-
PDF
- Split View
-
Views
-
Cite
Cite
Heike Kielstein, Mayuren Suntharalingam, Ronny Perthel, Rong Song, Sabrina M. Schneider, Jens Martens-Lobenhoffer, Kristin Jäger, Stefanie M. Bode-Böger, Jan T. Kielstein, Role of the endogenous nitric oxide inhibitor asymmetric dimethylarginine (ADMA) and brain-derived neurotrophic factor (BDNF) in depression and behavioural changes: clinical and preclinical data in chronic kidney disease, Nephrology Dialysis Transplantation, Volume 30, Issue 10, October 2015, Pages 1699–1705, https://doi.org/10.1093/ndt/gfv253
Close - Share Icon Share
Abstract
Patients with chronic kidney disease (CKD) exhibit a high prevalence of neuropsychiatric alterations, including depression and behavioural changes. CKD is also associated with decreased physical activity not fully explained by co-morbidities. In patients without CKD, the brain-derived neurotropic factor (BDNF) as well as the endogenous NOS inhibitor asymmetric dimethylarginine (ADMA) had been suspected to be involved in major depression. The aim of our study was to examine the role of ADMA and BDNF in the behaviour of haemodialysis patients (CKD5D) as well as in a rat model of 5/6 nephrectomy and chronic ADMA infusion alone.
Eleven (5F/6M) CKD5D patients underwent Beck Depression Inventory (BDI) testing along with analysis of ADMA and BDNF. Male Sprague–Dawley rats were randomly assigned to four groups: (i) saline infusion; (ii) ADMA (250 µg/kg/day) infusion via osmotic mini pumps; (iii) 5/6 nephrectomy; (iv) untreated controls. After 28 days, the animals underwent behavioural tests measuring anxiety, locomotion and investigative behaviour. Animals were sacrificed, blood samples were drawn and analysed and hippocampal immunohistology for BDNF was performed.
In CKD5D patients, decreased BDNF levels correlated with higher scores of depression (Pearson r = −0.8156, P = 0.002). ADMA infusion led to a significant decrease of BDNF while the decrease of BDNF in 5/6 nephrectomy was not significant. However, an attenuated hippocampal BDNF expression could be detected in 5/6 nephrecomized animals. Decreased spontaneous locomotor activity was shown in ADMA-infused rats [15.9 (13.5–26.1) lines crossed/min] and 5/6 nephrectomy [14.6 (6.1–20.2) lines crossed/min] when compared with controls [32.5 (15.3–42.4) lines crossed/min]. Anxiety-like behaviour tested by hole investigation time was significantly more pronounced in 5/6 nephrectomy [24 (6–44) s] when compared with ADMA infusion [64 (28–93) s] and controls [33 (26–65) s].
Progressive renal failure in rats is accompanied by a marked increase of ADMA and a decrease in BDNF. 5/6 nephrectomy leads to significantly decreased exploratory behaviour and locomotion. Both behaviours could be reproduced by ADMA infusion alone. Indicators of anxiety were more pronounced in ADMA-infused animals when compared with 5/6 nephrectomized rats. Furthermore, an inverse relationship of BDNF and BDI in 11 CKD5D patients was shown.
INTRODUCTION
The prevalence of chronic kidney disease (CKD) is increasing worldwide. About 14% of the population in industrialized countries is thought to suffer from some degree of renal impairment [1]. In the USA, 62.2% of subjects ≥80 years have an estimated glomerular filtration rate (eGFR) < 60 mL/min [2]. This number will increase in the next decades as the underlying illnesses (diabetes and hypertension) become more prevalent. Aside from cardiovascular disease, patients with CKD suffer from an excess risk of incident dementia [3] and depression. A recent cross-sectional study in 5800 patients revealed a 2-fold higher risk of depressive symptoms in patients with eGFR ≤ 29 mL/min/1.73 m2 in comparison to the population with normal renal function [4]. It is unclear whether depression itself is also responsible for the reduction of physical activity that has been documented in CKD [5]. The mechanism underlying both, diminished spontaneous physical activity and depression is poorly understood. Even though it is very likely that both processes are the result of various structural and functional changes, one uraemic toxin represents an interesting candidate for playing a role in this scenario—the endogenous NOS inhibitor asymmetric dimethylarginine (ADMA). Selley [6] was the first to point out the link between major depression and ADMA almost 10 years ago in 25 patients with major depression. Since then, ADMA has been shown to be increased in Alzheimers patients [7] and inherited forms of cerebral arteriopathy leading to subcortical infarcts and leucoencephalopathy [8]. Recently, a large prospective cohort study showed that ADMA was independently associated with incident depression at 6-years follow-up [9]. While there are only epidemiological data pointing to a potential role of ADMA in neuropsychiatric disease, both epidemiological and experimental data on brain-derived neurotrophic factor (BDNF) suggest that it plays an important role in depression [10]. BDNF, which orchestrates growth and maintenance of the neuronal system as well as neuronal plasticity like long-term potentiation of learning [11], has been shown to be decreased in major depressive disorders [12]. Severity of depression corresponds to the magnitude of BDNF decrease in the plasma [13]. Interestingly, the decrease of serum or plasma BDNF seems to be related to mechanisms of BDNF release from platelets [13], which appear to bind, store and release BDNF upon activation at the site of traumatic injury [14]. Further supporting the role of BDNF in major depressive disorders is the fact that BDNF increases after anti-depressive therapy [12], a finding in line with an autopsy study showing an increased expression of BDNF in hippocampal regions in patients treated with antidepressants compared with patients without treatment [15].
