-
PDF
- Split View
-
Views
-
Cite
Cite
Iain C. Macdougall, Nicole Casadevall, Francesco Locatelli, Christian Combe, Gerard M. London, Salvatore Di Paolo, Andreas Kribben, Danilo Fliser, Hans Messner, John McNeil, Paul Stevens, Antonio Santoro, Angel L.M. De Francisco, Paul Percheson, Anna Potamianou, Arnaud Foucher, Daniel Fife, Véronique Mérit, Els Vercammen, on behalf of the PRIMS study group, Incidence of erythropoietin antibody-mediated pure red cell aplasia: the Prospective Immunogenicity Surveillance Registry (PRIMS), Nephrology Dialysis Transplantation, Volume 30, Issue 3, March 2015, Pages 451–460, https://doi.org/10.1093/ndt/gfu297
Close - Share Icon Share
Abstract
Subcutaneous administration of Eprex® (epoetin alfa) in patients with chronic kidney disease (CKD) was contraindicated in the European Union between 2002 and 2006 after increased reports of anti-erythropoietin antibody-mediated pure red cell aplasia (PRCA). The Prospective Immunogenicity Surveillance Registry (PRIMS) was conducted to estimate the incidence of antibody-mediated PRCA with subcutaneous administration of a new coated-stopper syringe presentation of Eprex® and to compare this with the PRCA incidence with subcutaneous NeoRecormon® (epoetin beta) and Aranesp® (darbepoetin alfa).
PRIMS was a multicentre, multinational, non-interventional, parallel-group, immunogenicity surveillance registry. Adults with CKD receiving or about to initiate subcutaneous Eprex®, NeoRecormon® or Aranesp® for anaemia were enrolled and followed for up to 3 years. Unexplained loss or lack of effect (LOE), including suspected PRCA, was reported, with antibody testing for confirmation of PRCA.
Of the 15 333 patients enrolled, 5948 received Eprex® (8377 patient-years) and 9356 received NeoRecormon®/Aranesp® (14 286 patient-years). No treatment data were available for 29 patients. Among 23 patients with LOE, five cases of PRCA were confirmed (Eprex®, n = 3; NeoRecormon®, n = 1; Aranesp®, n = 1). Based on exposed time, PRCA incidence was 35.8/100 000 patient-years (95% CI 7.4–104.7) for Eprex® versus 14.0/100 000 patient-years (95% CI 1.7–50.6) for NeoRecormon®/Aranesp®. The incidence of PRCA with Eprex® was not significantly different versus comparator ESAs (rate ratio: 2.56; 95% CI 0.43–15.31). An analysis based on observed time produced similar findings.
This large, prospective registry demonstrates that PRCA is rare with subcutaneous administration of either the new coated-stopper syringe presentation of Eprex®, or NeoRecormon® or Aranesp®.
INTRODUCTION
Pure red cell aplasia (PRCA) is a rare haematological disorder characterized by severe and progressive normocytic, normochromic anaemia of sudden onset, reticulocytopenia and an almost complete absence of erythroid precursor cells in the bone marrow [1]. In patients receiving erythropoiesis-stimulating agents (ESAs), PRCA may occur secondary to the development of neutralizing anti-erythropoietin antibodies (Abs) [2], which block the interaction of both ESAs and endogenous erythropoietin (EPO) with the EPO receptor [1].
In the decade following the introduction of recombinant human EPO for treatment of renal anaemia in 1986, hundreds of thousands of patients received ESA therapy [3] and only three cases of ESA-associated PRCA were published [4]. However, the number of cases of neutralizing EPO Ab-mediated severe anaemia in CKD patients began to rise substantially in 1998 and increased progressively to peak in 2001, before declining in 2003 [5–7]. All cases during this period occurred in patients who received subcutaneous (SC) administration of an ESA and almost all had received Eprex® epoetin alfa [5, 6]. This transient increase between 1998 and 2003 with Eprex® was associated with the use of one product presentation—the polysorbate-80 (PS-80) formulation in prefilled syringes with uncoated rubber stoppers (1000–4000 and 10 000 IU strengths) [6, 8, 9]—which was introduced in 1998 to replace human serum albumin as a stabilizer and so avoid the hypothetical risk of virus/prion transmission [1].
For 2001–2003 the rate of reporting of PRCA in patients exposed to SC PS-80 Eprex® in prefilled syringes and uncoated stoppers was 46.1/100 000 patient-years (PY; 95% CI 38.8–54.3) versus 2.6/100 000 PY (95% CI 0.07–14.4; P < 0.0001) for coated-stopper syringe formulations [8]. In a study that reviewed US Food and Drug Administration reports of ESA-associated PRCA between January 1988 and April 2004, PRCA incidence was 18/100 000 PY for Eprex® with PS-80, 6/100 000 PY for Eprex® with human serum albumin, 1/100 000 PY for NeoRecormon® (epoetin beta), and 0.2/100 000 PY Epogen® epoetin alfa with human serum albumin [7]. Following the introduction of Aranesp® (darbepoetin alfa) in 2001 some cases of PRCA have been reported in patients with CKD who received this agent as their only ESA [10–12].
The mechanism of Ab-mediated PRCA remains elusive [13]. It is proposed that compounds with adjuvant activity leached by PS-80 from plastics and rubber materials in uncoated stoppers [8, 14] induced an anti-EPO immune response [1, 6, 15]. However, others have questioned whether adjuvants alone are sufficient for formation of self-reactive Abs [16]. Alternative potential explanations include protein denaturation and aggregation from tungsten contaminants, or lower stability of the PS-80 formulation versus the previous formulation and thus increased susceptibility to, for example, cold-chain interruption [16, 17].
Pending confirmatory evidence, SC administration of Eprex® to patients with CKD was contraindicated in the European Union (EU) in 2002 for interim risk mitigation. Following a worldwide withdrawal of Eprex® syringes with uncoated rubber stoppers in 2004, the Eprex® PS-80 formulation was reintroduced with coated-stopper presentations and in 2006 was approved in Europe for SC administration in patients with CKD for whom intravenous (IV) access was not readily available. Since that reintroduction and reinforcement of the cold-chain [17], the incidence of reported ESA Ab-mediated PRCA associated with SC administration of Eprex® in patients with CKD has fallen dramatically worldwide (except in Thailand, where local factors may explain a higher rate of PRCA reported with epoetin alfa [18–20]). In 2005, estimated PRCA rates reported with Eprex®, Epogen®, NeoRecormon® and Aranesp® were 0.2–0.3/100 000 PY, suggesting that PRCA is now rare with these agents [4]. However, post-marketing adverse event reporting is often incomplete and PRCA rates among CKD patients receiving SC ESAs have not been prospectively quantified.
The EU post-approval commitment following reinstatement of Eprex® for SC use included the Prospective Immunogenicity Surveillance Registry (PRIMS) as part of the Eprex® Risk Management Plan. PRIMS was designed to estimate the incidence of EPO Ab-mediated PRCA among patients with CKD and provide assurance that the Eprex® coated-stopper PS-80 formulation has an acceptable immunogenicity profile when administered subcutaneously in this setting. Reference therapies included the two recombinant ESAs marketed at the time of registry initiation: Aranesp® and NeoRecormon®.
MATERIALS AND METHODS
Study design and objectives
This non-interventional immunogenicity surveillance registry employed a prospective cohort design with enrolment of parallel groups across 751 sites in Europe and Australia (Figure 1; www.clinicaltrials.gov identifier, NCT00391287; registered 20 October 2006). The registry was conducted in accordance with the Declaration of Helsinki. Patients provided written, informed consent if required by local regulations.
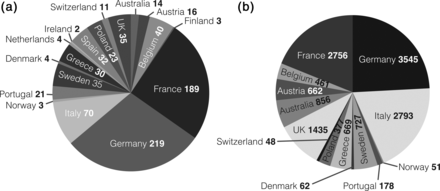
Distribution of (a) the 751 sites from Europe and Australia that participated in the registry and (b) patients enrolled per country.
The primary objective was to estimate the incidence rate of EPO Ab-mediated PRCA with SC administration of the coated-stopper syringe Eprex® PS-80 formulation and to compare this with the incidence rate with other marketed ESAs (NeoRecormon® and Aranesp®). The secondary objective was to employ sensitivity analyses to examine the effect of varying the assumed latency of PRCA onset on incidence rates and incidence rate ratios of EPO Ab-mediated PRCA.
