-
PDF
- Split View
-
Views
-
Cite
Cite
Aidar R. Gosmanov, Elvira O. Gosmanova, Csaba P. Kovesdy, Evaluation and management of diabetic and non-diabetic hypoglycemia in end-stage renal disease, Nephrology Dialysis Transplantation, Volume 31, Issue 1, January 2016, Pages 8–15, https://doi.org/10.1093/ndt/gfv258
Close - Share Icon Share
Abstract
Patients with end-stage renal disease (ESRD) regardless of diabetes status are at increased risk of hypoglycemia with a resultant array of adverse clinical outcomes. Therefore, hypoglycemia should be thoroughly evaluated in ESRD patients. In diabetic dialysis patients, hypoglycemic agents and nutritional alterations can trigger hypoglycemia in the background of diminished gluconeogenesis, reduced insulin clearance by the kidney and improved insulin sensitivity following initiation of renal replacement therapy. Detailed evaluation of antidiabetic regimen and nutritional patterns, patient education on self-monitoring of blood glucose and/or referral to a diabetes specialist may reduce risk of subsequent hypoglycemia. In certain situations, it is important to recognize the possibility of non-diabetic causes of hypoglycemia in patients with diabetes and to avoid treating pseudo-hyperglycemia caused by glucose- non-specific glucometers in patients utilizing icodextrin-based solutions for peritoneal dialysis. Adrenal insufficiency, certain medications, malnutrition and/or infection are among the most common causes of hypoglycemia in non-diabetic ESRD patients, and they should be suspected after exclusion of inadvertent use of hypoglycemic agents. The goal of this review article is to summarize approaches and recommendations for the work up and treatment of hypoglycemia in ESRD.
INTRODUCTION
Clinical care of patients with end-stage renal disease (ESRD) is complex and often involves management of conditions outside of dialysis provision. In 2012, nearly 40% of incident ESRD patients had diabetes mellitus listed as the primary diagnosis responsible for kidney failure [1]. Both diabetic and non-diabetic patients on renal replacement therapy (RRT) are at high risk for hypoglycemia. A growing body of evidence indicates that hypoglycemia caused by tight glycemic control in patients with diabetes on RRT should be regarded as a serious clinical sequela that affects mortality and morbidity of ESRD patients [2–5]. It is thought that advanced renal failure in diabetes may enhance glycemic variability posing a greater risk of hypoglycemia [6, 7]. Dysregulation of the physiological processes maintaining normal glucose metabolism contribute to the development of hypoglycemia in ESRD [3]. Improved insulin sensitivity following the institution of RRT, increased insulin clearance and diminished gluconeogenesis in the kidney and liver all result in the reduced rate of glucose appearance in the blood, which, in turn, increases dependence on the exogenous sources of carbohydrates [8, 9]. Malnutrition, weight loss and a high incidence of infections may further reduce appearance of glucose from exogenous and endogenous sources, thereby contributing to the development of hypoglycemia in ESRD.
The revived interest to hypoglycemia as a clinical marker of adverse clinical outcomes has been driven by a number of recent reports that showed increased mortality in patients with diabetes who received intensive glycemic therapy [10]. Glucose is a necessary fuel for the brain under physiological states [11]. Severe hypoglycemia may result in acute mental status changes, seizures, coma and death. One study suggested a link between hypoglycemia and increased risk of stroke in patients with renal failure [12]. Cardiac arrhythmias are another potential clinical manifestation of hypoglycemia [10]. ESRD patients can be more vulnerable to the neurological and cardiovascular effects of hypoglycemia due to the high prevalence of cardiovascular disease, dementia and administration of analgesics. The goal of this review is to summarize evidence and describe practical diagnostic and therapeutic approaches to reduce the risk of hypoglycemia in ESRD.
EVALUATION OF HYPOGLYCEMIA IN ESRD PATIENTS WITH DIABETES
Annually, each patient with type 1 diabetes is at risk of experiencing 1–3 episodes and every other person with type 2 diabetes may have at least one episode of severe hypoglycemia [10]. Patients with diabetes and declining kidney function are at even higher risk for hypoglycemia. Decreased insulin clearance, impaired renal gluconeogenesis, malnutrition and hypoglycemic agents are among the main factors predisposing to hypoglycemia in diabetic ESRD [7, 13–16]. It should be noted that current evidence suggests avoiding strict glycemic control in ESRD patients [4, 5]. Targeting hemoglobin A1c between 7.0 and 7.9% is associated with the lowest mortality in dialysis patients [4]. Maintenance hemodialysis can independently contribute to hypoglycemic events in ESRD, and glucose-free dialysate use for hemodialysis should be avoided [17]. The risk of asymptomatic hypoglycemia is highest during the first 24 h after dialysis independent of caloric intake [18]. Improved insulin sensitivity may partially explain this observation. Proper adjustment of the dose of hypoglycemic agents such as reduction of insulin dose, avoidance of missed meals and self-monitoring of blood glucose may lower risk of hypoglycemia.