The aim of our study was to investigate the potential role of ADMA and BDNF in behavioural changes and depression of chronic kidney disease in a cross-sectional clinical study of CKD5D patients as well as in a 4-week preclinical model of 5/6 nephrectomy and infusion of AMDA in otherwise healthy rats.
MATERIALS AND METHODS
Patients
The study was approved by the local Ethics Committee (reference # 2012–30). All patients gave written informed consent. The Beck Depression Inventory (BDI) [15, 16] was used to assess the severity of a mood disorder in 11 haemodialysis patients immediately before a dialysis session after a short dialysis interval. The patient characteristics are presented in Table 1. The BDI is a self-assessment questionnaire consisting of 21 multiple choice items related to affective, cognitive and somatic symptoms of depression such as hopelessness and sadness, guilt and punishment, weight loss, sleep disturbance and fatigue. Each item can be scored on a 4-point Likert-type scale indicating the severity of the symptom. A total sum score is computed and compared with cut-off values to indicate a clinical relevant amount of depressive symptoms. CKD patients scoring ≥15 are considered as at least mildly depressed [17].
| CKD5D patients (n = 11) . | . | |
|---|---|---|
| . | M ± SD . | . |
| Age (years) (M ± SD) | 52.6 ± 10.5 | |
| Gender (female/male) | 5/6 | |
| Weight (kg) | 76.7 ± 19.4 | |
| Laboratory results | Reference rangea | |
| Haemoglobin (g/dL) | 9.96 ± 1.25 | 12.0–16.0 | >10.0 − <11.5a |
| Leukocytes (Tsd/µL) | 7.96 ± 5.83 | 4.4–11.3 |
| Albumin (g/dL) | 34.33 ± 7.57 | 35–52 |
| MCV (fl) | 93.16 ± 8.39 | 80–100 |
| MCHC(g/dL) | 33.33 ± 0.99 | 31–37 |
| PTH (ng/L) | 188.50 ± 2.12 | 15–65 | 150–300a |
| TSH (mU/L) | 1.45 ± 1.18 | 0.27–4.2 |
| K+ (mmol/L) | 5.62 ± 0.83 | 3.6–5.4 |
| Na+ (mmol/L) | 138.2 ± 4.4 | 135–145 |
| Ca2+ (mmol/L) | 2.23 ± 0.28 | 2.15–2.60 |
| Phosphate (mmol/L) | 1.54 ± 0.53 | 0.83–1.67 |
| Creatinine (µmol/L) | 624.7 ± 241.8 | 59–104 |
| Urea (mmol/L) | 13.90 ± 5.07 | 2.8–7.2 |
| CKD5D patients (n = 11) . | . | |
|---|---|---|
| . | M ± SD . | . |
| Age (years) (M ± SD) | 52.6 ± 10.5 | |
| Gender (female/male) | 5/6 | |
| Weight (kg) | 76.7 ± 19.4 | |
| Laboratory results | Reference rangea | |
| Haemoglobin (g/dL) | 9.96 ± 1.25 | 12.0–16.0 | >10.0 − <11.5a |
| Leukocytes (Tsd/µL) | 7.96 ± 5.83 | 4.4–11.3 |
| Albumin (g/dL) | 34.33 ± 7.57 | 35–52 |
| MCV (fl) | 93.16 ± 8.39 | 80–100 |
| MCHC(g/dL) | 33.33 ± 0.99 | 31–37 |
| PTH (ng/L) | 188.50 ± 2.12 | 15–65 | 150–300a |
| TSH (mU/L) | 1.45 ± 1.18 | 0.27–4.2 |
| K+ (mmol/L) | 5.62 ± 0.83 | 3.6–5.4 |
| Na+ (mmol/L) | 138.2 ± 4.4 | 135–145 |
| Ca2+ (mmol/L) | 2.23 ± 0.28 | 2.15–2.60 |
| Phosphate (mmol/L) | 1.54 ± 0.53 | 0.83–1.67 |
| Creatinine (µmol/L) | 624.7 ± 241.8 | 59–104 |
| Urea (mmol/L) | 13.90 ± 5.07 | 2.8–7.2 |
aKDIGO (Kidney Disease: Improving Global Outcomes) recommendation if applicable.
