-
PDF
- Split View
-
Views
-
Cite
Cite
Alberto Ortiz, João P. Oliveira, Steven Waldek, David G. Warnock, Bruno Cianciaruso, Christoph Wanner, on behalf of the Fabry Registry, Nephropathy in males and females with Fabry disease: cross-sectional description of patients before treatment with enzyme replacement therapy, Nephrology Dialysis Transplantation, Volume 23, Issue 5, May 2008, Pages 1600–1607, https://doi.org/10.1093/ndt/gfm848
Close - Share Icon Share
Abstract
Background. Fabry disease, an X-linked genetic disorder with deficient α-galactosidase A activity, is characterized by kidney disease and kidney failure. The spectrum of kidney disease has not been well defined, especially in female patients.
Methods. We did a cross-sectional retrospective analysis of natural history of glomerular filtration rate (estimated— eGFR), albuminuria and proteinuria in 1262 adult patients (585 males, 677 females) from the Fabry Registry.
Results. Twenty-eight percent of males (age 20–79 years) and 13% of females (age 20–82 years) had chronic kidney disease (CKD) with eGFR < 60 ml/min/1.73 m 2 . Overt proteinuria (>300 mg/24 h) was demonstrated in 43 and 26% of males and females with CKD stage 1, respectively, and the proportions were higher with more severe kidney involvement. However, 11% of males and 28% of females with eGFR < 60 ml/min/1.73 m 2 had proteinuria <300 mg/ 24 h. Of eGFR ≥ 60 ml/min/1.73 m 2 patients without overt proteinuria ( n = 93), 55% of the males and 35% of the females had albuminuria >30 mg/24 h. Systemic blood pressure was ≥130/80 mmHg in 48% and 67% of patients with eGFR ≥ and <60 ml/min/1.73 m 2 , respectively, with no significant differences between males and females. Proteinuria values were significantly correlated with systolic blood pressure in both sexes.
Conclusions. Kidney involvement in Fabry disease is more prevalent and heterogeneous than previously reported. Proteinuria is an early complication, but may not be overt in patients with advanced kidney disease. This analysis, which includes more females than males, confirms that a significant proportion of females suffer moderate to severe kidney involvement in Fabry disease.
Introduction
Fabry disease is an X-linked genetic disorder of glyco- sphingolipid catabolism resulting from deficient activity of the lysosomal enzyme α-galactosidase A. As a consequence, neutral glycosphingolipids, mainly globotriaosylceramide (GL-3), accumulate in a variety of cells and tissues, leading to a wide clinical spectrum of clinical manifestations [ 1 ]. In hemizygous males, cutaneous angiokeratomas, acroparesthesias and decreased sweating are typically among the earliest symptoms to develop, usually beginning in childhood. Late life-threatening complications include cardiomyopathy, cerebrovascular disease and renal injury. The phenotype in heterozygous females is more variable, due, in part, to random X-chromosome inactivation. While classically described as a recessive X-linked disorder with infrequent clinical symptoms in females [ 1,2 ], recent data suggest that females may indeed be considerably burdened with Fabry disease manifestations [ 3–5 ].
Chronic kidney disease (CKD) is a prominent feature of Fabry disease [ 1 , 6 ] that accounts for 0.01% of end-stage renal disease (ESRD) patients enrolled in European and US dialysis registries [ 7,8 ]. However, enzymatic screening studies suggest that the true prevalence for male dialysis patients may be 10- to 100-fold higher [ 9,10 ]. Before the advent of dialysis and transplantation, males commonly died of CKD in the fifth decade of life. In a recent study of 106 male Fabry patients, all those surviving to the age of 56 developed ESRD and no patient survived beyond the age of 60 years [ 6 ].
Enzyme replacement therapy (ERT) with human recombinant α-galactosidase A has been available since 2001 [ 11,12 ]. Thus, Fabry disease is a treatable cause of CKD. Recent evidence suggests that ERT is more efficacious if started early before advanced kidney involvement with overt proteinuria has developed [ 13 ]. A better understanding of the entire spectrum of Fabry nephropathy will facilitate earlier diagnosis and guide decisions on the initiation of ERT in Fabry disease. Although family studies and case reports have disclosed some aspects of Fabry nephropathy, the rarity of this disorder has made it difficult to fully appreciate the spectrum of kidney involvement in male and female patients.
The Fabry Registry is a global outcomes assessment programme for Fabry disease. Here, we use the Fabry Registry to characterize the nephropathy in a large cohort of Fabry patients, including a high proportion of female patients, before they began receiving ERT.