Patients
Eligible patients were those aged ≥18 years with documented CKD (stages 1–5, including stage 5D [21]) receiving SC Eprex®, NeoRecormon® or Aranesp® at enrolment or who were due to start such treatment within 1 month of enrolment, and considered likely to continue treatment for ≥1 year. Peritoneal dialysis, haemodialysis and non-dialysis patients were eligible. Exclusion criteria included: >1 year since first SC exposure to any ESA; history of PRCA; prior loss or lack of effect (LOE) or unexplained, ongoing LOE with an ESA; and immunosuppressive therapy (including transplant recipients).
Patients were enrolled through their CKD healthcare provider and observed for up to 3 years. Following registry initiation, CKD healthcare providers were asked to review all non-enrolled patients seen subsequently for eligibility.
Treatment
All ESAs and concomitant medications were administered as part of standard treatment and were expected to be consistent with standard practice guidelines and local marketing authorizations. The sponsor (Janssen, Pharmaceutical Companies of Johnson & Johnson) did not supply any ESAs or other medications.
Completion and withdrawal
Patient participation was completed in the following circumstances: completion of 3 years of follow-up, completion of 1 year of follow-up after SC ESA discontinuation, permanent switching from SC to IV ESA administration, initiation of immunosuppressive therapy (e.g. following organ transplantation), or administration of any non-registry ESA. Patients in the registry at the time of its termination were also considered completers. Patients were withdrawn due to loss to follow-up, withdrawal of consent or death.
Outcomes
Data on ESA exposure, handling and storage; stage and treatment for CKD; and most recent Hb values were collected quarterly from patient notes. LOE was defined as loss or lack of therapeutic response, therapeutic response decrease, PRCA, or EPO Ab-positivity. In such cases, the treating physician completed a PRCA-specific questionnaire, including medical history, investigations for other causes of LOE/PRCA, and results of relevant tests including iron stores, complete blood count, Hb, reticulocytes, and bone marrow examination (if performed). All usual causes of anaemia or LOE were excluded before patients were deemed to have unexplained LOE. Unexplained LOE, including suspected PRCA, was reported as a serious adverse event (SAE).
EPO Ab testing was recommended for suspected PRCA. Confirmation of EPO Ab-mediated PRCA required demonstration of EPO Abs by radio-immunoprecipitation, neutralization and/or other validated assays in a patient with unexplained LOE. Potential (Ab-positive and Ab-borderline) cases of PRCA, including date of LOE onset, were adjudicated by a treatment-blinded Independent Case Adjudication Committee.
Only ESA drug-related SAE reporting was mandatory. Collection of other adverse events (AEs) and unrelated SAEs were left to investigator discretion or local health authority regulations.
Data analysis
Throughout this report, ‘ESA exposure’ refers to SC exposure unless otherwise stated. Incidence rates of LOE and PRCA were calculated based on both exposed time (time during the registry in which the patient received a specific ESA) and observed time (time at risk of development of PRCA assuming a latency of 1–12 months). Incidence rates of Ab-mediated PRCA were adjusted for duration of ESA exposure, with CIs calculated using the Poisson distribution for rare events. Statistical significance was assessed at the 5% level. A sample size of ≥20 000 PY of exposure to Eprex® and ≥20 000 PY for comparators was planned. Assuming a background rate of EPO Ab-mediated PRCA of 10/100 000 PY for SC exposure to all ESAs, this would provide 0.50 power (α = 0.05, one-sided, two-sample Poisson) to detect a 4-fold greater incidence of EPO Ab-mediated PRCA with PS-80 Eprex® versus comparators.
RESULTS
Patient population
Between June 2006 and December 2010 15 333 patients were enrolled (Figure 1), of whom 5948 received Eprex® and 9356 received Aranesp®/NeoRecormon®. Treatment data were unavailable for 29 patients. As agreed with health authorities, the registry was terminated early by concluding follow-up of all ongoing patients on 31 December 2010, due to decreasing recruitment, the impact of ESA switching, and the commercial availability of ESA biosimilars.
The median age of the patients was 73 years and 56.5% were male (Table 1). Most patients (80.5%) were non-dialysis at enrolment. Of those on dialysis, 74.5% received haemodialysis and 25.5% peritoneal dialysis (Figure 2). Except for differences related to dialysis, Eprex® and comparator subjects were similar at enrolment. At the initial visit, 43.3% of patients received the ESA by self-administration and 74.5% stored their ESA at home (Supplementary Table S1).
| . | Overall (N = 15 333) . | Eprex® (n = 5948) . | Aranesp® (n = 5974) . | NeoRecormon® (n = 3382) . | Aranesp® plus NeoRecormon® (n = 9356) . | No treatment data available at baseline (n = 29) . |
|---|---|---|---|---|---|---|
| Median age, years | 73.0 | 74.0 | 72.0 | 73.0 | 72.0 | 81.0 |
| Male, n (%) | 8669 (56.5) | 3360 (56.5) | 3395 (56.8) | 1898 (56.1) | 5293 (56.6) | 16 (55.2) |
| Dialysis, n (%) | ||||||
| No | 12 345 (80.5) | 4903 (82.4) | 4974 (83.3) | 2439 (72.1) | 7413 (79.2) | 29 (100) |
| Yes | 2988 (19.5) | 1045 (17.6) | 1000 (16.7) | 943 (27.9) | 1943 (20.8) | N/A |
| Haemodialysis | 2226 (74.5) | 876 (83.8) | 613 (61.3) | 737 (78.2) | 1350 (69.5) | N/A |
| Peritoneal dialysis | 762 (25.5) | 169 (16.2) | 387 (38.7) | 206 (21.8) | 593 (30.5) | N/A |
| Cause of CKD, n (%) | ||||||
| Analgesic drug abuse | 113 (0.7) | 56 (0.9) | 39 (0.7) | 17 (0.5) | 56 (0.6) | 1 (3.4) |
| Diabetic nephropathy | 4463 (29.1) | 1750 (29.4) | 1682 (28.2) | 1025 (30.3) | 2707 (28.9) | 6 (20.7) |