The choices of hypoglycemic agents for the treatment of diabetes in ESRD are limited (Table 1) [19]. Sulfonylureas, meglitinides and insulin are the most likely to be associated with hypoglycemia in ESRD patients. Glyburide use should be strongly avoided in ESRD as the medication itself and its active metabolites are cleared exclusively by the kidney. For those sulfonylureas and meglitinides that can be used in ESRD patients, the recommended initial dose should be lower than for those with normal renal function [16]. Recent study showed that the risk of hypoglycemia is increased in older type 2 diabetes patients treated with glyburide and glipizide who received antimicrobial agents such as quinolones, macrolides and sulfamethoxazole-trimethoprim [20]. Therefore, caution should be exercised when prescribing antimicrobial agents in older ESRD patients whose diabetes is managed by sulfonylureas.
| Recommended (dose range) . | Not recommended . |
|---|---|
| Sulfonylureas | Sulfonylureas |
| Glipizide IR or Glipizide XR (5–10 mg/d) | Glyburide Gliclizide |
| Glimepiride (1–4 mg/d) | |
| Meglitinides | Biguinides |
| Nateglinide (60–120 mg with meals) | Metformin |
| Repaglinide (0.5–1 mg with meals) | |
| Thiazolidinediones | α-Glucosidase inhibitors |
| Pioglitazone (15–30 mg/d) | Acarbose |
| Miglitol | |
| Voglibose | |
| DPP-4 inhibitors | GLP-1 agonists |
| Alogliptin (6.25 mg/d) | Exenatide |
| Linagliptin (5 mg/d) | Exenatide XR |
| Saxagliptin (2.5 mg/d) | Liraglutide |
| Sitagliptin (25 mg/d) | Albiglutide |
| Vildagliptin (50 mg/d) | Dilaglutide |
| Insulin | SGLT-2 inhibitors |
| Short acting (Human regular) | Canagliflozin |
| Rapid-acting analogs (Aspart, Glulisine, Lispro) | Dapagliflozin Empagliflozin |
| Intermediate- and long-acting (NPH, Glargine, Detemir) | |
| Premixed | |
| Other hypoglycemic agents | |
| Bile acid resin (Colesevalam) | |
| Amylin analog (Pramlitinide) | |
| Dopamine agonist (Bromociptine) (quick release) |
| Recommended (dose range) . | Not recommended . |
|---|---|
| Sulfonylureas | Sulfonylureas |
| Glipizide IR or Glipizide XR (5–10 mg/d) | Glyburide Gliclizide |
| Glimepiride (1–4 mg/d) | |
| Meglitinides | Biguinides |
| Nateglinide (60–120 mg with meals) | Metformin |
| Repaglinide (0.5–1 mg with meals) | |
| Thiazolidinediones | α-Glucosidase inhibitors |
| Pioglitazone (15–30 mg/d) | Acarbose |
| Miglitol | |
| Voglibose | |
| DPP-4 inhibitors | GLP-1 agonists |
| Alogliptin (6.25 mg/d) | Exenatide |
| Linagliptin (5 mg/d) | Exenatide XR |
| Saxagliptin (2.5 mg/d) | Liraglutide |
| Sitagliptin (25 mg/d) | Albiglutide |
| Vildagliptin (50 mg/d) | Dilaglutide |
| Insulin | SGLT-2 inhibitors |
| Short acting (Human regular) | Canagliflozin |
| Rapid-acting analogs (Aspart, Glulisine, Lispro) | Dapagliflozin Empagliflozin |
| Intermediate- and long-acting (NPH, Glargine, Detemir) | |
| Premixed | |
| Other hypoglycemic agents | |
| Bile acid resin (Colesevalam) | |
| Amylin analog (Pramlitinide) | |
| Dopamine agonist (Bromociptine) (quick release) |
DPP-4, dipeptidyl peptidase 4; GLP-1, glucagon-like peptide-1; SGLT-2, sodium-glucose cotransporter 2.