Methods
The Fabry registry
The Fabry Registry, sponsored by Genzyme, is an international observational and voluntary programme intended to track the natural course and outcomes of patients with Fabry disease (https://www.lsdregistry.net/fabryregistry/). Structured clinical and laboratory data are compiled based on a recommended schedule of assessments that are based on routine clinical practice using an online data capture programme or paper case-report forms. All Fabry patients are eligible for enrolment irrespective of their gender, geographical origin or treatment status; however, signed informed consent is mandatory. In accordance with patient privacy regulations, all patient data are kept anonymous and confidential.
All physicians managing patients with Fabry disease are encouraged to participate in the Registry. By assessing patients using the recommended schedule of assessments, key data related to significant clinical events and qualitative data (e.g. quality of life) are collected. Aggregate data can then be systematically analysed. In order to minimize the potential for gender bias, participating physicians have been contacted and specifically encouraged to enrol asymptomatic or mildly symptomatic females.
These efforts will enhance our understanding of the spectrum and natural history of Fabry disease, and can support efforts to define the overall disease severity, as well as the organ-specific manifestations.
Patient inclusions and exclusions
The information provided in this manuscript is based on the April 2007 Fabry Registry download; 2316 patients with Fabry disease were documented in the database. Of these patients, 1193 (52%) were males and 1123 (48%) were females. All adult (age ≥ 18 years) males and females who had serum creatinine (SCr) values and demographic data reported via the Registry were extracted from the database. The data used in the present cross-sectional analysis were defined as the most recent assessment for ERT-naïve patients, or the most recent assessment prior to start of ERT or renal replacement therapy. Paediatric patients ( n = 175), and patients for whom SCr was available only following initiation of dialysis or transplantation ( n = 80), or only following institution of ERT ( n = 264), were excluded from the present analysis.
Data analysed
As for any large clinical practice database, the Fabry Registry is not conducted under GCP regulations. However, data collection, data management, statistical analysis and review are conducted under many of the same strict operating paradigms used for clinical trials. Submitted data were reviewed against approved review guidelines for missing data points, incomplete information and discrepancies with previously submitted data. Fabry Registry data processed for publication purposes were subjected to a quality-control review and outlying values (SCr values >25 mg/dl, proteinuria <10 mg/24 h) were double-checked with the submitting centre to verify accuracy of data entry.
The eGFR was estimated from serum creatinine using the Modification of Diet in Renal Disease (MDRD) Study Group equation: eGFR (ml/min/1.73 m 2 ) = 186 × (SCr) −1.154 × (age in years) −0.203 × (0.742, if patient is female) × (1.212, if patient ethnicity is black) [ 14 ]. Patients with eGFR values >250 ml/min/1.73 m 2 or SCr values >25 mg/dl ( n = 7) were excluded from the present analysis.
Given the inborn nature of Fabry disease, it may be assumed that all Fabry male patients, and probably most females, have glycosphingolipid deposition in renal epithelial cells. They were all considered as having CKD [ 15 ], irrespective of the presence or absence of proteinuria. Patients were categorized according to CKD stage based on the eGFR values as follows: CKD stage 1, eGFR ≥ 90; stage 2, ≥60 to <90; stage 3, ≥30 to <60; stage 4, ≥15 to <30 and stage 5, <15 ml/min/1.73 m 2 [ 15 ]. Due to the limited number of patients, CKD stages 4 and 5 were combined. Since the Fabry Registry captures routine clinical and laboratory data, SCr concentrations were measured over several years in various laboratories throughout the world [most (>90%) of the patients were recruited in North America and Europe] raising potential issues with regard to data standardization. Therefore, we have followed the recommendations of the Laboratory Working Group of the National Kidney Disease Education Programme (NKDEP) [ 16 ] and analysed grouped data, i.e. groups of patients with eGFR ≥ 60 ml/min/1.73 m 2 compared to patients with eGFR < 60 ml/min/1.73 m 2 .
Systemic blood pressure, 24-h urine total protein and 24-h urine albumin, obtained closest to the eGFR assessment, were summarized for each patient using a window of ±13 months relative to the date of the most recent eGFR assessment. Urinary albumin was measured on the same day as the 24-h urinary total protein values.
For systolic blood pressure (sBP), values between 70 and 240 mmHg and for diastolic blood pressure (dBP), values between 40 and 160 mmHg were included in this analysis. All patients with sBP ≥ 130 mmHg or dBP ≥ 80 mmHg, either treated or not treated with anti-hypertensive medication, were defined as having a blood pressure above the recommended target to decrease cardiovascular disease and progression of kidney disease in CKD, according to JNC7 and K/DOQI clinical practice guidelines [ 17,18 ].