| Glomerulonephritis | 1620 (10.6) | 517 (8.7) | 714 (12.0) | 388 (11.5) | 1102 (11.8) | 1 (3.4) |
| Multifactorial | 28 (0.2) | 10 (0.2) | 7 (0.1) | 11 (0.3) | 18 (0.2) | N/A |
| Polycystic/multicystic kidney disease | 731 (4.8) | 274 (4.6) | 294 (4.9) | 162 (4.8) | 456 (4.9) | 1 (3.4) |
| Pyelonephritis/interstitial nephritis | 1122 (7.3) | 420 (7.1) | 462 (7.7) | 239 (7.1) | 701 (7.5) | 1 (3.4) |
| Renovascular disease/hypertension | 5028 (32.8) | 2081 (35.0) | 1877 (31.4) | 1058 (31.3) | 2935 (31.4) | 12 (41.4) |
| Other | 394 (2.6) | 134 (2.3) | 156 (2.6) | 104 (3.1) | 260 (2.8) | N/A |
| Unknown | 1834 (12.0) | 706 (11.9) | 743 (12.4) | 378 (11.2) | 1121 (12.0) | 7 (24.1) |
| . | Overall (N = 15 333) . | Eprex® (n = 5948) . | Aranesp® (n = 5974) . | NeoRecormon® (n = 3382) . | Aranesp® plus NeoRecormon® (n = 9356) . | No treatment data available at baseline (n = 29) . |
|---|---|---|---|---|---|---|
| Median age, years | 73.0 | 74.0 | 72.0 | 73.0 | 72.0 | 81.0 |
| Male, n (%) | 8669 (56.5) | 3360 (56.5) | 3395 (56.8) | 1898 (56.1) | 5293 (56.6) | 16 (55.2) |
| Dialysis, n (%) | ||||||
| No | 12 345 (80.5) | 4903 (82.4) | 4974 (83.3) | 2439 (72.1) | 7413 (79.2) | 29 (100) |
| Yes | 2988 (19.5) | 1045 (17.6) | 1000 (16.7) | 943 (27.9) | 1943 (20.8) | N/A |
| Haemodialysis | 2226 (74.5) | 876 (83.8) | 613 (61.3) | 737 (78.2) | 1350 (69.5) | N/A |
| Peritoneal dialysis | 762 (25.5) | 169 (16.2) | 387 (38.7) | 206 (21.8) | 593 (30.5) | N/A |
| Cause of CKD, n (%) | ||||||
| Analgesic drug abuse | 113 (0.7) | 56 (0.9) | 39 (0.7) | 17 (0.5) | 56 (0.6) | 1 (3.4) |
| Diabetic nephropathy | 4463 (29.1) | 1750 (29.4) | 1682 (28.2) | 1025 (30.3) | 2707 (28.9) | 6 (20.7) |
| Glomerulonephritis | 1620 (10.6) | 517 (8.7) | 714 (12.0) | 388 (11.5) | 1102 (11.8) | 1 (3.4) |
| Multifactorial | 28 (0.2) | 10 (0.2) | 7 (0.1) | 11 (0.3) | 18 (0.2) | N/A |
| Polycystic/multicystic kidney disease | 731 (4.8) | 274 (4.6) | 294 (4.9) | 162 (4.8) | 456 (4.9) | 1 (3.4) |
| Pyelonephritis/interstitial nephritis | 1122 (7.3) | 420 (7.1) | 462 (7.7) | 239 (7.1) | 701 (7.5) | 1 (3.4) |
| Renovascular disease/hypertension | 5028 (32.8) | 2081 (35.0) | 1877 (31.4) | 1058 (31.3) | 2935 (31.4) | 12 (41.4) |
| Other | 394 (2.6) | 134 (2.3) | 156 (2.6) | 104 (3.1) | 260 (2.8) | N/A |
| Unknown | 1834 (12.0) | 706 (11.9) | 743 (12.4) | 378 (11.2) | 1121 (12.0) | 7 (24.1) |
| . | Overall (N = 15 333) . | Eprex® (n = 5948) . | Aranesp® (n = 5974) . | NeoRecormon® (n = 3382) . | Aranesp® plus NeoRecormon® (n = 9356) . | No treatment data available at baseline (n = 29) . |
|---|---|---|---|---|---|---|
| Median age, years | 73.0 | 74.0 | 72.0 | 73.0 | 72.0 | 81.0 |
| Male, n (%) | 8669 (56.5) | 3360 (56.5) | 3395 (56.8) | 1898 (56.1) | 5293 (56.6) | 16 (55.2) |
| Dialysis, n (%) | ||||||
| No | 12 345 (80.5) | 4903 (82.4) | 4974 (83.3) | 2439 (72.1) | 7413 (79.2) | 29 (100) |
| Yes | 2988 (19.5) | 1045 (17.6) | 1000 (16.7) | 943 (27.9) | 1943 (20.8) | N/A |
| Haemodialysis | 2226 (74.5) | 876 (83.8) | 613 (61.3) | 737 (78.2) | 1350 (69.5) | N/A |
| Peritoneal dialysis | 762 (25.5) | 169 (16.2) | 387 (38.7) | 206 (21.8) | 593 (30.5) | N/A |
| Cause of CKD, n (%) | ||||||
| Analgesic drug abuse | 113 (0.7) | 56 (0.9) | 39 (0.7) | 17 (0.5) | 56 (0.6) | 1 (3.4) |
| Diabetic nephropathy | 4463 (29.1) | 1750 (29.4) | 1682 (28.2) | 1025 (30.3) | 2707 (28.9) | 6 (20.7) |
| Glomerulonephritis | 1620 (10.6) | 517 (8.7) | 714 (12.0) | 388 (11.5) | 1102 (11.8) | 1 (3.4) |
| Multifactorial | 28 (0.2) | 10 (0.2) | 7 (0.1) | 11 (0.3) | 18 (0.2) | N/A |
| Polycystic/multicystic kidney disease | 731 (4.8) | 274 (4.6) | 294 (4.9) | 162 (4.8) | 456 (4.9) | 1 (3.4) |
| Pyelonephritis/interstitial nephritis | 1122 (7.3) | 420 (7.1) | 462 (7.7) | 239 (7.1) | 701 (7.5) | 1 (3.4) |
| Renovascular disease/hypertension | 5028 (32.8) | 2081 (35.0) | 1877 (31.4) | 1058 (31.3) | 2935 (31.4) | 12 (41.4) |
| Other | 394 (2.6) | 134 (2.3) | 156 (2.6) | 104 (3.1) | 260 (2.8) | N/A |
| Unknown | 1834 (12.0) | 706 (11.9) | 743 (12.4) | 378 (11.2) | 1121 (12.0) | 7 (24.1) |
| . | Overall (N = 15 333) . | Eprex® (n = 5948) . | Aranesp® (n = 5974) . | NeoRecormon® (n = 3382) . | Aranesp® plus NeoRecormon® (n = 9356) . | No treatment data available at baseline (n = 29) . |
|---|---|---|---|---|---|---|
| Median age, years | 73.0 | 74.0 | 72.0 | 73.0 | 72.0 | 81.0 |
| Male, n (%) | 8669 (56.5) | 3360 (56.5) | 3395 (56.8) | 1898 (56.1) | 5293 (56.6) | 16 (55.2) |
| Dialysis, n (%) | ||||||
| No | 12 345 (80.5) | 4903 (82.4) | 4974 (83.3) | 2439 (72.1) | 7413 (79.2) | 29 (100) |
| Yes | 2988 (19.5) | 1045 (17.6) | 1000 (16.7) | 943 (27.9) | 1943 (20.8) | N/A |
| Haemodialysis | 2226 (74.5) | 876 (83.8) | 613 (61.3) | 737 (78.2) | 1350 (69.5) | N/A |
| Peritoneal dialysis | 762 (25.5) | 169 (16.2) | 387 (38.7) | 206 (21.8) | 593 (30.5) | N/A |
| Cause of CKD, n (%) | ||||||
| Analgesic drug abuse | 113 (0.7) | 56 (0.9) | 39 (0.7) | 17 (0.5) | 56 (0.6) | 1 (3.4) |
| Diabetic nephropathy | 4463 (29.1) | 1750 (29.4) | 1682 (28.2) | 1025 (30.3) | 2707 (28.9) | 6 (20.7) |
| Glomerulonephritis | 1620 (10.6) | 517 (8.7) | 714 (12.0) | 388 (11.5) | 1102 (11.8) | 1 (3.4) |
| Multifactorial | 28 (0.2) | 10 (0.2) | 7 (0.1) | 11 (0.3) | 18 (0.2) | N/A |
| Polycystic/multicystic kidney disease | 731 (4.8) | 274 (4.6) | 294 (4.9) | 162 (4.8) | 456 (4.9) | 1 (3.4) |
| Pyelonephritis/interstitial nephritis | 1122 (7.3) | 420 (7.1) | 462 (7.7) | 239 (7.1) | 701 (7.5) | 1 (3.4) |
| Renovascular disease/hypertension | 5028 (32.8) | 2081 (35.0) | 1877 (31.4) | 1058 (31.3) | 2935 (31.4) | 12 (41.4) |
| Other | 394 (2.6) | 134 (2.3) | 156 (2.6) | 104 (3.1) | 260 (2.8) | N/A |
| Unknown | 1834 (12.0) | 706 (11.9) | 743 (12.4) | 378 (11.2) | 1121 (12.0) | 7 (24.1) |
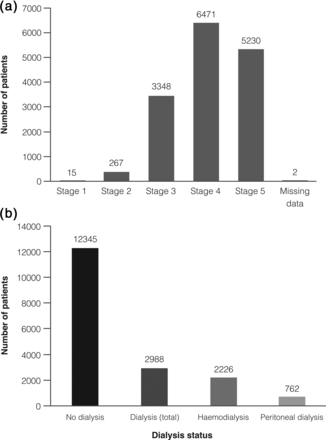
K/DOQI CKD stage (a) and dialysis status (b) at enrolment. CKD, chronic kidney disease; K/DOQI, Kidney Disease Outcomes Quality Initiative.