| CKD5D patients (n = 11) . | . | |
|---|---|---|
| . | M ± SD . | . |
| Age (years) (M ± SD) | 52.6 ± 10.5 | |
| Gender (female/male) | 5/6 | |
| Weight (kg) | 76.7 ± 19.4 | |
| Laboratory results | Reference rangea | |
| Haemoglobin (g/dL) | 9.96 ± 1.25 | 12.0–16.0 | >10.0 − <11.5a |
| Leukocytes (Tsd/µL) | 7.96 ± 5.83 | 4.4–11.3 |
| Albumin (g/dL) | 34.33 ± 7.57 | 35–52 |
| MCV (fl) | 93.16 ± 8.39 | 80–100 |
| MCHC(g/dL) | 33.33 ± 0.99 | 31–37 |
| PTH (ng/L) | 188.50 ± 2.12 | 15–65 | 150–300a |
| TSH (mU/L) | 1.45 ± 1.18 | 0.27–4.2 |
| K+ (mmol/L) | 5.62 ± 0.83 | 3.6–5.4 |
| Na+ (mmol/L) | 138.2 ± 4.4 | 135–145 |
| Ca2+ (mmol/L) | 2.23 ± 0.28 | 2.15–2.60 |
| Phosphate (mmol/L) | 1.54 ± 0.53 | 0.83–1.67 |
| Creatinine (µmol/L) | 624.7 ± 241.8 | 59–104 |
| Urea (mmol/L) | 13.90 ± 5.07 | 2.8–7.2 |
| CKD5D patients (n = 11) . | . | |
|---|---|---|
| . | M ± SD . | . |
| Age (years) (M ± SD) | 52.6 ± 10.5 | |
| Gender (female/male) | 5/6 | |
| Weight (kg) | 76.7 ± 19.4 | |
| Laboratory results | Reference rangea | |
| Haemoglobin (g/dL) | 9.96 ± 1.25 | 12.0–16.0 | >10.0 − <11.5a |
| Leukocytes (Tsd/µL) | 7.96 ± 5.83 | 4.4–11.3 |
| Albumin (g/dL) | 34.33 ± 7.57 | 35–52 |
| MCV (fl) | 93.16 ± 8.39 | 80–100 |
| MCHC(g/dL) | 33.33 ± 0.99 | 31–37 |
| PTH (ng/L) | 188.50 ± 2.12 | 15–65 | 150–300a |
| TSH (mU/L) | 1.45 ± 1.18 | 0.27–4.2 |
| K+ (mmol/L) | 5.62 ± 0.83 | 3.6–5.4 |
| Na+ (mmol/L) | 138.2 ± 4.4 | 135–145 |
| Ca2+ (mmol/L) | 2.23 ± 0.28 | 2.15–2.60 |
| Phosphate (mmol/L) | 1.54 ± 0.53 | 0.83–1.67 |
| Creatinine (µmol/L) | 624.7 ± 241.8 | 59–104 |
| Urea (mmol/L) | 13.90 ± 5.07 | 2.8–7.2 |
aKDIGO (Kidney Disease: Improving Global Outcomes) recommendation if applicable.
Animals
All research and animal care procedures had been approved by the Lower Saxony district government (registration # 10/0229EEC) and followed the guidelines set by the European Communities Council Directive of 24 November 1986. Male Sprague–Dawley rats (n = 23) were obtained from Charles RiverGmbH, Sulzfeld, Germany. The rats were held in cages of up to five rats for the first 3 days covered with standard cage bedding. They were then placed in plastic single cages bedded in cellulose in an air-conditioned facility under a 14-h light and 10-h dark cycle (lights on at 07:00 a.m.) at an ambient temperature of 24°C. Standard carbohydrate chow (50% carbohydrate, 19% protein, 12% water, 4% fat and 2.1 kcal/g; Altromin, Lage, Germany) and water were available ad libitum.