Statistical analysis
Fisher's exact test (two-sided) was used for categorical comparisons across CKD stages. The P -value test for trend in sBP and dBP and above target blood pressure was calculated using a Cochran–Armitage trend two-sided test. Differences of least-square means from regression analysis were used to test the null hypothesis that the distribution of disease severity was the same across the four CKD stages versus the alternative hypothesis that there were significant differences among the CKD stages in their respective ordering for each parameter studied. A regression analysis t -test was used to test mean differences between males and females with eGFR < 60 and with eGFR ≥ 60 ml/min/ 1.73 m 2 . Linear relationships between log proteinuria and sBP were examined by correlation analysis. A value of P < 0.05 was considered significant. Non-zero values of 24-h proteinuria reported as <3 mg ( n = 16) were excluded from the analysis, as non-plausible with current quantification methods. Zero and all other values <10 mg/24 h were pooled together for statistical purposes.
Data analysis was conducted using SAS software, version 8.2 (SAS Institute Inc., Cary, NC, USA).
Results
Distribution by CKD stages
There were 1262 adult patients in the Fabry Registry with ERT-naïve eGFR assessments; 585 (46%) patients were male and 677 (54%) were female. Table 1 displays summary statistics for all adult male and female patients by CKD stage. The majority, 72% (419/585) of males and 87% (588/677) of females, had CKD stages 1 or 2. A higher proportion of males than females (28% males versus 13% females, P < 0.0001) had eGFR < 60 ml/min/1.73 m 2 . In patients aged >40 years, the percentage rose to 45 and 20%, respectively (Fisher's Exact two-sided test, P < 0.0001; not shown in Table 1 ).
Age and estimated glomerular filtration rate (eGFR) by the CKD stage for adult Fabry patients
| . | All . | Stage 1 . | Stage 2 . | Stage 3 . | Stage 4/5 . |
|---|---|---|---|---|---|
| n (%) | 1262 | 582 | 425 | 155 | 100 |
| Males (%) | 585 (100) | 275 (47) | 144 (25) | 88 (15) | 78 (13) |
| Females (%) | 677 (100) | 307 (45) | 281 (42) | 67 (10) | 22 (3) |
| Age (years) | |||||
| Males | 30.2 ± 9.2 (18–68) | 42.1 ± 9.8 (19–66) | 45.6 ± 10.3 (20–72) | 43.8 ± 11.6 (21–79) | |
| Females | 35.8 ± 11.0 (18–70) | 46.8 ± 12.6 (18–83) | 56.5 ± 11.1 (24–82) | 46.6 ± 12.3 (20–74) | |
| eGFR (ml/min/1.73 m 2 ) | |||||
| Males | 116 ± 23.7 | 76 ± 8.8 | 46 ± 8.6 | 17 ± 7.8 | |
| Females | 108 ± 16.3 | 77 ± 8.1 | 51 ± 8.0 | 16 ± 8.5 |
| . | All . | Stage 1 . | Stage 2 . | Stage 3 . | Stage 4/5 . |
|---|---|---|---|---|---|
| n (%) | 1262 | 582 | 425 | 155 | 100 |
| Males (%) | 585 (100) | 275 (47) | 144 (25) | 88 (15) | 78 (13) |
| Females (%) | 677 (100) | 307 (45) | 281 (42) | 67 (10) | 22 (3) |
| Age (years) | |||||
| Males | 30.2 ± 9.2 (18–68) | 42.1 ± 9.8 (19–66) | 45.6 ± 10.3 (20–72) | 43.8 ± 11.6 (21–79) | |
| Females | 35.8 ± 11.0 (18–70) | 46.8 ± 12.6 (18–83) | 56.5 ± 11.1 (24–82) | 46.6 ± 12.3 (20–74) | |
| eGFR (ml/min/1.73 m 2 ) | |||||
| Males | 116 ± 23.7 | 76 ± 8.8 | 46 ± 8.6 | 17 ± 7.8 | |
| Females | 108 ± 16.3 | 77 ± 8.1 | 51 ± 8.0 | 16 ± 8.5 |
Age is given as mean ± SD (range); eGFR is given as mean ± SD.
