-
PDF
- Split View
-
Views
-
Cite
Cite
Olivier Ethgen, Antoine G. Schneider, Sean M. Bagshaw, Rinaldo Bellomo, John A. Kellum, Economics of dialysis dependence following renal replacement therapy for critically ill acute kidney injury patients, Nephrology Dialysis Transplantation, Volume 30, Issue 1, January 2015, Pages 54–61, https://doi.org/10.1093/ndt/gfu314
Close - Share Icon Share
Abstract
The obective of this study was to perform a cost-effectiveness analysis comparing intermittent with continuous renal replacement therapy (IRRT versus CRRT) as initial therapy for acute kidney injury (AKI) in the intensive care unit (ICU).
Assuming some patients would potentially be eligible for either modality, we modeled life year gained, the quality-adjusted life years (QALYs) and healthcare costs for a cohort of 1000 IRRT patients and a cohort of 1000 CRRT patients. We used a 1-year, 5-year and a lifetime horizon. A Markov model with two health states for AKI survivors was designed: dialysis dependence and dialysis independence. We applied Weibull regression from published estimates to fit survival curves for CRRT and IRRT patients and to fit the proportion of dialysis dependence among CRRT and IRRT survivors. We then applied a risk ratio reported in a large retrospective cohort study to the fitted CRRT estimates in order to determine the proportion of dialysis dependence for IRRT survivors. We conducted sensitivity analyses based on a range of differences for daily implementation cost between CRRT and IRRT (base case: CRRT day $632 more expensive than IRRT day; range from $200 to $1000) and a range of risk ratios for dialysis dependence for CRRT as compared with IRRT (from 0.65 to 0.95; base case: 0.80).
Continuous renal replacement therapy was associated with a marginally greater gain in QALY as compared with IRRT (1.093 versus 1.078). Despite higher upfront costs for CRRT in the ICU ($4046 for CRRT versus $1423 for IRRT in average), the 5-year total cost including the cost of dialysis dependence was lower for CRRT ($37 780 for CRRT versus $39 448 for IRRT on average). The base case incremental cost-effectiveness analysis showed that CRRT dominated IRRT. This dominance was confirmed by extensive sensitivity analysis.
Initial CRRT is cost-effective compared with initial IRRT by reducing the rate of long-term dialysis dependence among critically ill AKI survivors.
INTRODUCTION
Acute kidney injury (AKI) is a common condition among critically ill patients in the intensive care unit (ICU). Acute kidney injury results in rapid loss of kidney function and worsens the patient's prognosis. When severe, AKI may necessitate the provision of renal replacement therapy (RRT); RRT can be applied through two main modalities: continuous RRT (CRRT) or intermittent RRT (IRRT). Both modalities provide satisfactory metabolic control and neither has been found superior in terms of survival [1–4]. However, among those who survive critical illness requiring RRT, failure to recover kidney function and progression to end-stage kidney disease (ESKD) leading to dialysis dependence remains a significant medical and economic issue [5].
A recent systematic review and meta-analysis suggested that among AKI survivors, initial treatment with IRRT might be associated with higher rates of dialysis dependence than initial CRRT [6]. Pooled analyses from seven randomized controlled trials comparing 240 IRRT patients with 232 CRRT patients did not show a statistically significant difference in the risk of dialysis dependence between IRRT and CRRT. However, the point estimate was in the direction of better long-term renal outcomes with CRRT (relative risk 1.15 [95% CI: 0.78–1.68]). In addition, pooled analyses from 16 observational studies enrolling 1476 IRRT patients and 2023 CRRT patients demonstrated a significantly higher rate of dialysis dependence among survivors who initially received IRRT as compared with CRRT (relative risk 1.99 [95% CI: 1.53–2.59]) [6].
These results were confirmed in a large retrospective cohort study including 2315 CRRT recipients of whom 2004 (87%) were 1 : 1 matched to 2004 IRRT recipients [7]. The matching was comprehensive and based on the history of chronic kidney disease, receipt of mechanical ventilation within 7 days of the initiation of RRT and logit of the propensity score for receipt of CRRT (within ±0.2 SD). This study also showed that the risk of chronic dialysis was significantly lower among patients who initially received CRRT versus IRRT (hazard ratio 0.75 [95% CI: 0.62–0.87]) [7].