Experimental design
Twenty-three rats were randomly divided into four groups. On Day 1, Alzet-pumps (model 2ML4 with a flow rate of 2.5 µL/h) (Durect Corporation, Cupertino, CA) were subcutaneously implanted in rats of Group 1 and 2 under inhalation anaesthesia (isoflurane) as described previously [18]. Group 1 (n = 5) received 0.9% NaCl infusion and was used as control, Group 2 (n = 10) received 250 µmol/kg/day ADMA (Alexis Biochemicals, Lausen, Switzerland) which was dispensed in 0.9% NaCl and subsequently sterile filtered. Rats in Group 3 (n = 5) underwent 5/6 nephrectomy (Nx) as previously described [19]. Group 4 (n = 3) were untreated control rats for the immunohistochemical staining. In brief, we performed selective ligation of the extrarenal artery branches of the left renal artery under ketamine and xylazine anaesthesia to obtain a two-thirds renal infarction. The opposite kidney was then removed. Behavioural tests were performed after 4 weeks of ADMA infusion or 5/6 nephrectomy. All animals were sacrificed on Day 28.
Holeboard
The apparatus used in this study was a combination of holeboard and open-field apparatus and therefore a capable tool of measuring locomotor activity, anxiety and directed and undirected investigative behaviours. The arena (60 × 60 cm) consisted of a quadratic wooden board, which was divided by white lines into 25 square fields. It was enclosed by wooden walls (40 cm high) that hindered the rat from leaving the apparatus. At each intersection of two lines, a circular hole (2.5 cm in diameter) was drilled into the board (16 holes in the board in total). The dimension of each field was 12 × 12 cm. The 16 fields adjacent to the boundary walls were defined as perimeter fields, whereas the nine fields in the middle were defined as centre fields. Each rat was placed in a definite corner of the holeboard and supervised closely by two experienced experimenters for 8 min. The following parameters were measured: hole-pocking (directed investigative behaviour); rearing (undirected investigative behaviour); lines crossed (locomotor activity); time spent in centre and time until first hole investigation occurred (anxiety-like behaviour). Holeboard tests were carried out in the light cycle (04:00–07:00 p.m.)
Laboratory measurements
Blood samples for measurement of plasma ADMA, SDMA, BDNF and routine chemistry were drawn before sacrifice, immediately cooled on ice, centrifuged at 1500 g and 4°C for 10 min. Supernatants were stored in 500 µL aliquots at −80°C until further use. Plasma concentrations of ADMA and SDMA were measured applying a liquid chromatography-mass spectrometry method described elsewhere [20]. Since recently, a diurnal BDNF rhythm was demonstrated in healthy humans where plasma BDNF displayed highest concentrations in the morning, followed by a substantial decrease throughout the day and the lowest values at midnight [21]. Blood samples from dialysis patients were collected between 7 and 8 AM. BDNF serum levels in the rat study were measured using an Elisa (IBL, Minneapolis, MN). Human BDNF serum levels in the dialysis patients were measured using an Elisa Kit from Ray Biotech (Norcross, GA). All other measurements were performed with routine laboratory tests using certified assay methods.
Immunohistochemistry of rat brains
Brains of both, the control and the Nx group were coronally cut into seven series of 30 µm-thick sections using a freezing microtome and stored in antifreezer-solution (160 mL PBS, 120 mL ethyleneglycol, 120 mL glycerol) at −20°C until staining. Using a commercially available rabbit polyclonal antibody (ab101752) (1:1000 Abcam, Cambridge, UK), free-floating sections of the hippocampal area were stained for BDNF according to the manufacturer's instructions. Colour reaction for light microscopy was performed using ABC solution [VectaStain Elite Kit, PK-6101 (anti-rabbit), Vector Laboratories Inc., Burlingame, CA], followed by the DAB kit (Peroxidase Subtrate Kit, SK-4100, Vector Laboratories Inc., Burlingame, CA) according to the manufacturer's instructions. Sections were semi-quantitatively analysed with a Biozero 8100E Keyence microscope in a ×40 resolution.
Statistics
GraphPad Prism 5 (Informer Technologies, Inc., Dominica, CA) was used for statistical analysis. Data are expressed as median [interquartile range (IQR)] unless otherwise stated. To compare groups at different time points, ANOVA and Tukey's multiple comparison tests were used. The significance level was set at P < 0.05.
RESULTS
Depressed dialysis patients have decreased BDNF serum levels
In our cohort of CKD5D patients, there was a significant inverse correlation between the sum score BDI (Beck Depression Inventory) and BDNF (Figure 1). There was neither a relationship between BDNF and the l-arginine/ADMA ratio (Pearson r = 0.4891, NS; data not shown) nor between BDI and ADMA (Pearson r = 0.1709, NS).