Age and estimated glomerular filtration rate (eGFR) by the CKD stage for adult Fabry patients
| . | All . | Stage 1 . | Stage 2 . | Stage 3 . | Stage 4/5 . |
|---|---|---|---|---|---|
| n (%) | 1262 | 582 | 425 | 155 | 100 |
| Males (%) | 585 (100) | 275 (47) | 144 (25) | 88 (15) | 78 (13) |
| Females (%) | 677 (100) | 307 (45) | 281 (42) | 67 (10) | 22 (3) |
| Age (years) | |||||
| Males | 30.2 ± 9.2 (18–68) | 42.1 ± 9.8 (19–66) | 45.6 ± 10.3 (20–72) | 43.8 ± 11.6 (21–79) | |
| Females | 35.8 ± 11.0 (18–70) | 46.8 ± 12.6 (18–83) | 56.5 ± 11.1 (24–82) | 46.6 ± 12.3 (20–74) | |
| eGFR (ml/min/1.73 m 2 ) | |||||
| Males | 116 ± 23.7 | 76 ± 8.8 | 46 ± 8.6 | 17 ± 7.8 | |
| Females | 108 ± 16.3 | 77 ± 8.1 | 51 ± 8.0 | 16 ± 8.5 |
| . | All . | Stage 1 . | Stage 2 . | Stage 3 . | Stage 4/5 . |
|---|---|---|---|---|---|
| n (%) | 1262 | 582 | 425 | 155 | 100 |
| Males (%) | 585 (100) | 275 (47) | 144 (25) | 88 (15) | 78 (13) |
| Females (%) | 677 (100) | 307 (45) | 281 (42) | 67 (10) | 22 (3) |
| Age (years) | |||||
| Males | 30.2 ± 9.2 (18–68) | 42.1 ± 9.8 (19–66) | 45.6 ± 10.3 (20–72) | 43.8 ± 11.6 (21–79) | |
| Females | 35.8 ± 11.0 (18–70) | 46.8 ± 12.6 (18–83) | 56.5 ± 11.1 (24–82) | 46.6 ± 12.3 (20–74) | |
| eGFR (ml/min/1.73 m 2 ) | |||||
| Males | 116 ± 23.7 | 76 ± 8.8 | 46 ± 8.6 | 17 ± 7.8 | |
| Females | 108 ± 16.3 | 77 ± 8.1 | 51 ± 8.0 | 16 ± 8.5 |
Age is given as mean ± SD (range); eGFR is given as mean ± SD.
Table 1 shows that the patients’ mean age was greater for those with more advanced CKD and that the mean age at CKD stages 4/5 was not different for males and females. The age range at different CKD stages was rather wide for both sexes.
Proteinuria and CKD stage
Forty-eight percent (606/1262) of all adult patients with eGFR assessments had 24-h proteinuria values recorded in the Fabry Registry. The distribution by CKD stage of 24-h urine protein measurements is shown in Table 2 . Median proteinuria was greater with a more advanced CKD stage for both males and females ( Table 2 ). The proportion of patients with overt proteinuria (>300 mg/24 h) was higher with more severe kidney involvement, both in males and females. Nephrotic range proteinuria (>3.5 gm/24 h) was present in 11/306 (3.6%) of females and 22/300 (7.3%) of males, and its prevalence was greater with a more severe CKD stage ( Table 2 ). Although proteinuria was present in the majority of patients, 11/100 (11%) of males and 12/43 (28%) of females with eGFR < 60 ml/min/1.73 m 2 did not have overt proteinuria (<300 mg/24 h). Conversely, proteinuria was detected in a significant proportion of patients with CKD 1, more so in males (54/127; 43%) than in females (37/144; 26%). Among patients with early kidney disease (CKD stages 1 and 2) and without overt proteinuria, albuminuria was measured on the same day in 93 patients. Albuminuria >30 mg/24 h was detected in 17/31 (55%) of males and 22/62 (35%) of female patients.
| . | All . | Stage 1 . | Stage 2 . | Stage 3 . | Stage 4/5 . |
|---|---|---|---|---|---|
| n (%) | |||||
| Males | 300 (100) | 127 (42) | 73 (24) | 62 (21) | 38 (13) |
| Females | 306 (100) | 144 (47) | 119 (39) | 32 (10) | 11 (4) |
| Proteinuria (mg/24 h) | |||||
| Males | 629 ± 925 (240; 10–6000) | 1076 ± 1832 (600; 10–12 500) | 1829 ± 1383 (1644; 15–5566) | 2638 ± 2418 (2074; 160–13 840) | |
| Females | 402 ± 867 (140; 10–7548) | 449 ± 795 (180; 10–4760) | 1382 ± 1601 (879; 18–6403) | 2672 ± 1825 (2063; 80–6811) | |
| Overt proteinuria, n (%) | |||||
| Males | 54 (43) | 44 (60) | 54 (87) | 35 (92) | |
| Females | 37 (26) | 32 (27) | 21 (66) | 10 (91) | |
| Nephrotic range, n (%) | |||||
| Males | 2 (2) | 4 (5) | 9 (15) | 7 (18) | |
| Females | 3 (2) | 2 (2) | 3 (9) | 3 (27) | |
| . | All . | Stage 1 . | Stage 2 . | Stage 3 . | Stage 4/5 . |
|---|---|---|---|---|---|
| n (%) | |||||
| Males | 300 (100) | 127 (42) | 73 (24) | 62 (21) | 38 (13) |
| Females | 306 (100) | 144 (47) | 119 (39) | 32 (10) | 11 (4) |
| Proteinuria (mg/24 h) | |||||
| Males | 629 ± 925 (240; 10–6000) | 1076 ± 1832 (600; 10–12 500) | 1829 ± 1383 (1644; 15–5566) | 2638 ± 2418 (2074; 160–13 840) | |
| Females | 402 ± 867 (140; 10–7548) | 449 ± 795 (180; 10–4760) | 1382 ± 1601 (879; 18–6403) | 2672 ± 1825 (2063; 80–6811) | |
| Overt proteinuria, n (%) | |||||
| Males | 54 (43) | 44 (60) | 54 (87) | 35 (92) | |
| Females | 37 (26) | 32 (27) | 21 (66) | 10 (91) | |
| Nephrotic range, n (%) | |||||
| Males | 2 (2) | 4 (5) | 9 (15) | 7 (18) | |
| Females | 3 (2) | 2 (2) | 3 (9) | 3 (27) | |
Data for proteinuria are given as mean ± SD (median; range); overt proteinuria (>300 mg/24 h), shown as a percent of total for each sex and stage, nephrotic range >3.5 g/24 h.