| Recommended (dose range) . | Not recommended . |
|---|---|
| Sulfonylureas | Sulfonylureas |
| Glipizide IR or Glipizide XR (5–10 mg/d) | Glyburide Gliclizide |
| Glimepiride (1–4 mg/d) | |
| Meglitinides | Biguinides |
| Nateglinide (60–120 mg with meals) | Metformin |
| Repaglinide (0.5–1 mg with meals) | |
| Thiazolidinediones | α-Glucosidase inhibitors |
| Pioglitazone (15–30 mg/d) | Acarbose |
| Miglitol | |
| Voglibose | |
| DPP-4 inhibitors | GLP-1 agonists |
| Alogliptin (6.25 mg/d) | Exenatide |
| Linagliptin (5 mg/d) | Exenatide XR |
| Saxagliptin (2.5 mg/d) | Liraglutide |
| Sitagliptin (25 mg/d) | Albiglutide |
| Vildagliptin (50 mg/d) | Dilaglutide |
| Insulin | SGLT-2 inhibitors |
| Short acting (Human regular) | Canagliflozin |
| Rapid-acting analogs (Aspart, Glulisine, Lispro) | Dapagliflozin Empagliflozin |
| Intermediate- and long-acting (NPH, Glargine, Detemir) | |
| Premixed | |
| Other hypoglycemic agents | |
| Bile acid resin (Colesevalam) | |
| Amylin analog (Pramlitinide) | |
| Dopamine agonist (Bromociptine) (quick release) |
| Recommended (dose range) . | Not recommended . |
|---|---|
| Sulfonylureas | Sulfonylureas |
| Glipizide IR or Glipizide XR (5–10 mg/d) | Glyburide Gliclizide |
| Glimepiride (1–4 mg/d) | |
| Meglitinides | Biguinides |
| Nateglinide (60–120 mg with meals) | Metformin |
| Repaglinide (0.5–1 mg with meals) | |
| Thiazolidinediones | α-Glucosidase inhibitors |
| Pioglitazone (15–30 mg/d) | Acarbose |
| Miglitol | |
| Voglibose | |
| DPP-4 inhibitors | GLP-1 agonists |
| Alogliptin (6.25 mg/d) | Exenatide |
| Linagliptin (5 mg/d) | Exenatide XR |
| Saxagliptin (2.5 mg/d) | Liraglutide |
| Sitagliptin (25 mg/d) | Albiglutide |
| Vildagliptin (50 mg/d) | Dilaglutide |
| Insulin | SGLT-2 inhibitors |
| Short acting (Human regular) | Canagliflozin |
| Rapid-acting analogs (Aspart, Glulisine, Lispro) | Dapagliflozin Empagliflozin |
| Intermediate- and long-acting (NPH, Glargine, Detemir) | |
| Premixed | |
| Other hypoglycemic agents | |
| Bile acid resin (Colesevalam) | |
| Amylin analog (Pramlitinide) | |
| Dopamine agonist (Bromociptine) (quick release) |
DPP-4, dipeptidyl peptidase 4; GLP-1, glucagon-like peptide-1; SGLT-2, sodium-glucose cotransporter 2.
In stable diabetic patients on chronic insulin therapy who progressed to ESRD, insulin dose reduction should be advised to avoid hypoglycemia [21]. Some observed a need in 40–50% reduction in daily insulin requirements in attempt to avoid hypoglycemia in type 1 and insulin-dependent type 2 diabetic patients who were followed from the onset of overt nephropathy until the final stage of renal disease [22]. One recent study suggested reducing basal insulin by 25% on the day after dialysis to avoid hypoglycemia [23]. If initiation of insulin therapy is required in insulin-naïve ESRD patients with type 2 diabetes, starting basal insulin 10–12 units once daily with further titration according selected glycemic goals can be safe and efficacious [24]. To date, there are no published clinical studies that evaluated long-term safety and efficacy of basal insulin detemir or glargine in ESRD patients. In hospitalized patients with renal insufficiency, short-term administration of rapid- and long-acting insulin in the dose of 0.25 u/kg/day was safe and effective in the management of diabetes in acute setting [25].