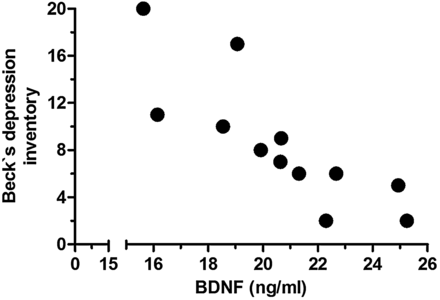
Inverse relationship of BDNF and Beck Depression Inventory (BDI) in 11 chronic haemodialysis patients (Pearson r −0.8156, P = 0.002).
Sole ADMA infusion leads to decreased BDNF serum levels
ADMA infusion increased plasma ADMA levels to 1.2 [1.08–1.47] µmol/L as compared to 0.7 [0.54–0.73] µmol/L in the control group. ADMA infusion significantly decreased BDNF levels compared with controls (Figure 2).
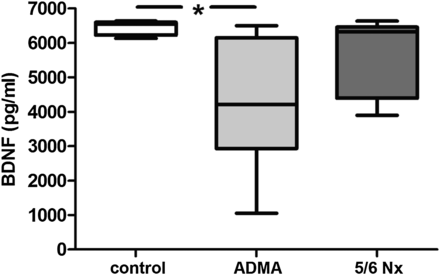
Effect of ADMA infusion (28 days) and 5/6 nephrectomy (28 days after surgical intervention; Nx) on serum BDNF levels in Sprague–Dawley rats. Data are compared with BDNF levels of saline injected control rats. Data presented as median (interquartile ranges); comparison between groups using ANOVA and Tukey's multiple comparison test, *P < 0.05.
5/6 nephrectomy led to a significant increase in urea [21.8 (17.79–29.59) mmol/L] when compared with controls [8.1 (7.74–8.52) mmol/L] and resulted in an increased ADMA level of 0.8 [0.72–0.90] µmol/L. The increased ADMA levels in 5/6 nephrectomy only tended to be associated with decreased BDNF levels. The effect of ADMA infusion as well as 5/6 nephrectomy are given in Table 2.
Dimethylarginine levels and parameters of renal function of animals in the different groups [28 days after start of infusion or nephrectomy (Nx)]
| . | NaCl (Control) . | ADMA . | 5/6 Nx . |
|---|---|---|---|
| Serum creatinine (µmol/L) | 37.4 [35.0–39.6] | 38.8 [36.8–40.5] | 84.6* [65.2–119.3] |
| Urea (mmol/L) | 8.08 [7.74–8.52] | 8.19 [7.52–9.09] | 21.78* [17.79–29.59] |
| SDMA (µmol/L) | 0.22 [0.19–0.28] | 0.37 [0.32–0.43] | 0.99*§ [0.74–1.43] |
| L-Arginine/ADMA | 302 [271–336] | 145*# [135–163] | 208* [189–254] |
| ADMA (µmol/L) | 0.66 [0.54–0.72] | 1.21*# [1.08–1.47] | 0.81 [0.72–0.90] |
| BDNF (pg/mL) | 6550 [6260–6600] | 4214* [3550–6093] | 6327 [4561–6400] |
| . | NaCl (Control) . | ADMA . | 5/6 Nx . |
|---|---|---|---|
| Serum creatinine (µmol/L) | 37.4 [35.0–39.6] | 38.8 [36.8–40.5] | 84.6* [65.2–119.3] |
| Urea (mmol/L) | 8.08 [7.74–8.52] | 8.19 [7.52–9.09] | 21.78* [17.79–29.59] |
| SDMA (µmol/L) | 0.22 [0.19–0.28] | 0.37 [0.32–0.43] | 0.99*§ [0.74–1.43] |
| L-Arginine/ADMA | 302 [271–336] | 145*# [135–163] | 208* [189–254] |
| ADMA (µmol/L) | 0.66 [0.54–0.72] | 1.21*# [1.08–1.47] | 0.81 [0.72–0.90] |
| BDNF (pg/mL) | 6550 [6260–6600] | 4214* [3550–6093] | 6327 [4561–6400] |
Data are presented as median [interquartile range].
*P < 0.05 versus control; analysis of variance confirmed by Tukey's multiple comparison test.
§P < 0.05 versus ADMA; analysis of variance confirmed by Tukey's multiple comparison test.
#P < 0.05 versus 5/6 Nx; analysis of variance confirmed by Tukey's multiple comparison test.