| . | All . | Stage 1 . | Stage 2 . | Stage 3 . | Stage 4/5 . |
|---|---|---|---|---|---|
| n (%) | |||||
| Males | 300 (100) | 127 (42) | 73 (24) | 62 (21) | 38 (13) |
| Females | 306 (100) | 144 (47) | 119 (39) | 32 (10) | 11 (4) |
| Proteinuria (mg/24 h) | |||||
| Males | 629 ± 925 (240; 10–6000) | 1076 ± 1832 (600; 10–12 500) | 1829 ± 1383 (1644; 15–5566) | 2638 ± 2418 (2074; 160–13 840) | |
| Females | 402 ± 867 (140; 10–7548) | 449 ± 795 (180; 10–4760) | 1382 ± 1601 (879; 18–6403) | 2672 ± 1825 (2063; 80–6811) | |
| Overt proteinuria, n (%) | |||||
| Males | 54 (43) | 44 (60) | 54 (87) | 35 (92) | |
| Females | 37 (26) | 32 (27) | 21 (66) | 10 (91) | |
| Nephrotic range, n (%) | |||||
| Males | 2 (2) | 4 (5) | 9 (15) | 7 (18) | |
| Females | 3 (2) | 2 (2) | 3 (9) | 3 (27) | |
| . | All . | Stage 1 . | Stage 2 . | Stage 3 . | Stage 4/5 . |
|---|---|---|---|---|---|
| n (%) | |||||
| Males | 300 (100) | 127 (42) | 73 (24) | 62 (21) | 38 (13) |
| Females | 306 (100) | 144 (47) | 119 (39) | 32 (10) | 11 (4) |
| Proteinuria (mg/24 h) | |||||
| Males | 629 ± 925 (240; 10–6000) | 1076 ± 1832 (600; 10–12 500) | 1829 ± 1383 (1644; 15–5566) | 2638 ± 2418 (2074; 160–13 840) | |
| Females | 402 ± 867 (140; 10–7548) | 449 ± 795 (180; 10–4760) | 1382 ± 1601 (879; 18–6403) | 2672 ± 1825 (2063; 80–6811) | |
| Overt proteinuria, n (%) | |||||
| Males | 54 (43) | 44 (60) | 54 (87) | 35 (92) | |
| Females | 37 (26) | 32 (27) | 21 (66) | 10 (91) | |
| Nephrotic range, n (%) | |||||
| Males | 2 (2) | 4 (5) | 9 (15) | 7 (18) | |
| Females | 3 (2) | 2 (2) | 3 (9) | 3 (27) | |
Data for proteinuria are given as mean ± SD (median; range); overt proteinuria (>300 mg/24 h), shown as a percent of total for each sex and stage, nephrotic range >3.5 g/24 h.
The distribution of 24-h urine protein with eGFR is shown in Figure 1 for males ( Figure 1 A) and females ( Figure 1 B). The median value for eGFR was 81 and 88 ml/min/1.73 m 2 for males and females, respectively (solid vertical lines). The median value for urinary protein was 572 mg/24 h for males and 180 mg/24 h for females (solid horizontal lines). The top-left quadrants show the individual values for patients with increased urinary protein and decreased eGFR, with distributions that were very similar for both sexes. The bottom-left quadrants show the individual values for patients who had decreased eGFR and urinary protein excretion rates that were not greatly elevated despite definite reductions in eGFR.