Erythropoietin-stimulating agent exposure in 12 months before enrolment
Overall, 69% of patients had received prior ESA therapy (Eprex®, n = 3317; Aranesp®, n = 4564; NeoRecormon®, n = 2698; Table 2). Among patients initiated on Eprex® at enrolment, 49.7% were SC-ESA naive and 45.4% had received Eprex® within the previous 12 months. Note that SC administration of Eprex® in CKD patients remained contraindicated in the EU until a few months before registry initiation. Of the patients receiving Aranesp® and/or NeoRecormon® at enrolment, 24.3% were SC-ESA naive and 74.8% had received Aranesp® and/or NeoRecormon® in the preceding 12 months. At enrolment, 9.6% of patients were receiving no ESA, of whom 95.9% were SC-ESA naive.
| ESA exposure in 12 months before enrolmenta . | ESA at time of enrolment, n (%) . | Total, n (%) . | ||
|---|---|---|---|---|
| Eprex® . | Aranesp® or NeoRecormon® . | Noneb . | ||
| Eprex® | 2319 (45.4) | 12 (0.1) | 14 (1.0) | 2345 (15.3) |
| Other (Aranesp® and/or NeoRecormon®) | 104 (2.0) | 6545 (74.8) | 45 (3.0) | 6694 (43.7) |
| Both (Eprex® and ‘other’) | 146 (2.9) | 64 (0.7) | 2 (0.1) | 212 (1.4) |
| No ESA | 2536 (49.7) | 2130 (24.3) | 1416 (95.9) | 6082 (39.7) |
| Total | 5105 (100) | 8751 (100) | 1477 (100) | 15 333 (100) |
| ESA exposure in 12 months before enrolmenta . | ESA at time of enrolment, n (%) . | Total, n (%) . | ||
|---|---|---|---|---|
| Eprex® . | Aranesp® or NeoRecormon® . | Noneb . | ||
| Eprex® | 2319 (45.4) | 12 (0.1) | 14 (1.0) | 2345 (15.3) |
| Other (Aranesp® and/or NeoRecormon®) | 104 (2.0) | 6545 (74.8) | 45 (3.0) | 6694 (43.7) |
| Both (Eprex® and ‘other’) | 146 (2.9) | 64 (0.7) | 2 (0.1) | 212 (1.4) |
| No ESA | 2536 (49.7) | 2130 (24.3) | 1416 (95.9) | 6082 (39.7) |
| Total | 5105 (100) | 8751 (100) | 1477 (100) | 15 333 (100) |
aNote that Eprex® SC administration in the treatment of CRF was still contraindicated a few months before the start of the registry.
bNote that patients not receiving ESA treatment, but due to be initiated onto an approved ESA treatment within 1 month of enrolment were eligible for inclusion.
| ESA exposure in 12 months before enrolmenta . | ESA at time of enrolment, n (%) . | Total, n (%) . | ||
|---|---|---|---|---|
| Eprex® . | Aranesp® or NeoRecormon® . | Noneb . | ||
| Eprex® | 2319 (45.4) | 12 (0.1) | 14 (1.0) | 2345 (15.3) |
| Other (Aranesp® and/or NeoRecormon®) | 104 (2.0) | 6545 (74.8) | 45 (3.0) | 6694 (43.7) |
| Both (Eprex® and ‘other’) | 146 (2.9) | 64 (0.7) | 2 (0.1) | 212 (1.4) |
| No ESA | 2536 (49.7) | 2130 (24.3) | 1416 (95.9) | 6082 (39.7) |
| Total | 5105 (100) | 8751 (100) | 1477 (100) | 15 333 (100) |
| ESA exposure in 12 months before enrolmenta . | ESA at time of enrolment, n (%) . | Total, n (%) . | ||
|---|---|---|---|---|
| Eprex® . | Aranesp® or NeoRecormon® . | Noneb . | ||
| Eprex® | 2319 (45.4) | 12 (0.1) | 14 (1.0) | 2345 (15.3) |
| Other (Aranesp® and/or NeoRecormon®) | 104 (2.0) | 6545 (74.8) | 45 (3.0) | 6694 (43.7) |
| Both (Eprex® and ‘other’) | 146 (2.9) | 64 (0.7) | 2 (0.1) | 212 (1.4) |
| No ESA | 2536 (49.7) | 2130 (24.3) | 1416 (95.9) | 6082 (39.7) |
| Total | 5105 (100) | 8751 (100) | 1477 (100) | 15 333 (100) |
aNote that Eprex® SC administration in the treatment of CRF was still contraindicated a few months before the start of the registry.
bNote that patients not receiving ESA treatment, but due to be initiated onto an approved ESA treatment within 1 month of enrolment were eligible for inclusion.
Treatment switches
Of the 15 333 participants, 3086 switched to a non-registry ESA, resulting in their early completion. Among patients who switched, 37.6% had previously received Aranesp®, 34.5% Eprex® and 27.9% NeoRecormon®.
Chronic kidney disease stage and dialysis status during the registry period
At enrolment, patients were predominantly at CKD stage 4 (42.2%) or CKD stage 5 (34.1%), with very few at CKD stage 1 (0.1%), based on Kidney Disease Outcomes Quality Initiative (K/DOQI) definitions (Figure 2) [21]. At 36 months, 25.4 and 56.7% of patients were at CKD stages 4 and 5, respectively. The proportion of patients receiving dialysis increased from 19.5% at enrolment to 47.3% at 36 months.
Cumulative erythropoietin-stimulating agent and renin-angiotensin antagonist exposure
Cumulative SC ESA exposure from enrolment to completion/onset of PRCA was 8377 PY for Eprex® and 14 286 PY for the two comparator ESAs (Table 3). In calculating cumulative SC ESA exposure, missing data relating to exposure data, non-registry ESAs or non-SC administration were censored. Over the registry period, 52.3–57.9% of patients regularly received renin-angiotensin antagonists.
| ESA product . | Patients ESA-naive at enrolment, n (%) . | ESA treatment at last visit before completion, n (%) . | Cumulative ESA exposure from enrolment to completion/PRCA onset, PY . | Mean exposure per patient, months . | Cases of LOE for which Ab testing was available, n (n = 11) . | Confirmed PRCA cases, n (n = 5) . |
|---|---|---|---|---|---|---|
| Eprex® | 2631 (44.2) | 4242 (27.7) | 8376.8 | 15.4 | 7 | 3 |
| Aranesp® | 1410 (23.6) | N/A | N/A | 17.0 | 2 | 1 |
| NeoRecormon® | 682 (20.2) | N/A | N/A | 15.7 | 2 | 1 |
| Aranesp® and/or NeoRecormon® | 2092 (22.4) | 6240 (40.7) | 14 286.3 | 17.3 | 4 | 2 |
| No ESA | – | 4851 (31.6)a | 4614.2 | – | – | – |
| ESA product . | Patients ESA-naive at enrolment, n (%) . | ESA treatment at last visit before completion, n (%) . | Cumulative ESA exposure from enrolment to completion/PRCA onset, PY . | Mean exposure per patient, months . | Cases of LOE for which Ab testing was available, n (n = 11) . | Confirmed PRCA cases, n (n = 5) . |
|---|---|---|---|---|---|---|
| Eprex® | 2631 (44.2) | 4242 (27.7) | 8376.8 | 15.4 | 7 | 3 |
| Aranesp® | 1410 (23.6) | N/A | N/A | 17.0 | 2 | 1 |
| NeoRecormon® | 682 (20.2) | N/A | N/A | 15.7 | 2 | 1 |
| Aranesp® and/or NeoRecormon® | 2092 (22.4) | 6240 (40.7) | 14 286.3 | 17.3 | 4 | 2 |
| No ESA | – | 4851 (31.6)a | 4614.2 | – | – | – |
aPatients were observed for 12 months following permanent cessation of ESA treatment.
| ESA product . | Patients ESA-naive at enrolment, n (%) . | ESA treatment at last visit before completion, n (%) . | Cumulative ESA exposure from enrolment to completion/PRCA onset, PY . | Mean exposure per patient, months . | Cases of LOE for which Ab testing was available, n (n = 11) . | Confirmed PRCA cases, n (n = 5) . |
|---|---|---|---|---|---|---|
| Eprex® | 2631 (44.2) | 4242 (27.7) | 8376.8 | 15.4 | 7 | 3 |
| Aranesp® | 1410 (23.6) | N/A | N/A | 17.0 | 2 | 1 |
| NeoRecormon® | 682 (20.2) | N/A | N/A | 15.7 | 2 | 1 |
| Aranesp® and/or NeoRecormon® | 2092 (22.4) | 6240 (40.7) | 14 286.3 | 17.3 | 4 | 2 |
| No ESA | – | 4851 (31.6)a | 4614.2 | – | – | – |
| ESA product . | Patients ESA-naive at enrolment, n (%) . | ESA treatment at last visit before completion, n (%) . | Cumulative ESA exposure from enrolment to completion/PRCA onset, PY . | Mean exposure per patient, months . | Cases of LOE for which Ab testing was available, n (n = 11) . | Confirmed PRCA cases, n (n = 5) . |
|---|---|---|---|---|---|---|
| Eprex® | 2631 (44.2) | 4242 (27.7) | 8376.8 | 15.4 | 7 | 3 |
| Aranesp® | 1410 (23.6) | N/A | N/A | 17.0 | 2 | 1 |
| NeoRecormon® | 682 (20.2) | N/A | N/A | 15.7 | 2 | 1 |
| Aranesp® and/or NeoRecormon® | 2092 (22.4) | 6240 (40.7) | 14 286.3 | 17.3 | 4 | 2 |
| No ESA | – | 4851 (31.6)a | 4614.2 | – | – | – |
aPatients were observed for 12 months following permanent cessation of ESA treatment.