Dimethylarginine levels and parameters of renal function of animals in the different groups [28 days after start of infusion or nephrectomy (Nx)]
| . | NaCl (Control) . | ADMA . | 5/6 Nx . |
|---|---|---|---|
| Serum creatinine (µmol/L) | 37.4 [35.0–39.6] | 38.8 [36.8–40.5] | 84.6* [65.2–119.3] |
| Urea (mmol/L) | 8.08 [7.74–8.52] | 8.19 [7.52–9.09] | 21.78* [17.79–29.59] |
| SDMA (µmol/L) | 0.22 [0.19–0.28] | 0.37 [0.32–0.43] | 0.99*§ [0.74–1.43] |
| L-Arginine/ADMA | 302 [271–336] | 145*# [135–163] | 208* [189–254] |
| ADMA (µmol/L) | 0.66 [0.54–0.72] | 1.21*# [1.08–1.47] | 0.81 [0.72–0.90] |
| BDNF (pg/mL) | 6550 [6260–6600] | 4214* [3550–6093] | 6327 [4561–6400] |
| . | NaCl (Control) . | ADMA . | 5/6 Nx . |
|---|---|---|---|
| Serum creatinine (µmol/L) | 37.4 [35.0–39.6] | 38.8 [36.8–40.5] | 84.6* [65.2–119.3] |
| Urea (mmol/L) | 8.08 [7.74–8.52] | 8.19 [7.52–9.09] | 21.78* [17.79–29.59] |
| SDMA (µmol/L) | 0.22 [0.19–0.28] | 0.37 [0.32–0.43] | 0.99*§ [0.74–1.43] |
| L-Arginine/ADMA | 302 [271–336] | 145*# [135–163] | 208* [189–254] |
| ADMA (µmol/L) | 0.66 [0.54–0.72] | 1.21*# [1.08–1.47] | 0.81 [0.72–0.90] |
| BDNF (pg/mL) | 6550 [6260–6600] | 4214* [3550–6093] | 6327 [4561–6400] |
Data are presented as median [interquartile range].
*P < 0.05 versus control; analysis of variance confirmed by Tukey's multiple comparison test.
§P < 0.05 versus ADMA; analysis of variance confirmed by Tukey's multiple comparison test.
#P < 0.05 versus 5/6 Nx; analysis of variance confirmed by Tukey's multiple comparison test.
Significantly decreased locomotor activity and investigative behaviour after 5/6 nephrectomy and chronic ADMA infusion
The lines crossed by the animal in a given time are a measure of spontaneous locomotor activity. In contrast to controls, both rats with 5/6 nephrectomy and rats in the ADMA infusion group showed significantly decreased spontaneous locomotor activity (Figure 3). The direct exploratory behaviour, assessed by the number of holes visited in a given time, was less in the 5/6 nephrectomy group when compared with the ADMA group (Figure 4). Both, ADMA infusion and 5/6 nephrectomy, led to a decrease in the time spent rearing compared with the control group, indicating a decrease in undirected investigative behaviour (Figure 4). Hole investigation time, a marker of anxiety like behaviour, was significantly decreased in 5/6 nephrectomy when compared with animals in the ADMA infusion group (Figure 5). Centre time and self-grooming were neither affected by 5/6 nephrectomy nor by ADMA infusion (Supplementary data, Figure).
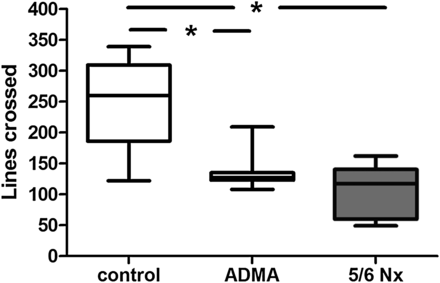
Effect of ADMA infusion (28 days) and 5/6 nephrectomy (28 days after surgical intervention; Nx) on locomotion of rats measured by the lines crossed in the holeboard test. Data are compared with locomotion of saline-injected control rats. Data presented as median (interquartile ranges); comparison between groups using ANOVA and Tukey's multiple comparison test, *P < 0.05.
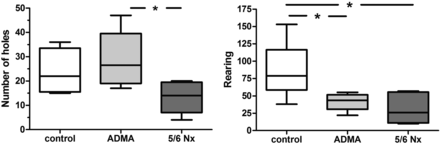
Direct (number of holes) and indirect (rearing) exploratory behaviour of rats in holeboard testing after 28 days of infusion of ADMA or 5/6 nephrectomy (Nx) compared with saline-injected controls. Data presented as median (interquartile ranges); comparison between groups using ANOVA and Tukey's multiple comparison test, *P < 0.05.