Thiazolidinedione pioglitazone and dipeptidyl peptidase-4 (DPP-4) inhibitors (Table 1) are oral antidiabetic medications characterized by overall the lowest hypoglycemia risk [26]. Pioglitazone prescribed as add-on or monotherapy can reduce hemoglobin A1c by 0.5–1.0% independent of diabetes duration in ESRD patients [27]. History of heart failure, osteoporosis or concerns regarding the worsening of peripheral edema may limit the use of pioglitazone in ESRD [24]. DPP-4 inhibitors appear to be relatively safe and modestly efficient in dialysis patients with type 2 diabetes [26]; however, their clinical utilization can be hampered by their high cost [24]. Studies assessing glycemic efficacy and safety of DPP-4 inhibitors in diabetic ESRD are lacking as dialysis patients are excluded from the long-term outcome studies. In a recent study, a 2-year administration of saxagliptin in pre-dialysis patients reduced hemoglobin A1c to the same extent as in patients with normal renal function, caused less hypoglycemia compared with placebo and was not associated with increase in the risk of ischemic cardiovascular events [28].
Managing hypoglycemia in diabetic ESRD patients can be challenging. ESRD patients with difficult glycemic control should be referred to a diabetes specialist when possible. In addition, providers should address patient education about antidiabetic regimen and hypoglycemia, dietary interventions, medication adjustments and self-monitoring of blood glucose. Reduction in insulin dose and adjustment in oral hypoglycemic agents should be considered in all diabetic ESRD patients experiencing hypoglycemia. It is also possible that some type 2 diabetes patients on dialysis may discontinue all hypoglycemic agents—a phenomenon termed as ‘burnt-out diabetes' [3]. Use of antidiabetic agents with low hypoglycemic potential in the early stages of diabetes management or reduction of the dose of secretagogues can be a safe approach to minimize the risk of hypoglycemia in ESRD. Diabetes education is a valuable strategy for all diabetes patients to create a consistent meal plan matching the administration of hypoglycemic agents and to develop appropriate strategies for self-monitoring of blood glucose [10]. Intensive diabetes education and care management conducted in a dialysis unit can be effective in providing significant improvements in patient outcomes, glycemic control and better quality of life [29]. In patients with recurrent hypoglycemia, other causes of hypoglycemia outside of diabetes management issues should be considered such as adrenal insufficiency, infection and/or non-diabetic medications.
EVALUATION OF HYPOGLYCEMIA IN THE NON-DIABETIC ESRD PATIENT
In a non-diabetic state, biochemical hypoglycemia is defined by a plasma glucose concentration below 55 mg/dL [30]. At this glycemic threshold, counter-regulatory biochemical and clinical responses take place to help restore euglycemia. The presence of biochemical hypoglycemia by itself is not sufficient for the diagnosis of hypoglycemic disorder. The Endocrine Society clinical practice guideline suggests that the diagnosis of hypoglycemic disorder should be made only in those patients in whom Whipple's triad—(i) symptoms and/or signs of hypoglycemia, (ii) plasma glucose concentration below 55 mg/dL and (iii) resolution of the signs or symptoms after the correction of hypoglycemia—is documented [30]. Unfortunately, there is a paucity of literature defining hypoglycemia events in ESRD. During the work-up of hypoglycemia in dialysis patients, we recommend to use guidance from the Endocrine Society recommendations on the evaluation and management of hypoglycemia in adults considering several caveats that are discussed below.
The first event during the physiological response to hypoglycemia is an inhibition of insulin secretion by pancreatic β-cells. Initial biochemical evaluation should include the measurement of blood glucose, insulin and C-peptide. The Endocrine Society considers an insulin concentration of <3 μU/mL and a C-peptide level of <0.2 nmol/L as an expected physiologic response to a blood glucose of <55 mg/dL in a person with normal renal function [30]. However, the interpretation of insulin and C-peptide levels in hypoglycemic patients with ESRD can be challenging. Most of the circulating insulin is metabolized by the kidney and up to 70% of C-peptide, a marker of insulin production, is cleared by the kidney [21]. Fasting levels of insulin and C-peptide in non-diabetic ambulatory ESRD patients are higher than in non-diabetic individuals with normal kidney function. The high baseline insulin level may decrease by 50% following a hemodialysis session likely related to its accelerated clearance [31, 32].