Based on these recent findings, we hypothesized that initial CRRT might be economically superior compared with IRRT and performed a cost-effectiveness analysis of CRRT versus IRRT in critically ill AKI patients.
MATERIALS AND METHODS
This study followed the CHEERS statement for reporting economic evaluation [8].
Decision problem
We assumed a cohort of AKI patients who would potentially be eligible for either IRRT or CRRT in the ICU setting. We then modeled potential health gains and cost savings, comparing patients receiving IRRT as the initial modality with those receiving CRRT as the initial modality, all else being the same.
Decision analytic model
Using Microsoft Excel, we designed a decision analytic Markov model. A Markov model is a recursive modeling approach that spreads a cohort of patients through a series of transition probabilities across multiple health states [9, 10]. Markov models are used to project the outcomes associated with specific treatment options. Health outcomes and healthcare costs are accumulated cyclically as the cohort evolves over time through the different health states. Our model had two health states for AKI survivors in the ICU (CRRT and IRRT) and two health states for AKI survivors discharged from the hospital (DD: dialysis dependence and DI: dialysis independence) (Figure 1). Daily cycle was used over the 5 first years after RRT initiation in the ICU and yearly cycle afterward. Health outcomes were expressed in terms of life year gained (LYG) and quality-adjusted life years (QALYs). Costs encompassed direct medical costs from a US third-party public payer perspective. Health outcomes and healthcare costs were simulated and averaged for a cohort of 1000 patients initiated on CRRT and a cohort of 1000 patients initiated on IRRT. Discount rate was set at 3% per annum.
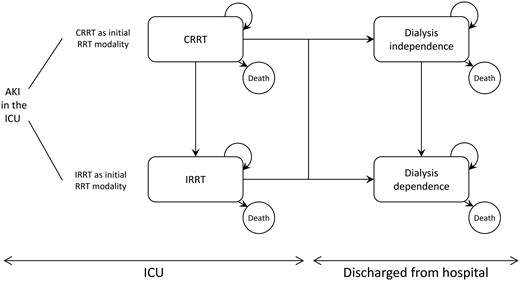
Schematic representation of the Markov model. When AKI occurs in the ICU, patients are initiated on CRRT or IRRT. The ICU and hospital lengths of stay are supposed to be the same between both modalities. Continuous renal replacement therapy and IRRT patients can be discharged dialysis dependent or independent. The model assumed that once patients become dialysis dependent, they cannot recover their renal function and can only remain dialysis dependent or die.
Survival
As there is no evidence of survival differences between CRRT and IRRT [1–4], we stipulated the same survival pattern would apply for both initial CRRT and IRRT. Transition probabilities toward death were not assumed constant over time. They were modeled with a single survival curve for both modalities using a Weibull regression on three survival proportions, assumed to be 60% at discharge [11, 12], 46% at Day 60 [11, 12] and 37% at Day 180 [13] (Table 1 and Figure 2A).