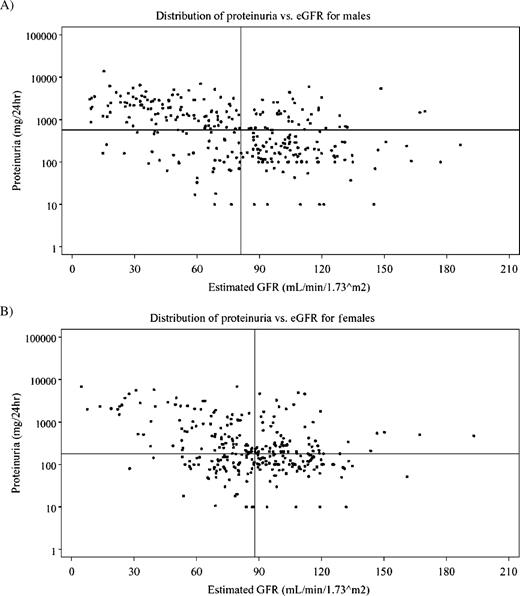
Distribution of proteinuria and eGFR. The median value for eGFR is shown as the solid vertical line, and the median value for 24-h urinary protein is shown as the solid horizontal line. ( A ) Males, n = 300, median eGFR 81.0 ml/min/1.73 m 2 , median proteinuria 572 mg/24 h. ( B ) Females, n = 306, median eGFR 88.0 ml/min/1.73 m 2 , median proteinuria 180 mg/24 h.
The bottom-right quadrants show the individual values for patients with increased eGFR and urine protein excretion that was less than the median for the gender strata. Of note, there were a substantial number of males and females who had distinctly elevated eGFRs.
The upper-right quadrants show the individual values for patients with increased eGFR and increased urine protein excretion. Of note, a substantial number of males and females had distinctly elevated eGFRs in combination with striking increases in urinary protein excretion.
Male patients showed higher mean 24-h urine total protein values than female patients in each CKD stage, except CKD stage 4/5. In CKD stages 1 and 2, mean 24-h urine total protein was 792 mg/24 h for males and 423 mg/24 h for females (difference of 369 mg/24 h, P = 0.0026). In CKD stages 3–5, mean 24-h urine total protein was 2136 mg/24 h for males and 1712 mg/24 h for females (difference of 424 mg/24 h, P = 0.0741). A significantly higher proportion of males than females had proteinuria >300 mg/24 h both in CKD stages 1 and 2 ( P < 0.0001, Figure 2 ) and in CKD stages 3–5 ( P = 0.0232).
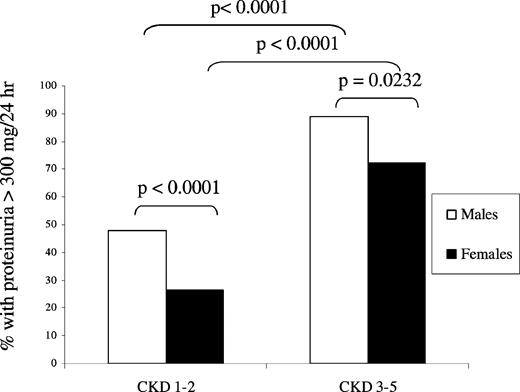
Proteinuria and eGFR. Percentage of male and female patients with proteinuria >300 mg/24 h in CKD stages 1 and 2 and 3–5. Prevalence of proteinuria was significantly higher in males. Males, n = 300; females, n = 306.
Systemic blood pressure in Fabry disease
Eighty-four percent (1066/1262) of all adult patients with eGFR assessments prior to ERT had sBP and dBP data reported in the registry ( Table 3 ). Mean sBP and dBP were higher in patients with eGFR < 60 than in those with eGFR ≥ 60 ml/min/1.73 m 2 , but did not differ between both genders in the two eGFR groups ( Table 4 ).
Blood pressure and prevalence of above target blood pressure (≥130/80 mmHg) by the CKD stage for adult Fabry patients
| . | All . | Stage 1 . | Stage 2 . | Stage 3 . | Stage 4/5 . |
|---|---|---|---|---|---|
| n (%) | |||||
| Males | 502 (100) | 241 (48) | 129 (26) | 79 (16) | 53 (11) |
| Females | 564 (100) | 266 (47) | 224 (40) | 57 (10) | 17 (3) |
| Systolic blood pressure (mmHg) | |||||
| Males | 123 ± 14 | 128 ± 16 | 127 ± 16 | 135 ± 18 | |
| Females | 120 ± 15 | 126 ± 20 | 130 ± 17 | 135 ± 17 | |
| Diastolic blood pressure (mmHg) | |||||
| Males | 72 ± 10 | 78 ± 10 | 78 ± 11 | 78 ± 10 | |
| Females | 74 ± 10 | 76 ± 11 | 77 ± 12 | 80 ± 12 | |
| Above target blood pressure, n (%) | |||||
| Males | 111 (46) | 81 (63) | 43 (54) | 42 (79) | |
| Females | 106 (40) | 118 (53) | 41 (72) | 12 (71) | |
| . | All . | Stage 1 . | Stage 2 . | Stage 3 . | Stage 4/5 . |
|---|---|---|---|---|---|
| n (%) | |||||
| Males | 502 (100) | 241 (48) | 129 (26) | 79 (16) | 53 (11) |
| Females | 564 (100) | 266 (47) | 224 (40) | 57 (10) | 17 (3) |
| Systolic blood pressure (mmHg) | |||||
| Males | 123 ± 14 | 128 ± 16 | 127 ± 16 | 135 ± 18 | |
| Females | 120 ± 15 | 126 ± 20 | 130 ± 17 | 135 ± 17 | |
| Diastolic blood pressure (mmHg) | |||||
| Males | 72 ± 10 | 78 ± 10 | 78 ± 11 | 78 ± 10 | |
| Females | 74 ± 10 | 76 ± 11 | 77 ± 12 | 80 ± 12 | |
| Above target blood pressure, n (%) | |||||
| Males | 111 (46) | 81 (63) | 43 (54) | 42 (79) | |
| Females | 106 (40) | 118 (53) | 41 (72) | 12 (71) | |
Data for blood pressure is given as mean ± SD.