Blood glucose-level evaluation in clinical practice is done with point-of-care glucose meters (POC) or in a central laboratory. Although POC capillary blood glucose measurement provides a rapid result, the analytical accuracy of many POC meters for glucose levels <75 mg/dL is ±15 mg/dL [33]. Also, several clinical variables frequently observed in ESRD such as hypothermia, hypotension, anemia, pH changes and hypoxia can affect the accuracy of the POC method [34]. Therefore, to establish a proper diagnosis of biochemical hypoglycemia, glucose measurement, if possible, should be performed in a central laboratory. The recovery of adrenergic and neuroglycopenic symptoms of hypoglycemia such as palpitations, anxiety, sweating, tremors, slurred speech, hunger, dizziness and/or fainting along with the increase in blood glucose concentration following the provision of exogenous dextrose will further ascertain the diagnosis of hypoglycemia [30]. Patients with ESRD undergoing peritoneal dialysis using icodextrin must use glucose-specific POC glucometers. Maltose is the main icodextrin metabolite and can be measured as ‘glucose’ by nonselective POC devices resulting in falsely elevated blood glucose with subsequent risk of hypoglycemia if patients intensify their insulin treatment in response to the measured hyperglycemia [35].
The etiology of hypoglycemia in non-diabetic ESRD patient can be grouped in conditions associated with decreased or undetectable insulin level, and those with inappropriately high insulin concentration. Malnutrition, alcohol abuse, organ failure, infections, drugs and/or adrenal insufficiency are among the frequently encountered clinical states in which hypoglycemia is most likely to be associated with hypoinsulinemia. Importantly, in clinical practice, hypoglycemia in non-diabetic ESRD is often multifactorial and triggered by more than one event. A review of medical history and physical examination are necessary to identify early clues, suggesting potential etiologies of hypoglycemia in ESRD.
Adrenal insufficiency is one of the clinically important etiologies of hypoglycemia in the general population and, if suspected, requires a prompt diagnosis, as the institution of steroid replacement therapy will result in the immediate improvement of plasma glucose level [36]. Adrenal failure is not infrequent in patients with ESRD. In one study, biochemical diagnosis of adrenal insufficiency was made in 18% of dialysis patients with unexplained hypotension [37]. Primary adrenal insufficiency is caused by direct effect of pathogenic factors such as metastases, infections, hemorrhage, autoimmune adrenalitis and drugs such as ketoconazole, etomidate, phenytoin, phenobarbital, heparin and warfarin [36, 38]. Secondary adrenal insufficiency results from hypothalamic and/or pituitary axis dysfunction. A prior history of exogenous steroid administration, brain injury, pituitary pathology and use of megestrol acetate may provide initial clues towards the diagnosis of hypocortisolism. Sepsis can be associated with relative adrenal insufficiency [39]. Autoimmune forms of adrenal insufficiency are rare and usually associated with other autoimmune diseases such as type 1 diabetes mellitus or Hashimoto's thyroiditis. In addition to the acute onset of hypoglycemia, other clinical manifestations of adrenal insufficiency may include nausea, vomiting, anorexia, abdominal pain and unexplained reductions in blood pressure. The vast majority of ESRD patients have concomitant hypertension requiring drug therapy. Therefore, unexplained reduction in blood pressure requiring reduction of antihypertensive medications along with hypoglycemia could be a sign of adrenal insufficiency. Hence, a high level of clinical suspicion is required to initiate work-up for adrenal insufficiency in ESRD patients.