| . | CRRT . | IRRT . | Reference . |
|---|---|---|---|
| ICU stay | |||
| ICU LoS (days) | 12 | 12 | [13] |
| RRT duration (days) | 7 | 7 | [13] |
| Switch from CRRT to IRRT (%) | 30 | — | Assumption |
| Survival (all cause death) | |||
| Discharged alive from ICU (%) | 60.0 | 60.0 | [11, 12] |
| Alive at 60 days (%) | 46.0 | 46.0 | [11,12] |
| Alive at 180 days (%) | 37.0 | 37.0 | [13] |
| DD among AKI survivorsa | |||
| DD at 90 days (%) | 16.4 | 20.8 | [7] |
| DD at 3 years (%) | 21.7 | 26.6 | [7] |
| Cost of implementing RRT | |||
| Acute RRT cost/day | $858 | $226 | [14, 15] |
| Health utilities | |||
| ICU stay | 0.13 | 0.13 | [16] |
| DI | 0.84 | 0.84 | [17] |
| DD | 0.62 | 0.62 | [17] |
| Healthcare costs | |||
| DI cost/day | $31 | $31 | [5] |
| DD cost/day | $211 | $211 | [5] |
| . | CRRT . | IRRT . | Reference . |
|---|---|---|---|
| ICU stay | |||
| ICU LoS (days) | 12 | 12 | [13] |
| RRT duration (days) | 7 | 7 | [13] |
| Switch from CRRT to IRRT (%) | 30 | — | Assumption |
| Survival (all cause death) | |||
| Discharged alive from ICU (%) | 60.0 | 60.0 | [11, 12] |
| Alive at 60 days (%) | 46.0 | 46.0 | [11,12] |
| Alive at 180 days (%) | 37.0 | 37.0 | [13] |
| DD among AKI survivorsa | |||
| DD at 90 days (%) | 16.4 | 20.8 | [7] |
| DD at 3 years (%) | 21.7 | 26.6 | [7] |
| Cost of implementing RRT | |||
| Acute RRT cost/day | $858 | $226 | [14, 15] |
| Health utilities | |||
| ICU stay | 0.13 | 0.13 | [16] |
| DI | 0.84 | 0.84 | [17] |
| DD | 0.62 | 0.62 | [17] |
| Healthcare costs | |||
| DI cost/day | $31 | $31 | [5] |
| DD cost/day | $211 | $211 | [5] |
LoS, length of stay; ICU, intensive care unit; CRRT/IRRT, continuous/intermittent renal replacement therapy; DI, dialysis independence; DD, dialysis dependence.
aThis corresponds to a risk ratio of ∼0.80 for the base case (16.4/20.8% = 0.79 and 21.7/26.6% = 0.82).
| . | CRRT . | IRRT . | Reference . |
|---|---|---|---|
| ICU stay | |||
| ICU LoS (days) | 12 | 12 | [13] |
| RRT duration (days) | 7 | 7 | [13] |
| Switch from CRRT to IRRT (%) | 30 | — | Assumption |
| Survival (all cause death) | |||
| Discharged alive from ICU (%) | 60.0 | 60.0 | [11, 12] |
| Alive at 60 days (%) | 46.0 | 46.0 | [11,12] |
| Alive at 180 days (%) | 37.0 | 37.0 | [13] |
| DD among AKI survivorsa | |||
| DD at 90 days (%) | 16.4 | 20.8 | [7] |
| DD at 3 years (%) | 21.7 | 26.6 | [7] |
| Cost of implementing RRT | |||
| Acute RRT cost/day | $858 | $226 | [14, 15] |
| Health utilities | |||
| ICU stay | 0.13 | 0.13 | [16] |
| DI | 0.84 | 0.84 | [17] |
| DD | 0.62 | 0.62 | [17] |
| Healthcare costs | |||
| DI cost/day | $31 | $31 | [5] |
| DD cost/day | $211 | $211 | [5] |
| . | CRRT . | IRRT . | Reference . |
|---|---|---|---|
| ICU stay | |||
| ICU LoS (days) | 12 | 12 | [13] |
| RRT duration (days) | 7 | 7 | [13] |
| Switch from CRRT to IRRT (%) | 30 | — | Assumption |
| Survival (all cause death) | |||
| Discharged alive from ICU (%) | 60.0 | 60.0 | [11, 12] |
| Alive at 60 days (%) | 46.0 | 46.0 | [11,12] |
| Alive at 180 days (%) | 37.0 | 37.0 | [13] |
| DD among AKI survivorsa | |||
| DD at 90 days (%) | 16.4 | 20.8 | [7] |
| DD at 3 years (%) | 21.7 | 26.6 | [7] |
| Cost of implementing RRT | |||
| Acute RRT cost/day | $858 | $226 | [14, 15] |
| Health utilities | |||
| ICU stay | 0.13 | 0.13 | [16] |
| DI | 0.84 | 0.84 | [17] |
| DD | 0.62 | 0.62 | [17] |
| Healthcare costs | |||
| DI cost/day | $31 | $31 | [5] |
| DD cost/day | $211 | $211 | [5] |
LoS, length of stay; ICU, intensive care unit; CRRT/IRRT, continuous/intermittent renal replacement therapy; DI, dialysis independence; DD, dialysis dependence.
aThis corresponds to a risk ratio of ∼0.80 for the base case (16.4/20.8% = 0.79 and 21.7/26.6% = 0.82).