Completion and discontinuation
Among all patients, 62.6% (9602 patients) completed the registry. Reasons for discontinuation included death (n = 2627, 17.1% of all patients), loss to follow-up (n = 2547, 16.6%), AEs (n = 321, 2.1%), administrative reasons (n = 161, 1.1%), withdrawn consent (n = 68, 0.4%) or medical reasons (n = 7, <0.1%). The proportion of patients lost to follow-up was similar for Eprex® (16.8%) and comparators (16.3%).
Haemoglobin values over time
At ESA initiation, mean Hb was lower among patients receiving Eprex® (10.9 g/dL) compared with patients receiving NeoRecormon®/Aranesp® (11.3 g/dL). Mean Hb values were similar between these treatments after 3 months (Eprex®, 11.7 g/dL; comparator ESAs, 11.8 g/dL), and remained similar throughout registry period (Eprex®, 11.6 g/dL; comparator ESAs, 11.5 g/dL at 36 months).
Mean Hb at ESA initiation was lower among ESA-naive patients (10.1 g/dL) than non-ESA-naive patients (11.2 g/dL), but was the same in both groups after 3 months (11.8 g/dL), and remained similar throughout the registry period (11.5 g/dL at 36 months). There were no substantial variations in Hb by treatment.
Loss/lack of effect and antibody-mediated pure red cell aplasia
Of the 28 LOE reports during the registry, 5 were subsequently withdrawn, leaving 23 LOE cases (Figure 3). Anti-EPO Ab testing was not performed at the time of LOE for 12 of these patients, most commonly because another cause of LOE was identified (n = 7; Table 4). Iron deficiency was reported as the cause of LOE for one of these cases. Although this could not be confirmed by laboratory data available from the time of LOE, bone marrow investigation was not suggestive of PRCA. In four of the 12 patients, the cause of LOE was not established, but PRCA was excluded based on high reticulocyte count or short duration of LOE. A further patient developed LOE after approximately 5 months of SC exposure to methoxy polyethylene glycol-epoetin beta (Mircera®), which was preceded by ∼2 years of NeoRecormon® treatment. Although the cause of LOE was not identified and Ab testing was not performed at the time, a sample taken ∼6 months after LOE onset tested negative for anti-EPO Abs (Supplementary data).
Demographics, treatment, and bone marrow and anti-EPO Ab testing results in patients with LOE (n = 23)
| Number . | Age at initial visit (years)/gender . | Race . | Country . | ESA(s)a . | Bone marrow findings . | EPO Ab status . | LOE cause . |
|---|---|---|---|---|---|---|---|
| 1 | 80/Female | White | Germany | Eprex® | Not performed | Negative | Haemorrhage |
| 2 | 75/Male | White | Sweden | Aranesp® | Suggestive of PRCA | Positive | Ab-mediated PRCA |
| 3 | 58/Male | White | France | NeoRecormon® | Suggestive of PRCA | Negative | Methotrexate |
| 4 | 77/Female | White | Italy | Eprex® | Suggestive of PRCA | Negative | Folate deficiencyb |
| 5 | 62/Female | White | France | NeoRecormon® | Suggestive of PRCA | Positive | Ab-mediated PRCA |
| 6 | 72/Male | White | Austria | Aranesp®, NeoRecormon®, Eprex® | Not performed | Not performed | Iron deficiency and haemorrhage |
| 7 | 76/Male | White | Germany | Eprex® | Not performed | Not performed | Chemotherapy |
| 8 | 75/Male | White | France | Aranesp® | Not performed | Not performed | Unknown; PRCA excluded based on short duration of LOE |
| 9 | 76/Female | White | France | NeoRecormon® | Not performed | Not performed | Inadequate ESA dose |
| 10 | 76/Female | White | Italy | Eprex® | Not performed | Negative | Iron deficiencyb |
| 11 | 57/Female | White | France | Aranesp® | Not performed | Not performed | Unknown; PRCA excluded based on short duration of LOE |
| 12 | 54/Female | White | Great Britain | Eprex® | Not performed | Not performed | Unknown; PRCA excluded based on high reticulocyte count at the time of LOE |
| 13 | 73/Female | White | France | Eprex® | Not performed | Not performed | Unknown; PRCA excluded based on short duration of LOE, which occurred during hospitalization for sub-acute pulmonary oedema |
| 14 | 65/Male | White | Great Britain | NeoRecormon®, Aranesp® | Not performed | Not performed | Myelodysplastic syndrome |
| 15 | 84/Male | White- Indonesian | Netherlands | Eprex® | Not performed | Positive | Ab-mediated PRCA very probable |
| 16 | 91/Male | White | France | Eprex® | Suggestive of PRCA | Positive | Ab-mediated PRCA |
| 17 | 82/Female | White | Great Britain | NeoRecormon® | Not performed | Not performed | Sepsis |
| 18 | 75/Female | White | Belgium | NeoRecormon®, Aranesp® | Not suggestive of PRCA | Not performed | Iron deficiencyb |
| 19 | 66/Male | White | Spain | Eprex® | Not performed | Negative | Haemorrhage |
| 20 | 65/Male | White | France | Eprex® | Suggestive of PRCA | Positive | Ab-mediated PRCA |
| 21 | 31/Male | White | Ireland | NeoRecormon®, Mircera® | Not performed | Not performed | Unknown; a sample taken 6 months after LOE tested negative for anti-EPO Abs |
| 22 | 86/Female | White | France | NeoRecormon®, Eprex® | Not performed | Not performed | Cancer |
| 23 | 71/Male | White | France | Aranesp® | Not performed | Negative | Iron deficiency |
| Number . | Age at initial visit (years)/gender . | Race . | Country . | ESA(s)a . | Bone marrow findings . | EPO Ab status . | LOE cause . |
|---|---|---|---|---|---|---|---|
| 1 | 80/Female | White | Germany | Eprex® | Not performed | Negative | Haemorrhage |
| 2 | 75/Male | White | Sweden | Aranesp® | Suggestive of PRCA | Positive | Ab-mediated PRCA |
| 3 | 58/Male | White | France | NeoRecormon® | Suggestive of PRCA | Negative | Methotrexate |
| 4 | 77/Female | White | Italy | Eprex® | Suggestive of PRCA | Negative | Folate deficiencyb |
| 5 | 62/Female | White | France | NeoRecormon® | Suggestive of PRCA | Positive | Ab-mediated PRCA |
| 6 | 72/Male | White | Austria | Aranesp®, NeoRecormon®, Eprex® | Not performed | Not performed | Iron deficiency and haemorrhage |
| 7 | 76/Male | White | Germany | Eprex® | Not performed | Not performed | Chemotherapy |
| 8 | 75/Male | White | France | Aranesp® | Not performed | Not performed | Unknown; PRCA excluded based on short duration of LOE |
| 9 | 76/Female | White | France | NeoRecormon® | Not performed | Not performed | Inadequate ESA dose |
| 10 | 76/Female | White | Italy | Eprex® | Not performed | Negative | Iron deficiencyb |
| 11 | 57/Female | White | France | Aranesp® | Not performed | Not performed | Unknown; PRCA excluded based on short duration of LOE |
| 12 | 54/Female | White | Great Britain | Eprex® | Not performed | Not performed | Unknown; PRCA excluded based on high reticulocyte count at the time of LOE |
| 13 | 73/Female | White | France | Eprex® | Not performed | Not performed | Unknown; PRCA excluded based on short duration of LOE, which occurred during hospitalization for sub-acute pulmonary oedema |
| 14 | 65/Male | White | Great Britain | NeoRecormon®, Aranesp® | Not performed | Not performed | Myelodysplastic syndrome |
| 15 | 84/Male | White- Indonesian | Netherlands | Eprex® | Not performed | Positive | Ab-mediated PRCA very probable |
| 16 | 91/Male | White | France | Eprex® | Suggestive of PRCA | Positive | Ab-mediated PRCA |
| 17 | 82/Female | White | Great Britain | NeoRecormon® | Not performed | Not performed | Sepsis |
| 18 | 75/Female | White | Belgium | NeoRecormon®, Aranesp® | Not suggestive of PRCA | Not performed | Iron deficiencyb |
| 19 | 66/Male | White | Spain | Eprex® | Not performed | Negative | Haemorrhage |
| 20 | 65/Male | White | France | Eprex® | Suggestive of PRCA | Positive | Ab-mediated PRCA |
| 21 | 31/Male | White | Ireland | NeoRecormon®, Mircera® | Not performed | Not performed | Unknown; a sample taken 6 months after LOE tested negative for anti-EPO Abs |
| 22 | 86/Female | White | France | NeoRecormon®, Eprex® | Not performed | Not performed | Cancer |
| 23 | 71/Male | White | France | Aranesp® | Not performed | Negative | Iron deficiency |
aAll SC ESAs administered during the study are shown. SC ESA administered at the time of LOE is highlighted in bold, if known.