Blood pressure and prevalence of above target blood pressure (≥130/80 mmHg) by the CKD stage for adult Fabry patients
| . | All . | Stage 1 . | Stage 2 . | Stage 3 . | Stage 4/5 . |
|---|---|---|---|---|---|
| n (%) | |||||
| Males | 502 (100) | 241 (48) | 129 (26) | 79 (16) | 53 (11) |
| Females | 564 (100) | 266 (47) | 224 (40) | 57 (10) | 17 (3) |
| Systolic blood pressure (mmHg) | |||||
| Males | 123 ± 14 | 128 ± 16 | 127 ± 16 | 135 ± 18 | |
| Females | 120 ± 15 | 126 ± 20 | 130 ± 17 | 135 ± 17 | |
| Diastolic blood pressure (mmHg) | |||||
| Males | 72 ± 10 | 78 ± 10 | 78 ± 11 | 78 ± 10 | |
| Females | 74 ± 10 | 76 ± 11 | 77 ± 12 | 80 ± 12 | |
| Above target blood pressure, n (%) | |||||
| Males | 111 (46) | 81 (63) | 43 (54) | 42 (79) | |
| Females | 106 (40) | 118 (53) | 41 (72) | 12 (71) | |
| . | All . | Stage 1 . | Stage 2 . | Stage 3 . | Stage 4/5 . |
|---|---|---|---|---|---|
| n (%) | |||||
| Males | 502 (100) | 241 (48) | 129 (26) | 79 (16) | 53 (11) |
| Females | 564 (100) | 266 (47) | 224 (40) | 57 (10) | 17 (3) |
| Systolic blood pressure (mmHg) | |||||
| Males | 123 ± 14 | 128 ± 16 | 127 ± 16 | 135 ± 18 | |
| Females | 120 ± 15 | 126 ± 20 | 130 ± 17 | 135 ± 17 | |
| Diastolic blood pressure (mmHg) | |||||
| Males | 72 ± 10 | 78 ± 10 | 78 ± 11 | 78 ± 10 | |
| Females | 74 ± 10 | 76 ± 11 | 77 ± 12 | 80 ± 12 | |
| Above target blood pressure, n (%) | |||||
| Males | 111 (46) | 81 (63) | 43 (54) | 42 (79) | |
| Females | 106 (40) | 118 (53) | 41 (72) | 12 (71) | |
Data for blood pressure is given as mean ± SD.
Blood pressure values in males and females with eGFR > or <60 ml/min/1.73 m 2
| . | eGFR (ml/min/1.73 m 2 ) . | |
|---|---|---|
| . | ≥60 (CKD 1–2) . | <60 (CKD 3–5) . |
| Systolic blood pressure (mmHg) | ||
| Males | 125 ± 15 | 130 ± 17 a |
| Females | 123 ± 18 | 131 ± 17 b |
| Diastolic blood pressure (mmHg) | ||
| Males | 74 ± 11 | 77 ± 11 c |
| Females | 75 ± 11 | 78 ± 12 d |
| . | eGFR (ml/min/1.73 m 2 ) . | |
|---|---|---|
| . | ≥60 (CKD 1–2) . | <60 (CKD 3–5) . |
| Systolic blood pressure (mmHg) | ||
| Males | 125 ± 15 | 130 ± 17 a |
| Females | 123 ± 18 | 131 ± 17 b |
| Diastolic blood pressure (mmHg) | ||
| Males | 74 ± 11 | 77 ± 11 c |
| Females | 75 ± 11 | 78 ± 12 d |
Data for blood pressure are given as mean ± SD.
aP = 0.0021 CKD 1–2 versus CKD 3–5 sBP in males.
bP < 0.0001 CKD 1–2 versus CKD 3–5 sBP in females.
cP = 0.0011 CKD 1–2 versus CKD 3–5 dBP in males.
dP = 0.015 CKD 1–2 versus CKD 3–5 dBP in females.