In ambulatory patients without ESRD, adrenal insufficiency is diagnosed if morning (6–8 am) cortisol level is below 3–5 µg/dL (80–138 nmol/L) [36, 38, 39]. Based on previous evidence of extensive biochemical evaluation of adrenal function in seemingly well subjects, a peak morning or a random cortisol level of >18–20 µg/dL (500–550 nmol/L) essentially excludes the diagnosis of adrenal insufficiency [40]. In critically ill patients, a random cortisol level below 10 µg/dL (275 nmol/L) confirms the diagnosis of adrenal insufficiency [39]. Unless basal cortisol results and the clinical picture are absolutely unequivocal, a corticotropin (ACTH) stimulation test should be performed to confirm the diagnosis of adrenal insufficiency. When administered intravenously or intramuscularly in an individual with normal adrenal function, a pharmacological dose (250 µg) of exogenous corticotropin should result in a 30- or 60-min post-infusion cortisol level above 18–20 µg/dL (500–550 nmol/L) [39, 41]. The results of the test are not affected by diurnal cortisol variations, and hence, it can be performed without time constraints [42]. Caution is recommended in the interpretation of cortisol levels in certain clinical states that alter levels of cortisol-binding globulin (CBG), a major cortisol transporting protein, because standard laboratory assays measure total cortisol level that mostly reflects CBG-bound cortisol [39]. Liver cirrhosis, hypoalbuminemia in the malnourished, critical illness, nephrotic syndrome and high estrogen states during pregnancy and estrogen replacement alter affect CBG level and may result in inappropriately low or high plasma cortisol concentration [39, 43]. Once the diagnosis of adrenal insufficiency is established, glucocorticosteroid replacement will restore euglycemia along with other clinical parameters. Acutely ill patients with adrenal insufficiency will require intravenous administration of hydrocortisone in the dose of no more than 50 mg every 6 h followed by the reassessment of steroid replacement in 2–3 days after the commencement of therapy; such empiric hydrocortisone replacement regimen should not cause significant side effects [41]. In patients with known history of glucocorticosteroid deficiency, the decision on the intensity of hydrocortisone therapy during the acute illness should be made based on a combination of factors that include patients' past history and severity of intercurrent illness [44]. Clinicians should consider giving lower stress doses of hydrocortisone in the amount of 25 mg every 8–12 h for those patients who experience a mild to moderate degree of stress. In chronic adrenal failure patients, the recommended replacement regimen is hydrocortisone at the dose equivalent to 10–12 mg/m2 divided in 2–3 doses per day [41]. Fludrocortisone replacement in the dose 0.1 mg daily is advised for non-ESRD patients with primary adrenal insufficiency to substitute for the lost mineralocorticoid function [39]. However, small studies showed lack of therapeutic efficacy of fludrocortisone in the management of hyperkalemia in ESRD [45]. Future prospective studies should aim to evaluate prevalence of adrenal insufficiency in non-diabetic ESRD patients with history of otherwise unexplained hypoglycemia.
Once adrenal insufficiency has been ruled out in non-diabetic ESRD patients with low plasma insulin levels, an assessment for other causes of hypoglycemia should be performed. In the presence of appropriately suppressed insulin and C-peptide levels, insulin-like growth factor-II producing tumors and insulin receptor stimulating antibodies can cause hypoglycemia, but these conditions are extremely rare in the general population [30, 46]. In our opinion, in the absence of adrenal insufficiency, hypoinsulinemic hypoglycemia in ESRD patients will be multifactorial due to causes such as malnutrition or multiorgan dysfunction. Pending the implementation of multifaceted therapies to improve underlying medical conditions, nutrition therapy should be started to restore and maintain euglycemia, such as initiation of nutritional supplements containing 100–150 g of carbohydrates daily [47]. Reduced appetite is common in dialysis patients and can contribute to malnutrition. Management of anorexia should be initiated by addressing the treatment of the underlying medical problems when possible [48]. The appetite stimulant megestrol acetate has been shown to increase serum albumin levels and anthropometric indexes [49]. However, clinicians should be aware of the possibility of adrenal insufficiency in patients who receive chronic megestrol acetate which can occur even after discontinuation of megestrol [50, 51]. Anecdotal evidence suggests that antidepressant mirtazapine and antiemetic agent dronabinol can be used as appetite stimulants without risk of hypoglycemia.
High levels of plasma insulin level in a patient with non-diabetic hypoglycemia suggest the presence of endogenous or exogenous hyperinsulinism [30]. The etiologies of hypoglycemia associated with overproduction of endogenous insulin are insulinoma, hyperplasia of pancreatic β-cells and endogenous insulin release due to inadvertent or surreptitious use of insulin secreatogogues. Diagnostic clues suggesting the presence of endogenous hyperinsulinemia include hypoglycemia and simultaneously elevated insulin and C-peptide levels. In subjects with the normal renal function in whom insulinoma is suspected, plasma insulin above 3 μU/mL when blood glucose is below 55 mg/dL may suggest inappropriate insulin production. To our knowledge, insulinoma has been reported in a man with Stage 4 chronic kidney disease whose insulin concentration during symptomatic hypoglycemia episode was 52 μU/mL [52] and in a man with renal insufficiency on peritoneal dialysis [53]. No cases of insulinoma were reported in hemodialysis patients. To further reflect on the challenges in the interpretation of insulin levels in hemodialysis patients, it was shown that the average pre-dialysis fasting plasma insulin concentration was 21.6 μU/mL that was 3- to 4-fold higher than in control patients with normal renal function [32].