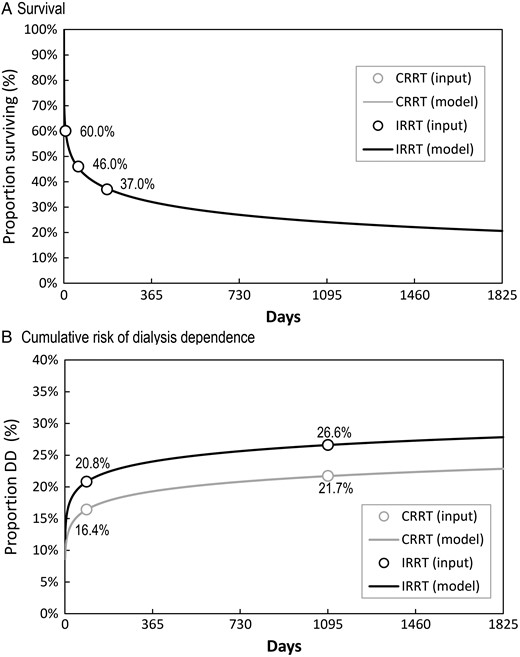
Survival and cumulative risk of dialysis dependence assumptions per initial RRT modality in the ICU. DD, dialysis dependence.
Dialysis independence among AKI survivors
For both initial CRRT and IRRT, the time-dependent proportion of AKI survivors becoming dialysis dependent was fitted from the study by Wald et al. [7] using a Weibull regression on two data points: at Day 90 and at the median follow-up of 3 years, i.e. Day 1095 (Table 1 and Figure 1B). Continuous renal replacement therapy dialysis independence estimates were then obtained by applying a risk ratio to IRRT estimates. In the base case, this risk ratio was ∼0.80 (16.4/20.8% = 0.79 and 21.7/26.6 = 0.82). Wald et al. reported a hazard ratio of 0.75 (95% CI: 0.65–0.87) [7].
Switch from initial CRRT to IRRT over the ICU stay
We accounted for the fact that it is relatively common practice to switch AKI patients initiated on CRRT to IRRT as their hemodynamic status evolves favorably [11]. In doing so, we assumed that switches from CRRT to IRRT will not alter the subsequent risk of dialysis dependence but would reduce the RRT daily implementation cost. In other words, initial CRRT patients switched to IRRT were applied the initial CRRT outcomes. Indeed, most of the studies retrieved in the literature compared initial RRT modalities only. We varied this proportion of CRRT patients being switched to IRRT over the ICU stay from 0 to 60%, with a base case at 30% and assuming a constant daily rate of switch over the ICU stay.
Health utility and cost
All health state utilities and costs were assumed to be the same between CRRT and IRRT except the daily cost of the RRT implementation in the ICU. Therefore, the model only accounted for the cost of implementing CRRT or IRRT in the ICU, the daily cost of dialysis independence and the daily cost of dialysis dependence in the outpatient setting. Health utilities and costs assigned to each health state were taken from literature [5, 14–17] (Table 1). All costs were inflated to 2013 $US using the Consumer Price Index for Medical Care Services from the US Bureau of Labor Statistics [18].
