study. Data collection and interpretation: all authors reviewed the data and participated in discussions related to interpretation. Preparation, review or approval of the manuscript: M.H., L.M.O., L.S.C., J.S. and J.A.K. wrote the paper. All authors reviewed and edited the paper and have seen and approved the final draft.
CONFLICT OF INTEREST STATEMENT
M.H., L.M.O. and R.G.W. report no related interests. L.S.C. is employed by La Jolla Pharmaceutical Company and has received consulting fees from Astute Medical, Alere Medical, AM Pharma and Baxter Medical and has licensed unrelated technologies through George Washington University to Astute Medical. W.H.S. has received consulting fees from BioFire Diagnostics, Venaxis and Abbott Point of Care and has received research funding from Astute Medical, Pfizer, BRAHMS, bioMerieux, Venaxis and Sphingotec. J.L.K. has received research funding from Astute Medical, Abbott, NxStage and Satellite Health Care and has received consulting fees from Pfizer and Astute Medical. J.S. has received consulting fees from Astute Medical. J.A.K. has received consulting fees from Astute Medical, Alere, Aethlon, AM Pharma, Cytosorbents, Bard, Fresenius, Baxter, Abbott Diagnostics and Spectral Diagnostics. J.A.K. has also received research grants from Astute Medical, Alere, Cytosorbents, Bard, Baxter and Spectral Diagnostics and has licensed unrelated technologies through the University of Pittsburgh to Astute Medical and Cytosorbents.
ACKNOWLEDGEMENTS
The authors acknowledge many staff, coordinators and investigators whose work was essential for completion of this study. Results of this study were presented in abstract form at the 2015 International Conference on Advances in Critical Care Nephrology (CRRT 2015), San Diego, CA, USA. Funding for this study was provided by Astute Medical, San Diego, CA, USA.
REFERENCES
Author notes
A complete list of Sapphire and Topaz investigators and support personnel is available online (http://www.ccm.pitt.edu/sapphire-investigators and http://www.ccm.pitt.edu/topaz-investigators).





Comments