Careful evaluation of past medical and family history may prompt investigations regarding iatrogenic or inadvertent administration of oral hypoglycemic agents in non-diabetic patients. A serum hypoglycemia screen that detects sulfonylureas and meglitinides metabolites is indicated in those patients in whom hypoglycemia is suspected to be due to the accidental or surreptitious use of these hypoglycemic agents [30]. An accumulating body of evidence demonstrates that antimicrobial therapy may result in non-diabetic hypoglycemia [54]. Although it is unclear whether underlying infectious processes contribute to the dysglycemic effect of antibiotics, some evidence suggests a direct effect of these medications on glucose homeostasis [54]. About 1–6% of patients exposed to quinolones can develop hypoglycemia. It is thought that quinolones exert direct β-cell action that results in hyperinsulinemia [55]. Quinine and pentamidine use is also associated with hypoglycemia, but the quality of evidence supporting this association is weak [54]. Severe hypoglycemia associated with elevated concentration of plasma insulin due to trimethoprim–sulfamethoxazole administration has been recently described in two patients with liver and renal insufficiency [56]. Therefore, elevation of both plasma insulin and C-peptide concentration may prompt one to suspect the diagnosis of antibiotic-mediated hypoglycemia in ESRD patients who recently initiated antimicrobial agents.
Medication errors or surreptitious use of insulin in non-diabetic individuals can result in hypoglycemia due to exogenous hyperinsulinemia. Biochemical characteristics that distinguish hypoglycemia due to exogenous insulin administration from any other causes of hypoglycemia include the presence of very high plasma insulin level in association with suppressed C-peptide concentration [30]. Of note, currently used plasma insulin assays can measure both endogenous and exogenous insulin. Non-diabetic patients with ESRD are very sensitive to insulin. This is especially important for those ESRD patients who can receive insulin during medical treatment of hyperkalemia. It has been reported that hypoglycemia may occur in 13% of ESRD patients during hyperkalemia treatment [57]. Therefore, when administering the standard 25 g of dextrose parenterally (50 mL of 50% dextrose), the dose of intravenous insulin should be reduced to 5–6 units of regular insulin followed by close blood glucose monitoring every 1 h during the first 2–3 h after insulin administration. Concentration of glucose in dialysate solution may also affect blood glucose levels in both diabetic and non-diabetic ESRD patients. The majority of dialysis chains utilize dialyzate with a glucose concentration of 100 mg/dL based on the data suggesting that glucose-free dialyzate predisposes to hypoglycemia [17]; the use of dialyzate with a higher glucose concentration (200 mg/dL) is declining due to increased risk of associated adverse metabolic effects including risk of hyperglycemia [58]. In patients with hypoglycemia, providers may consider using a higher dialyzate glucose concentration to raise blood glucose level secondary to positive glucose flux during dialysis.
In summary, several clinical states known to potentially cause hypoglycemia in non-diabetic ESRD patients should be recognized. Adrenal insufficiency, infection, malnutrition and/or administration of certain medications can reduce plasma glucose (Table 2). These conditions are not mutually exclusive and can occur simultaneously in the same patient. In the outpatient setting, in individuals who appear seemingly well, Whipple's triad should be documented to confirm the hypoglycemic disorder before initiation of extensive work-up. On the other hand, in acutely ill patients, the evaluation of signs and symptoms of hypoglycemia is difficult; hence, extensive biochemical evaluation of low plasma glucose should be commenced as soon as possible.
Common clinical conditions that may result in hypoglycemia in non-diabetic ESRD
|
|
Common clinical conditions that may result in hypoglycemia in non-diabetic ESRD
|
|
GENERAL PRINCIPLES OF ACUTE HYPOGLYCEMIA MANAGEMENT IN ESRD
Antidiabetic agents are likely to be the most common cause of hypoglycemia in hospitalized ESRD patients with diabetes. A culprit agent should be discontinued permanently if deemed appropriate or temporarily with subsequent dose reduction. Acute situations, whether in the hospital or in a dialysis unit, dictate the use of any available sugar source to remedy acute hypoglycemia independent of the subject's diabetes status. Approximately 15–20 g of dextrose typically should restore mild to moderate hypoglycemia in adults (Table 3). Examples of oral treatment are 1 tube of glucose gel, 3–4 glucose tablets or 4 oz. beverages such as fruit juice, regular soft drink or 8 oz. skimmed milk. It is important to remember that a single treatment may not reverse hypoglycemia. All patients should be retested in 15 min after 15 g of dextrose therapy. If subsequent blood glucose remains below 70 mg/dL, the treatment should be repeated. In patients who experience recurrent hypoglycemia in a dialysis unit, consideration should be given for their transfer to a higher level of medical care.