Base case analysis
Base case values are summarized in Tables 1 and 2. All values were the same for both modalities, CRRT and IRRT, except the daily implementation cost [14, 15] and the cumulative risk for dialysis dependence among survivors (Figure 2B) [7]. The daily cost of CRRT was set at $858, and the daily cost of IRRT at $226 based on estimates from Manns et al. [14] converted and inflated to US$ 2013. For the cost of CRRT, we used conservatively the high estimate reported by Manns et al. in lieu of the low one ($586). The resulting cost difference of $632 between the two RRT modalities falls within the interquartile range of the cost difference reported by Srisawat et al. from $134 to $950 with a median at $331 ($US 2013) [15]. In the base case analysis, we used a 5-year time horizon to compute the ICER of initial CRRT versus initial IRRT and to compare the 5-year cumulative total cost and cost of dialysis dependence between the two initial RRT modalities.
Five-year cost-effectiveness analysis of initial CRRT versus initial IRRT (undiscounted)
| . | CRRT . | IRRT . |
|---|---|---|
| Health outcomes | ||
| LYG | 1.387 | 1.387 |
| QALYs | 1.093 | 1.078 |
| Costs | ||
| RRT modality | $4046 | $1423 |
| Dialysis independence (DI) | $12 380 | $11 642 |
| Dialysis dependence (DD) | $21 354 | $26 383 |
| Total | $37 780 | $39 448 |
| Cost-effectiveness analysis | ||
| Cost/LYG | $27 248 | $28 451 |
| Cost/QALYs | $34 578 | $36 586 |
| ICER CRRT versus IRRTa | −$116 121 | — |
| ‘(CRRT dominates)’ | ||
| . | CRRT . | IRRT . |
|---|---|---|
| Health outcomes | ||
| LYG | 1.387 | 1.387 |
| QALYs | 1.093 | 1.078 |
| Costs | ||
| RRT modality | $4046 | $1423 |
| Dialysis independence (DI) | $12 380 | $11 642 |
| Dialysis dependence (DD) | $21 354 | $26 383 |
| Total | $37 780 | $39 448 |
| Cost-effectiveness analysis | ||
| Cost/LYG | $27 248 | $28 451 |
| Cost/QALYs | $34 578 | $36 586 |
| ICER CRRT versus IRRTa | −$116 121 | — |
| ‘(CRRT dominates)’ | ||
aICER: incremental cost-effectiveness ratio = (CostCRRT – CostIRRT)/(QALYCRRT – QALYIRRT).
CRRT dominates IRRT as QALYCRRT > QALYIRRT and CostCRRT < CostIRRT.
Five-year cost-effectiveness analysis of initial CRRT versus initial IRRT (undiscounted)
| . | CRRT . | IRRT . |
|---|---|---|
| Health outcomes | ||
| LYG | 1.387 | 1.387 |
| QALYs | 1.093 | 1.078 |
| Costs | ||
| RRT modality | $4046 | $1423 |
| Dialysis independence (DI) | $12 380 | $11 642 |
| Dialysis dependence (DD) | $21 354 | $26 383 |
| Total | $37 780 | $39 448 |
| Cost-effectiveness analysis | ||
| Cost/LYG | $27 248 | $28 451 |
| Cost/QALYs | $34 578 | $36 586 |
| ICER CRRT versus IRRTa | −$116 121 | — |
| ‘(CRRT dominates)’ | ||
| . | CRRT . | IRRT . |
|---|---|---|
| Health outcomes | ||
| LYG | 1.387 | 1.387 |
| QALYs | 1.093 | 1.078 |
| Costs | ||
| RRT modality | $4046 | $1423 |
| Dialysis independence (DI) | $12 380 | $11 642 |
| Dialysis dependence (DD) | $21 354 | $26 383 |
| Total | $37 780 | $39 448 |
| Cost-effectiveness analysis | ||
| Cost/LYG | $27 248 | $28 451 |
| Cost/QALYs | $34 578 | $36 586 |
| ICER CRRT versus IRRTa | −$116 121 | — |
| ‘(CRRT dominates)’ | ||
aICER: incremental cost-effectiveness ratio = (CostCRRT – CostIRRT)/(QALYCRRT – QALYIRRT).
CRRT dominates IRRT as QALYCRRT > QALYIRRT and CostCRRT < CostIRRT.