bCause of LOE could not be confirmed by available laboratory data from the time of LOE.
Demographics, treatment, and bone marrow and anti-EPO Ab testing results in patients with LOE (n = 23)
| Number . | Age at initial visit (years)/gender . | Race . | Country . | ESA(s)a . | Bone marrow findings . | EPO Ab status . | LOE cause . |
|---|---|---|---|---|---|---|---|
| 1 | 80/Female | White | Germany | Eprex® | Not performed | Negative | Haemorrhage |
| 2 | 75/Male | White | Sweden | Aranesp® | Suggestive of PRCA | Positive | Ab-mediated PRCA |
| 3 | 58/Male | White | France | NeoRecormon® | Suggestive of PRCA | Negative | Methotrexate |
| 4 | 77/Female | White | Italy | Eprex® | Suggestive of PRCA | Negative | Folate deficiencyb |
| 5 | 62/Female | White | France | NeoRecormon® | Suggestive of PRCA | Positive | Ab-mediated PRCA |
| 6 | 72/Male | White | Austria | Aranesp®, NeoRecormon®, Eprex® | Not performed | Not performed | Iron deficiency and haemorrhage |
| 7 | 76/Male | White | Germany | Eprex® | Not performed | Not performed | Chemotherapy |
| 8 | 75/Male | White | France | Aranesp® | Not performed | Not performed | Unknown; PRCA excluded based on short duration of LOE |
| 9 | 76/Female | White | France | NeoRecormon® | Not performed | Not performed | Inadequate ESA dose |
| 10 | 76/Female | White | Italy | Eprex® | Not performed | Negative | Iron deficiencyb |
| 11 | 57/Female | White | France | Aranesp® | Not performed | Not performed | Unknown; PRCA excluded based on short duration of LOE |
| 12 | 54/Female | White | Great Britain | Eprex® | Not performed | Not performed | Unknown; PRCA excluded based on high reticulocyte count at the time of LOE |
| 13 | 73/Female | White | France | Eprex® | Not performed | Not performed | Unknown; PRCA excluded based on short duration of LOE, which occurred during hospitalization for sub-acute pulmonary oedema |
| 14 | 65/Male | White | Great Britain | NeoRecormon®, Aranesp® | Not performed | Not performed | Myelodysplastic syndrome |
| 15 | 84/Male | White- Indonesian | Netherlands | Eprex® | Not performed | Positive | Ab-mediated PRCA very probable |
| 16 | 91/Male | White | France | Eprex® | Suggestive of PRCA | Positive | Ab-mediated PRCA |
| 17 | 82/Female | White | Great Britain | NeoRecormon® | Not performed | Not performed | Sepsis |
| 18 | 75/Female | White | Belgium | NeoRecormon®, Aranesp® | Not suggestive of PRCA | Not performed | Iron deficiencyb |
| 19 | 66/Male | White | Spain | Eprex® | Not performed | Negative | Haemorrhage |
| 20 | 65/Male | White | France | Eprex® | Suggestive of PRCA | Positive | Ab-mediated PRCA |
| 21 | 31/Male | White | Ireland | NeoRecormon®, Mircera® | Not performed | Not performed | Unknown; a sample taken 6 months after LOE tested negative for anti-EPO Abs |
| 22 | 86/Female | White | France | NeoRecormon®, Eprex® | Not performed | Not performed | Cancer |
| 23 | 71/Male | White | France | Aranesp® | Not performed | Negative | Iron deficiency |
| Number . | Age at initial visit (years)/gender . | Race . | Country . | ESA(s)a . | Bone marrow findings . | EPO Ab status . | LOE cause . |
|---|---|---|---|---|---|---|---|
| 1 | 80/Female | White | Germany | Eprex® | Not performed | Negative | Haemorrhage |
| 2 | 75/Male | White | Sweden | Aranesp® | Suggestive of PRCA | Positive | Ab-mediated PRCA |
| 3 | 58/Male | White | France | NeoRecormon® | Suggestive of PRCA | Negative | Methotrexate |
| 4 | 77/Female | White | Italy | Eprex® | Suggestive of PRCA | Negative | Folate deficiencyb |
| 5 | 62/Female | White | France | NeoRecormon® | Suggestive of PRCA | Positive | Ab-mediated PRCA |
| 6 | 72/Male | White | Austria | Aranesp®, NeoRecormon®, Eprex® | Not performed | Not performed | Iron deficiency and haemorrhage |
| 7 | 76/Male | White | Germany | Eprex® | Not performed | Not performed | Chemotherapy |
| 8 | 75/Male | White | France | Aranesp® | Not performed | Not performed | Unknown; PRCA excluded based on short duration of LOE |
| 9 | 76/Female | White | France | NeoRecormon® | Not performed | Not performed | Inadequate ESA dose |
| 10 | 76/Female | White | Italy | Eprex® | Not performed | Negative | Iron deficiencyb |
| 11 | 57/Female | White | France | Aranesp® | Not performed | Not performed | Unknown; PRCA excluded based on short duration of LOE |
| 12 | 54/Female | White | Great Britain | Eprex® | Not performed | Not performed | Unknown; PRCA excluded based on high reticulocyte count at the time of LOE |
| 13 | 73/Female | White | France | Eprex® | Not performed | Not performed | Unknown; PRCA excluded based on short duration of LOE, which occurred during hospitalization for sub-acute pulmonary oedema |
| 14 | 65/Male | White | Great Britain | NeoRecormon®, Aranesp® | Not performed | Not performed | Myelodysplastic syndrome |
| 15 | 84/Male | White- Indonesian | Netherlands | Eprex® | Not performed | Positive | Ab-mediated PRCA very probable |
| 16 | 91/Male | White | France | Eprex® | Suggestive of PRCA | Positive | Ab-mediated PRCA |
| 17 | 82/Female | White | Great Britain | NeoRecormon® | Not performed | Not performed | Sepsis |
| 18 | 75/Female | White | Belgium | NeoRecormon®, Aranesp® | Not suggestive of PRCA | Not performed | Iron deficiencyb |
| 19 | 66/Male | White | Spain | Eprex® | Not performed | Negative | Haemorrhage |
| 20 | 65/Male | White | France | Eprex® | Suggestive of PRCA | Positive | Ab-mediated PRCA |
| 21 | 31/Male | White | Ireland | NeoRecormon®, Mircera® | Not performed | Not performed | Unknown; a sample taken 6 months after LOE tested negative for anti-EPO Abs |
| 22 | 86/Female | White | France | NeoRecormon®, Eprex® | Not performed | Not performed | Cancer |
| 23 | 71/Male | White | France | Aranesp® | Not performed | Negative | Iron deficiency |
aAll SC ESAs administered during the study are shown. SC ESA administered at the time of LOE is highlighted in bold, if known.
bCause of LOE could not be confirmed by available laboratory data from the time of LOE.