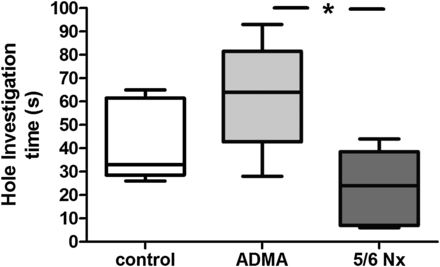
Anxiety measured by the hole investigation time in the holeboard test after 28 days of infusion of ADMA or 5/6 nephrectomy (5/6 Nx) compared with saline-injected controls. Data presented as median (interquartile ranges); comparison between groups using ANOVA and Tukey's multiple comparison test, *P < 0.05.
Decreased BDNF expression in 5/6 nephrectomized rats
Semi-quantitative assessment of hippocampal BDNF expression performed by an experienced examiner blinded to the treatment group showed a significant lower BDNF staining intensity (relative units) in the hippocampi from 5/6 nephrectomized rats when compared with control animals (1.5 ± 0.2 versus 2.3 ± 0.3, P < 0.05). Representative sections are shown in Figure 6.
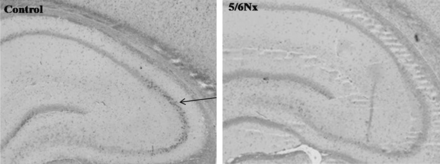
Immunohistochemical staining (anti-BDNF ab101752; 1:1000; Abcam, Cambridge, UK) of hippocampal BDNF in a control and 5/6 nephrectomized (5/6 Nx) rat; magnification: ×40. The arrow depicts positive BDNF immunoreactivity.
DISCUSSION
The key findings of our study in CKD5D patients were that BDNF levels were inversely related to the degree of depression measured by BDI. In rats, ADMA infusion led to significantly decreased BDNF levels. Within 4 weeks, 5/6 nephrectomy led to (i) decreased spontaneous locomotion, (ii) increased anxiety and (iii) decreased exploratory behaviour. All of these effects can be induced by long-term, i.e. 4-week continuous infusion of ADMA to rats with normal renal function.
Depression and BDNF
Our study shows for the first time a marked significant inverse correlation between BDNF and intensity of depression in CKD5D patients, i.e. lower BDNF levels implicate higher depression scores in these patients. Karege et al. [22] described a correlation between serum and cortical BDNF levels [13]. As BDNF is able to cross the blood–brain barrier, alterations in serum BDNF levels may have an impact of BDNF levels in the brain while altered brain BDNF might be reflected in lower serum BDNF. This might be an approach to understand the importance of BDNF in the pathophysiology in major depressive disorder. Several cross-sectional studies showed a tight correlation between low BDNF and the severity of depression [12, 13]. Serum BDNF levels of antidepressant-naïve patients with depression were reduced compared with those in treated patients and healthy controls [12, 23]. The magnitude of the difference in BDNF levels corresponds to the magnitude of the depression. After treatment with antidepressants, BDNF levels increased to those of healthy controls [23]. This is further supported by a study by Chen et al. [15] who found increased hippocampal BDNF immunoreactivity in patients with depression that were treated when compared with untreated patients. So far only one study explored BDNF levels in CKD5D patients. This study solely investigated the effect of a single haemodialysis session on BDNF levels [24]. This study also showed that basal BDNF levels in haemodialysis patients were significantly lower than in healthy individuals, which is in line with our findings.
CKD and behavioural changes
Up to 27% of urban haemodialysis patients have been reported to suffer a major anxiety disorder [25], a finding not universally found in smaller sample sizes [26]. The signs of anxiety we found in our animal model were in line with previous preclinical studies using models of renal impairment [27]. Interestingly, ADMA infusion alone was able to reproduce the effect of chronic renal impairment on almost all investigated parameters of behaviour. ADMA is an endogenous NOS inhibitor known to accumulate in renal failure [28]. Experimental data on the effect of NOS inhibition on anxiety are controversially discussed. While Volke et al. [29] showed that L-NAME, a synthetic NOS inhibitor had an anxiolytic effect in the elevated plus maze test, de Oliveira et al. [30] demonstrated increased anxiety by NOS inhibition with L-NOARG in rats using the elevated plus maze test. The increase in anxiety could be overcome by administration of L-arginine, i.e. increasing NO production [30]. Further supporting the importance of NO for anxiolysis is the study by Caton et al. [31] who reported that the anxiolytic effect of NO was significantly attenuated by inhibition of nNOS production. Given these preclinical data, it is very well conceivable that ADMA administration alone has the potential to induce anxiety like behaviour. Our data point to the fact that ADMA alone is powerful enough to replicate the behavioural changes seen in 5/6 nephrectomy.