Principles of hypoglycemia management in non-diabetic and diabetic ESRD patients
| 1. Managing acute hypoglycemia: |
|
| 2. Preventing and managing recurrent hypoglycemia: |
|
| 1. Managing acute hypoglycemia: |
|
| 2. Preventing and managing recurrent hypoglycemia: |
|
Principles of hypoglycemia management in non-diabetic and diabetic ESRD patients
| 1. Managing acute hypoglycemia: |
|
| 2. Preventing and managing recurrent hypoglycemia: |
|
| 1. Managing acute hypoglycemia: |
|
| 2. Preventing and managing recurrent hypoglycemia: |
|
In patients who are not able to swallow, intravenous glucose infusion of 25 g of concentrate dextrose should raise blood glucose; the maneuver should be repeated in 15 min if the patient remains hypoglycemic. Sulfonylurea-induced hypoglycemia is particularly difficult to treat in ESRD patients and may require administration of a continuous dextrose infusion. A higher dextrose concentration (e.g. a 10% solution) may be better suited for ESRD patients to avoid water overload. In subjects with normal renal function, octreotide therapy is effective in managing hypoglycemia after sulfonylurea overdose; however, in ESRD patients, octreotide should be administered at a reduced dose and very briefly to avoid hyperkalemia [59]. If intravenous access is not available, 1-mg glucagon injection is preferred. In malnourished patients, glucagon efficacy may be limited due to poor liver glycogen reserves. Once adrenal failure is diagnosed in non-diabetic hypoglycemia, initiation of glucocorticosteroid therapy will result in rapid restoration of blood glucose levels. Identification bracelets for adrenal insufficiency patients and education of caregivers about glucagon administration should be considered in outpatients. At the time of hospital discharge, diabetic ESRD patients should have their glycemic regimen reviewed and adjusted, as blunt discontinuation of all antidiabetic drugs can lead to subsequent hyperglycemia.
CONCLUSION
Hypoglycemia evaluation in ESRD patients is challenging due to its multifactorial nature related to renal disease and associated medical conditions. Figure 1 summarizes the proposed flow of diagnostic steps in the work-up of hypoglycemia in ESRD. In ESRD patients with diabetic hypoglycemia, reassessment of hypoglycemic therapy, optimization of nutrition, and search for concomitant conditions that may cause low blood glucose such as infections and adrenal insufficiency is imperative. Screening for adrenal insufficiency and evaluation for malnutrition and infections are central in the evaluation of hypoglycemia in non-diabetic patients. Measurement of insulin and C-peptide levels will help to determine whether administration of medications that raise insulin level has occurred. In the absence of better clinical trial data, the practice of hypoglycemia evaluation and management in ESRD should be based on individualized decision making.
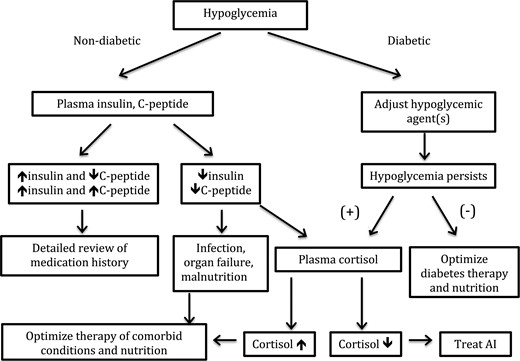
Algorithm for the diagnostic approach to hypoglycemia in ESRD patients. AI, adrenal insufficiency.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this paper have not been published previously in whole or part, except in abstract format.
SEARCH CRITERIA
Full-text articles written in English and published between January 1970 and December 2014 were identified using the PubMed database. The combinations of the following search terms were used: ‘hypoglycemia’, ‘end-stage renal disease’, ‘diabetes' and ‘adrenal insufficiency’.
ACKNOWLEDGEMENTS
A.R.G. has received research support (to the University of Tennessee HSC) for studies from Sanofi and Novo Nordisk and has received honoraria for lectures from AstraZeneca and Janssen Pharmaceuticals. E.O.G. and C.P.K. reported no potential conflicts of interest relevant to this article.





Comments