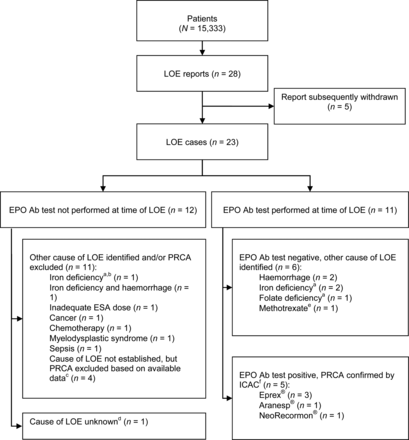
Outcomes of investigations of LOE reports. Ab, antibody; ICAC, Independent Case Adjudication Committee; EPO, erythropoietin; LOE, loss or lack of effect. aCause of LOE could not be confirmed by available laboratory data from the time of LOE for two cases with iron deficiency (one Ab testing not performed, one Ab-negative) and the Ab-negative case with folate deficiency reported as the cause of LOE. bBone marrow investigation was not suggestive of PRCA. cPRCA was excluded based on short duration of LOE (n = 3) or high reticulocyte count at time of LOE (n = 1). dPatient tested negative for anti-EPO Abs ∼6 months after LOE onset. eScreening failure. fBone marrow suggestive of PRCA in four cases; no bone marrow test for one case.
Evaluation for anti-EPO Abs was performed at the time of LOE for the remaining 11 cases, of whom 6 tested negative and had another cause of LOE identified: haemorrhage (n = 2); iron deficiency (n = 2); folate deficiency (n = 1); or methotrexate use (n = 1). However, laboratory data available from the time of the LOE could not confirm the cause of LOE for one of the patients with iron deficiency and the patient with folate deficiency. Since use of immunosuppressive therapy was an exclusion criterion, the patient with methotrexate use represents a screening failure.
The remaining five cases undergoing anti-EPO Ab testing at the time of LOE tested Ab-positive and were adjudicated as PRCA by the ICAC (Table 5; Supplementary data). Four cases of confirmed PRCA occurred after ESA initiation at enrolment in previously ESA-naive patients (Eprex®, n = 3; NeoRecormon®, n = 1). One case affected a patient with Aranesp® exposure prior to enrolment that continued throughout the registry. Four of the five PRCA cases affected men, three occurred in France and all five patients stored their ESA at home.
| Case . | Age at onset of LOE (years)/gender . | Race . | Primary CKD cause . | CKD stage . | Product . | ESA storage . | Duration of exposure up to LOE . | Haematological features/ESA dose (before diagnosis of PRCA) . | PRCA treatment . | PRCA outcome . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 76/Male | White | Renovascular disease and hypertension | 4 | Aranesp® | Home | 14 months | Unexplained LOE with low reticulocyte count/20 µg QW increased to 20 μg twice per week | Transfusions | Unknown |
| 2 | 63/Female | White | Renovascular disease and hypertension | 5 | NeoRecormon® | Home | 6 months | Unexplained LOE with a Hb of 8.0 g/dL/4000 IU QW increased to 10 000 IU QW | Transfusions | Not recovered |
| 3 | 92/Male | White | Unspecified | 3 | Eprex® | Home | 11 months | Unexplained LOE with a Hb of 6.1 g/dL/5000 IU QW increased to 10 000 IU QW | Corticosteroids, transfusions | Recovered |
| 4 | 66/Male | White | Unspecified | 5 | Eprex® | Home | 21 months | Unexplained LOE with a Hb of 6.4 g/dL/5000 IU QW decreased to 5000 IU Q2Wa | Transfusions | Recovered |
| 5 | 85/Male | White- Indonesian | Polycystic kidney disease, renovascular disease and hypertension | 4 | Eprex® | Home | 11 Months | Unexplained LOE with a Hb of 6.3 g/dL/3000 IU QW increased to 8000 IU QW | Transfusions | Not recovered |
| Case . | Age at onset of LOE (years)/gender . | Race . | Primary CKD cause . | CKD stage . | Product . | ESA storage . | Duration of exposure up to LOE . | Haematological features/ESA dose (before diagnosis of PRCA) . | PRCA treatment . | PRCA outcome . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 76/Male | White | Renovascular disease and hypertension | 4 | Aranesp® | Home | 14 months | Unexplained LOE with low reticulocyte count/20 µg QW increased to 20 μg twice per week | Transfusions | Unknown |
| 2 | 63/Female | White | Renovascular disease and hypertension | 5 | NeoRecormon® | Home | 6 months | Unexplained LOE with a Hb of 8.0 g/dL/4000 IU QW increased to 10 000 IU QW | Transfusions | Not recovered |
| 3 | 92/Male | White | Unspecified | 3 | Eprex® | Home | 11 months | Unexplained LOE with a Hb of 6.1 g/dL/5000 IU QW increased to 10 000 IU QW | Corticosteroids, transfusions | Recovered |
| 4 | 66/Male | White | Unspecified | 5 | Eprex® | Home | 21 months | Unexplained LOE with a Hb of 6.4 g/dL/5000 IU QW decreased to 5000 IU Q2Wa | Transfusions | Recovered |
| 5 | 85/Male | White- Indonesian | Polycystic kidney disease, renovascular disease and hypertension | 4 | Eprex® | Home | 11 Months | Unexplained LOE with a Hb of 6.3 g/dL/3000 IU QW increased to 8000 IU QW | Transfusions | Not recovered |
aOnset of LOE occurred 5 months after reduction of ESA dose.
| Case . | Age at onset of LOE (years)/gender . | Race . | Primary CKD cause . | CKD stage . | Product . | ESA storage . | Duration of exposure up to LOE . | Haematological features/ESA dose (before diagnosis of PRCA) . | PRCA treatment . | PRCA outcome . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 76/Male | White | Renovascular disease and hypertension | 4 | Aranesp® | Home | 14 months | Unexplained LOE with low reticulocyte count/20 µg QW increased to 20 μg twice per week | Transfusions | Unknown |
| 2 | 63/Female | White | Renovascular disease and hypertension | 5 | NeoRecormon® | Home | 6 months | Unexplained LOE with a Hb of 8.0 g/dL/4000 IU QW increased to 10 000 IU QW | Transfusions | Not recovered |
| 3 | 92/Male | White | Unspecified | 3 | Eprex® | Home | 11 months | Unexplained LOE with a Hb of 6.1 g/dL/5000 IU QW increased to 10 000 IU QW | Corticosteroids, transfusions | Recovered |
| 4 | 66/Male | White | Unspecified | 5 | Eprex® | Home | 21 months | Unexplained LOE with a Hb of 6.4 g/dL/5000 IU QW decreased to 5000 IU Q2Wa | Transfusions | Recovered |
| 5 | 85/Male | White- Indonesian | Polycystic kidney disease, renovascular disease and hypertension | 4 | Eprex® | Home | 11 Months | Unexplained LOE with a Hb of 6.3 g/dL/3000 IU QW increased to 8000 IU QW | Transfusions | Not recovered |
| Case . | Age at onset of LOE (years)/gender . | Race . | Primary CKD cause . | CKD stage . | Product . | ESA storage . | Duration of exposure up to LOE . | Haematological features/ESA dose (before diagnosis of PRCA) . | PRCA treatment . | PRCA outcome . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 76/Male | White | Renovascular disease and hypertension | 4 | Aranesp® | Home | 14 months | Unexplained LOE with low reticulocyte count/20 µg QW increased to 20 μg twice per week | Transfusions | Unknown |
| 2 | 63/Female | White | Renovascular disease and hypertension | 5 | NeoRecormon® | Home | 6 months | Unexplained LOE with a Hb of 8.0 g/dL/4000 IU QW increased to 10 000 IU QW | Transfusions | Not recovered |
| 3 | 92/Male | White | Unspecified | 3 | Eprex® | Home | 11 months | Unexplained LOE with a Hb of 6.1 g/dL/5000 IU QW increased to 10 000 IU QW | Corticosteroids, transfusions | Recovered |
| 4 | 66/Male | White | Unspecified | 5 | Eprex® | Home | 21 months | Unexplained LOE with a Hb of 6.4 g/dL/5000 IU QW decreased to 5000 IU Q2Wa | Transfusions | Recovered |
| 5 | 85/Male | White- Indonesian | Polycystic kidney disease, renovascular disease and hypertension | 4 | Eprex® | Home | 11 Months | Unexplained LOE with a Hb of 6.3 g/dL/3000 IU QW increased to 8000 IU QW | Transfusions | Not recovered |
aOnset of LOE occurred 5 months after reduction of ESA dose.