Sensitivity analysis
First, we conducted a series of one-way sensitivity analysis and presented results as Tornado diagram. Second, we ran a two-way deterministic sensitivity analysis by varying the two key model parameters distinguishing CRRT from IRRT: the daily implementation cost difference and the cumulative risk of dialysis dependence. The daily implementation cost difference between CRRT and IRRT varied from $200 to $1000 (base case at $632) and the risk ratio for dialysis dependence between CRRT and IRRT varied from 0.65 to 0.95 (base case around 0.80). Finally, we re-ran all analyses twice, with a 1-year time horizon and a lifetime time horizon.
RESULTS
Base case analysis
Table 2 displays the undiscounted 5-year results for the base case analysis. Continuous renal replacement therapy was associated with a marginally greater gain in QALY as compared with IRRT (1.093 versus 1.078). Despite higher upfront average costs for CRRT patients in the ICU ($4046 for CRRT versus $1423 for IRRT), the 5-year total cost including the cost of dialysis dependence was lower for a CRRT patient ($37 780 for CRRT versus $39 448 for IRRT on average).
The 5-year undiscounted cumulative total cost of the initial IRRT cohort exceeded that of the initial CRRT cohort within 2-year post-RRT initiation (Figure 3). The cost of dialysis dependence in the initial CRRT cohort was constantly lower than that for the initial IRRT cohort (Figure 3).
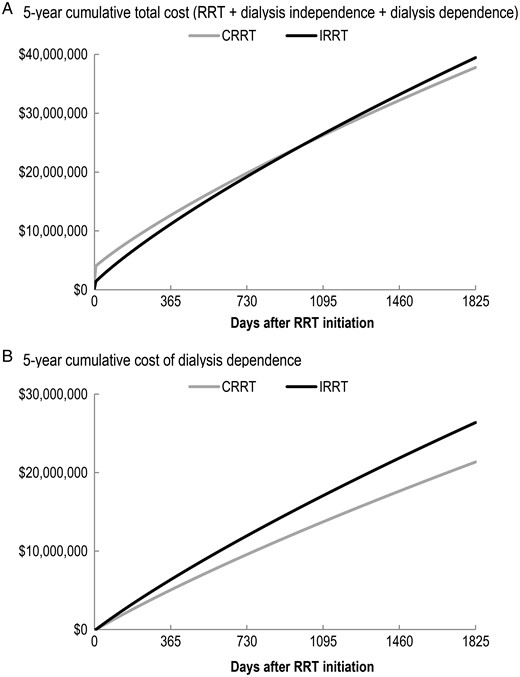
Five-year cumulative cost difference between initial CRRT and initial IRRT (undiscounted).
The ICER of CRRT versus IRRT was negative (−$116 121), meaning that CRRT dominated IRRT (QALYCRRT > QALYIRRT and CostCRRT < CostIRRT) and was thus cost-saving (Table 2). This dominance was confirmed in the 5-year discounted analysis (ICER = −$106 527) and the lifetime discounted analysis (ICER = −$196 956) but not in the 1-year analysis (ICER = $400 701).
Sensitivity analysis
Figure 4 shows the tornado diagrams obtained by varying seven parameters from low to high base. The risk ratio of dialysis dependence of initial CRRT as compared with initial IRRT and the health utility attributed to the dialysis independence state contributed the most to the variability of the ICER. The latter is explained by the fact that as this utility (low case at 0.67) gets closer to one of the dialysis dependence states (base case at 0.62), the denominator of the ICER approaches zero (i.e. nearly identical QALY gained between initial CRRT and initial IRRT). The other key parameter contributing the most to the variability of the ICER was the daily cost difference between CRRT and IRRT. The risk of dialysis dependence was the only parameter that noticeably resulted in an ICER of >0 (QALYCRRT > QALYIRRT and CostCRRT > CostIRRT).