CKD and NOS inhibition decreases spontaneous locomotion
Although distinct movement disorders such as tremor and myoclonus have been described in dialysis patients [32], a general assessment of spontaneous motor activity in patients with CKD has so far not been reported. Even a lack of physical activity could be attributed to several factors such as advanced age and underlying diseases as well as co-morbidities such as amputation.
The decreased spontaneous locomotion seen in our CKD model is in line with data from Topczewska-Bruns et al. [27]. These authors found a decrease in line crossings of more than 50% and in rearings a decrease of 83%. However, these effects became evident only after 8 weeks of 5/6 nephrectomy, while we saw significant changes already after 28 days in this model. Interestingly, a 4-week infusion of ADMA alone was able to induce the effects on spontaneous locomotion as seen in 5/6 nephrectomy. This effect on locomotion might also explain why ADMA infused animals investigated more of holes (direct exploratory behaviour) but showed less rearing (indirect exploratory behaviour) when compared with controls. The later activity requires strength and coordination which might be hampered by ADMA infusion. Several lines of evidence support the notion that NO plays an important role in the control of motor behaviour. Mice mutant for the neuronal NOS isoform have altered locomotor abilities [33]. Rats and mice treated with various NOS inhibitors show problems with fine motor control [34]. Moreover, NOS inhibitor compounds reduce spontaneous locomotor activity [35]. The nNOS-specific NOS inhibitor 7NI decreased locomotion in the open field-test [36]. Araki et al. [36] showed that motor-deficits caused by L-NAME and 7-NI were noted in doses higher than 160 mg/kg. The motor deficit caused by nNOS specific inhibitor 7-NI was more pronounced than the deficit caused by L-NAME, which inhibits all three NOS isoforms. This is in line with the findings concerning the potent inhibitory effect of ADMA on nNOS when compared with eNOS. The IC50 of ADMA for nNOS in one study was 1.5 µM while it was almost an order of magnitude higher (12 µM) for eNOS, i.e. at a given ADMA concentration, the inhibitory effect of ADMA on nNOS is higher than on eNOS [37]. Behavioural changes and effects on spontaneous locomotion caused in our study were just due to ADMA infusion in otherwise healthy rats. Thus, even without co-morbidities seen in CKD, rats exhibited less spontaneous locomotion, which sheds new light in this problem in CKD patients.
We wish to point out important limitations of our study. The number of patients included was limited, as this was not the main focus of our work. This limited number could, however, be responsible for the partial discordance of animal and human data, i.e. for the relationship of decreased BDNF levels and increased ADMA in animals, which could not be found in patients. An additional problem could arise from the measurement of BDNF in serum only. Although it has been described that serum BDNF levels were 14-fold higher than plasma BDNF levels [38], this might be different in rats. Furthermore, a potential variation of ADMA effects would have warranted a larger number of animals, yet the number was based on previous animal studies investigating the long-term effect of ADMA. Moreover, we compared different stages of renal impairment in the animals and humans. Our chronic renal failure model was limited to 28 days as the continuous infusion using an osmotic mini pump is limited to 28 days. Extension of the experiments would have required a second surgical procedure with anaesthesia, While ADMA infusion led to a significant decrease in BDNF levels in rats, there was no such relationship in humans. This could be due to the fact that several other risk factors increase ADMA thus potentially masking an existing relationship. The most unfortunate limitation is the absence of brain histology from the ADMA-infused animals, that were lost in freezer accident.
In conclusion, we show for the first time that the marked behavioural changes of CKD in an animal model of 5/6 nephrectomy can be reproduced by ADMA infusion alone, pointing to a new role of this uraemic toxin. Furthermore, our clinical and preclinical data suggest that BDNF plays a role in depression found in patients with CKD. For the first time, ADMA infusion was shown to induce a marked reduction in BDNF levels, known to be inversely correlated with depressive behavioural changes. Lastly, both, experimental uraemia as well as ADMA infusion led to decreased spontaneous locomotion, indicating that co-morbidities of CKD alone do not lead to physical inactivity.
FUNDING
This study is supported by a grant of the Werner Jackstädt-Stiftung to J.T.K. and H.K.
CONFLICT OF INTEREST STATEMENT
Dr J.T. K. owns and hosts the website www.adma.com.
REFERENCES
Author notes
H.K. and M.S. contributed equally to this work and are both considered first authors.





Comments