Based on exposed time, the rate of PRCA was 35.8/100 000 PY (95% CI 7.4–104.7) for Eprex® versus 14.0/100 000 PY (95% CI 1.7–50.6) for Aranesp®/NeoRecormon® combined. The PRCA incidence rate ratio with Eprex® versus comparator ESAs was not statistically significant (2.56; 95% CI 0.43–15.31). Based on observed time, the PRCA rate was 37.6/100 000 PY (95% CI 7.8–109.9) for Eprex® versus 13.9/100 000 PY (95% CI 1.7–50.3) for the pooled comparator ESAs, yielding a non-significant rate ratio of 2.70 (95% CI 0.45–16.15). Varying the lag time between exposure and PRCA did not substantially change the rate ratios.
Safety
Drug-related SAEs were reported for 25 of the 15 333 participants. The most commonly reported SAEs were blood and lymphatic system disorders (n = 8, including PRCA, suspected PRCA, unexpected anaemia and erythroblastopenia) and general disorders and administration site conditions (n = 5). Review of SAEs, including sensitivity analyses, revealed no new or unexpected safety concerns. Of the 2936 deaths during registry conduct, one was assessed to have Eprex® as a possible contributory factor (bronchial cancer; not pathologically confirmed).
DISCUSSION
PRIMS is the first international registry to estimate the incidence of PRCA in ESA-treated patients with CKD, which had not been quantified in a large prospective study since the 2006 EU reinstatement of SC Eprex® use in patients without readily available IV access. Few PRCA cases were detected during the 4-year registry period, indicating that PRCA is a rare complication with the ESAs under evaluation.
Although the PRIMS PRCA incidence rates are not substantially lower than those based on post-marketing surveillance of the uncoated-stopper Eprex® presentation for 2001–2003 [8], those estimates relied on spontaneous reporting and are widely believed to underestimate the true incidence. Since patients were prospectively followed, with systematic LOE investigation and easy access to anti-EPO Ab testing, PRIMS should capture all PRCA cases occurring in the population. Moreover, the prospective cohort design allowed accurate recording of individual patient ESA exposure over time. Therefore, PRIMS is expected to provide a more reliable estimate of PRCA incidence than spontaneous reporting. No significant difference in PRCA rates was observed between Eprex® and comparators (rate ratio 2.7; 95% CI 0.45–16.15). Although this could in part reflect the low number of PRCA cases, which limited the registry's power, PRCA was sufficiently rare that it would take an immense study to detect any potential difference in rates.
The results of PRIMS cannot be generalized to estimate or compare the immunogenicity risk of non-registry ESA treatments, including biosimilars. In a recent clinical trial of the epoetin alfa biosimilar HX575, two cases of EPO Ab-mediated PRCA were reported among the 337 participants [22].
The low number of registry PRCA cases hinders evaluation of potential associations with patient or treatment characteristics. The overrepresentation of males is consistent with previous data, as is the possible clustering in France, which could reflect the requirement for patients to collect ESAs from the pharmacy and associated risk of breaking the cold chain [2, 6, 7, 17, 22, 23]. All patients with PRCA received ESAs via SC administration, which is an established risk factor [13, 17]. Along with the known increased risk of eliciting an immune response with SC versus IV protein administration [24], SC delivery also permits home ESA administration, which might also increase the likelihood of cold-chain interruption and thereby facilitate the formation of immunogenic aggregates [13, 17]. All five registry patients with PRCA stored their ESA at home. Protein aggregation has been associated with product mishandling during illegal trade of epoetin alfa in Thailand [18]. Along with the continued occurrence of PRCA in clusters (e.g. in Singapore in 2013 [25]), these findings suggest the involvement of an environmental factor in its pathogenesis [13].
The limited power of the registry was due partly to its early termination, the impact of treatment switches, the low incidence of PRCA and the relatively small number of patients meeting the entry criterion of ≤12 months of prior SC ESA exposure. Moreover, the registry was subject to the limitations inherent in its non-interventional, non-randomized design, which meant that between-group imbalances in baseline characteristics could have affected the results. For example, a lower proportion of patients in the Eprex® and NeoRecormon® groups versus the Aranesp® group were receiving peritoneal dialysis, which may reflect the preferential prescription of a long-acting ESA for these patients. Enrolment of non-ESA-naive patients into the registry could have introduced survivor bias. However, SC administration of Eprex® had been newly reinstated in the EU at the time of study initiation, leading to a greater proportion of ESA-naive patients in the Eprex® group (∼50%) than the comparator group (∼25%). Therefore, any such survivor bias would tend to favour the comparator group due to its greater proportion of non-naive ‘survivor’ patients and resulting underestimate of PRCA occurrence. Although we could not exclude PRCA for one patient with LOE, this patient did not receive Eprex®; hence, even if this case did represent PRCA, it would not affect the estimated incidence rate for Eprex®. We also note the relatively high rate of loss to follow-up (16.6%), although this was comparable between Eprex® and NeoRecormon®/Aranesp®.
Advances are being made in delineating the cause of EPO Ab-mediated PRCA in ESA-treated patients and cases continue to emerge across the product class [13]. This registry indicates that PRCA was a rare adverse event with SC administration of the new Eprex® presentation and its comparator treatments.
FUNDING
This study was funded by Janssen, Pharmaceutical Companies of Johnson & Johnson.
CONFLICT OF INTEREST STATEMENT
A.L.M.D.F. has acted as a speaker for Abbott, Amgen, Fresenius, Roche and Gambro. A.S. reports no conflict of interest. A.K. has acted as an adviser for Janssen and declares sponsorship from Amgen, Roche and Janssen. G.M.L. has acted as an adviser for Janssen. H.M. has received research funding from Janssen and has acted as an adviser and speaker for Janssen. F.L. has acted as an adviser for Amgen-Dompé, Vifor-Fresenius Pharma, Janssen, Roche and Takeda and as a speaker for Amgen-Dompé, Janssen, Roche and Takeda. C.C. has acted as an adviser for Janssen and as a clinical trials investigator for Amgen and Roche. He has received conferences fees from Amgen, Janssen, Novartis and Roche. S.D.P. has acted as an adviser for Janssen. D.F. has acted as an adviser for Amgen, Janssen-Cilag and Roche and as a speaker for Amgen and Roche. He has received research funding from Janssen-Cilag and Roche. N.C. has acted as an adviser for Johnson & Johnson, Shire, Novartis and Sandoz, as a consultant for Sandoz and as a clinical trial investigator for Qiagen. She has received honoraria from Shire and Novartis and research support from Amgen. P.S. has acted as an adviser for Janssen. I.C.M. has received honoraria and consulting fees from Janssen, Roche and Amgen. J.M. has acted as an adviser for Johnson & Johnson and Pfizer and has received an honorarium and travel expenses from Bayer. P.P. holds stock in Johnson & Johnson, the parent company of Janssen Cilag, which markets Eprex®, and is an employee of Janssen, an operating company within Johnson & Johnson. A.P. is an employee of Janssen. A.F. is an employee of Janssen. D.Fi. holds stock in Johnson & Johnson and is a full-time employee of Janssen PRD. V.M. GTM from INC Research, mandated by Janssen. E.V. holds stock in and is an employee of Johnson & Johnson.
ACKNOWLEDGEMENTS
The authors thank all the PRIMS group investigators, nurses, patients and their families. Editorial assistance was provided by apothecom scopemedical ltd and funded by Janssen. Data from PRIMS have been previously presented as a poster at the 49th European Renal Association–European Dialysis and Transplant Association Congress, 24–27 May 2012, Paris, France: I.C.M., N.C., F.L., C.C., G.M.L., S.D.P., A.K., D.Fl., H.M., J.M., A.S., P.P., A.P., A.F., D.Fi., E.V., PRIMS Study Group. A prospective, immunogenicity surveillance registry (PRIMS) to estimate the incidence of erythropoietin antibody-mediated pure red cell aplasia among subjects with chronic renal failure and subcutaneous exposure to recombinant erythropoietin products (abstract FP217).





Comments