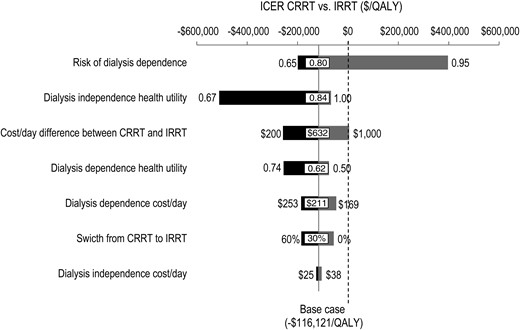
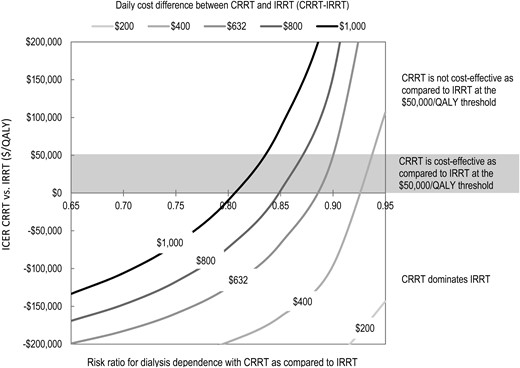
Figure 5 presents a two-way sensitivity analysis, varying the risk ratio for dialysis dependence and the daily implementation cost at the same time. Initial CRRT was predominantly cost-effective as compared with initial IRRT at the generally prevailing $50 000/QALY threshold.
DISCUSSION
Initial treatment with CRRT is cost-effective when compared with IRRT among critically ill patients with AKI by reducing the costs associated with the greater rate of long-term dialysis dependence. To our knowledge, this is the first economic analysis focusing on dialysis dependence among AKI survivors. In the light of a recent large observational cohort study [7], confirming previous meta-analytic findings [6], we demonstrated through a comprehensive model the potential economic advantage of CRRT compared with IRRT, despite the higher CRRT upfront cost in the ICU.
Our analysis contradicts previous economic findings. Both Klarenbach et al. [17] and De Smedt et al. [16] concluded that CRRT presented neither health nor economic advantage over IRRT. Using a lifetime horizon, Klarenbach et al. reported that CRRT resulted in equivalent health outcomes with IRRT but was CAN$3679 (≈$3309) more costly due to the higher direct costs of providing CRRT [17]. Using a 2-year life time horizon, De Smedt et al. reported that the supplementary cost per additional QALY associated with CRRT reached 114 012 €/QALY (≈$156 600), far exceeding the willingness to pay a threshold of 30 000 €/QALY (≈$41 200) [16].
In contrast, we found that CRRT dominated IRRT. In our analysis, only the 1-year time horizon yielded an ICER above the 50 000 $/QALY threshold, but both the 5-year and the lifetime horizons resulted in CRRT dominance over IRRT. At 400 701 $/QALY in the 1-year analysis, the ICER reached the 50 000 $/QALY threshold a Day 821 (2.2 years) and then became negative from Day 990 (2.7 years) indicating CRRT dominance over IRRT. Continuous renal replacement therapy was associated with a marginally greater gain in QALYs as compared with IRRT. This was due to the lower dialysis dependence among survivors. In addition, and despite higher ICU costs for CRRT, the cumulative total cost including the cost of dialysis dependence was lower for CRRT.
Our model explicitly includes the effect of both initial modalities on renal recovery among survivors. To model the risk of chronic dialysis between the two initial modalities, we used latest observational estimates as obtained by robust propensity score matching methodology on a large sample of patients [7]. This research confirmed previous meta-analysis findings [6] and consolidated the fact that initial CRRT may actually lead to less dialysis dependence as compared with initial IRRT. We translated this important clinical outcome into an economic assessment and reported an economic advantage of initial CRRT over IRRT.
Several reasons have led us to use the large observational study [7] instead of the meta-analysis [6] to document the risk of chronic dialysis between initial IRRT and initial CRRT in our model. First, the study is to our knowledge the only original study addressing the issue with direct data rather than derived data. Second, the study is of high quality, using propensity matching for treatment allocation and running multiple sensitivity analyses to evaluate the robustness of the data. Propensity matching for treatment allocation is precisely meant to minimize the risk of allocation bias, one of the principal limitations of observational studies on which the meta-analysis is largely based. Third, the effect seen in the large observational study is less than that in the meta-analysis, allowing our analysis to be more conservative. Finally, the meta-analysis included only papers published before 2012 and thus omitted the study by Wald et al., [7] which is by far the largest and most recent study published to date on the topic.
Our model has a number of limitations and assumptions that should be discussed. Our analysis assumed a cohort of AKI patients who would potentially be eligible for either IRRT or CRRT in the ICU. As such, our findings do not apply to those clinical circumstances where a specific RRT modality is particularly recommended or preferable. This may limit the generalizability of our findings.
In modeling the post-AKI dialysis-dependent and dialysis-independent patients' quality-of-life and costs, we used as surrogates ESKD patients and non-ESKD patients who otherwise share similar characteristics. We have used these surrogates to compute the ongoing costs of dialysis dependence and independence. However, post-AKI patients may actually have worse quality-of-life than standard ESKD patients [19]. Therefore, they may possibly have higher medical costs as well.
For reasons of clarity, we only accounted for the costs driven by the choice of RRT in the ICU and by the subsequent daily medical costs for dialysis dependence and dialysis independence for outpatients, as all other costs were assumed to be the same between both modalities. As health economics investigates the incremental ratio between multiple strategies, those costs were not indispensable. Their inclusion in the ICER computation would not have changed the conclusion of the model.
With regard to the cost difference between CRRT and IRRT in the ICU, we used the estimates reported by Srisawat et al. from the BEST Kidney Investigators, probably one of the most complete and global cost assessments of acute RRT [15]. A number of other cost studies have been done over the years, but they were all consistent with the BEST study, as summarized elsewhere [20]. Costs of chronic dialysis for outpatients encompassed only direct medical costs from an US third-party public payer perspective. It should be noted that these costs are similar to and consistent with public sector costs in Germany [21] and in Australia [22].
Patients initiated on CRRT can be switched to IRRT when clinical conditions permit, in particular once patients become hemodynamically stable while acute RRT is still required. Therefore, switching from initial CRRT to IRRT during the ICU stay is relatively common in settings where both modalities are available. As there is a paucity of data on that specific issue, we modeled the possibility of switch from initial CRRT to IRRT over the ICU stay as sensitivity analysis. We applied the same renal outcome of those patients receiving initial CRRT only to those initial CRRT patients being switched to IRRT. In effect, the results presented in the literature compared initial modalities only. This has only enhanced the economic attractiveness of CRRT versus IRRT, proving similar renal and health outcomes but at a slightly decreased cost of acute RRT, IRRT being less expensive than CRRT.
We assumed a constant daily rate of switch from CRRT to IRRT but varying the overall switch rate during the ICU stay from 0 to 60%. In all likelihood, the switch rate from CRRT to IRRT during the ICU stay greatly depends on the local setting as well as the availability of modalities and staff. In some countries, no CRRT patient will be switched to IRRT before the patient is ready for discharge whereas in others, CRRT patients will be transitioned to IRRT when hemodynamically stable, generally close to discharge but not necessarily. The data from the ATN trial suggest switching rates from initial CRRT to IRRT between 20 and 40% [23].
Our findings put a figure on recent observational studies that showed the potential protective effect of initial CRRT against dialysis dependence among AKI survivors. We also echo a recent editorial, challenging the economic case for using initial conventional IRRT in the ICU [24]. We show that CRRT might be less costly in the long run by diminishing the necessity of chronic dialysis among survivors. This also translates into quality-of-life benefits for survivors knowing the psychosocial burden of chronic dialysis.
CONCLUSIONS
In conclusion, our analysis suggests that initial CRRT is cost-effective compared with initial IRRT by reducing the rate of long-term dialysis dependence among critically ill AKI survivors. Knowledge of such economic advantages has implications for public health planning, resource allocation and physician choices.
ACKNOWLEDGEMENTS
This study was supported by a grant from Gambro. Dr. Bagshaw is supported by a ‘Canada Research Chair’ in Critical Care Nephrology and Clinical Investigator Award from Alberta Innovates–Health Solutions.





Comments