Blood pressure values in males and females with eGFR > or <60 ml/min/1.73 m 2
| . | eGFR (ml/min/1.73 m 2 ) . | |
|---|---|---|
| . | ≥60 (CKD 1–2) . | <60 (CKD 3–5) . |
| Systolic blood pressure (mmHg) | ||
| Males | 125 ± 15 | 130 ± 17 a |
| Females | 123 ± 18 | 131 ± 17 b |
| Diastolic blood pressure (mmHg) | ||
| Males | 74 ± 11 | 77 ± 11 c |
| Females | 75 ± 11 | 78 ± 12 d |
| . | eGFR (ml/min/1.73 m 2 ) . | |
|---|---|---|
| . | ≥60 (CKD 1–2) . | <60 (CKD 3–5) . |
| Systolic blood pressure (mmHg) | ||
| Males | 125 ± 15 | 130 ± 17 a |
| Females | 123 ± 18 | 131 ± 17 b |
| Diastolic blood pressure (mmHg) | ||
| Males | 74 ± 11 | 77 ± 11 c |
| Females | 75 ± 11 | 78 ± 12 d |
Data for blood pressure are given as mean ± SD.
aP = 0.0021 CKD 1–2 versus CKD 3–5 sBP in males.
bP < 0.0001 CKD 1–2 versus CKD 3–5 sBP in females.
cP = 0.0011 CKD 1–2 versus CKD 3–5 dBP in males.
dP = 0.015 CKD 1–2 versus CKD 3–5 dBP in females.
For the population as a whole, 52% of patients (554/1066) with blood pressure assessments exhibited values above those recommended for CKD patients (sBP ≥ 130 mmHg or dBP ≥ 80 mmHg). The proportion of patients with above target blood pressure did not differ between males and females with either CKD stage 1 or 2 (51.9% and 45.7%, respectively) (Fisher's exact two-sided test, P = 0.07) or CKD stages 3–5 (64.4% and 71.6%, respectively) (Fisher's exact two-sided test, P = 0.57). However, in the under 40-year-old population, there was a significantly higher prevalence of above target blood pressure among the 248 males (51%) as compared to the 229 females (35%, P ≤ 0.0003) with CKD stage 1 or 2 ( Table 3 ).
Figure 3 shows the relation between the log-transformed proteinuria values with sBP for male ( Figure 3 A) and female Fabry patients ( Figure 3 B). Statistically significant correlations were found for both sexes ( r = 0.22121, P = 0.0003 for males; r = 0.16959, P = 0.0055 for females). The vertical lines indicate the median sBP for males (125 mmHg) and females (122 mmHg). The horizontal lines indicate the median values for 24-h urine protein indicated for males (600 mg/24 h) and females (180 mg/24 h).
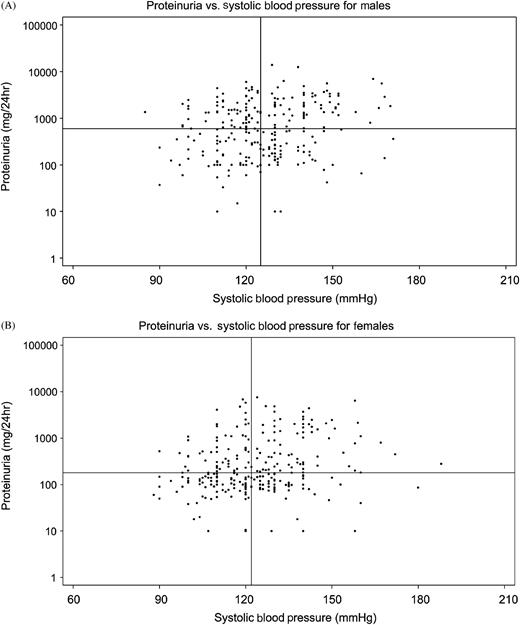
Log-transformed proteinuria values and systolic blood pressure for males ( A ) and females ( B ). The median value for sBP is shown as the solid vertical line, and the median value for 24-h urinary protein is shown as the solid horizontal line. ( A ) Males, n = 261, median sBP 125 mmHg, median proteinuria 600 mg/24 h. ( B ) Females, n = 267, sBP 122 mmHg, median proteinuria 180 mg/24 h.
Discussion
Proteinuria and CKD are important manifestations of Fabry disease. However, data obtained from large series, especially in females, are scarce. We report the results from a cross-sectional study in a large series of Fabry Registry patients who were stratified according to the CKD stage. The study cohort included a high percentage of female patients.
An important observation is the wide age range for patients at both ends of the CKD spectrum. The wide age range for males with GFR > 90 ml/min (18–68 years) contrasts with the observation in a series of 105 males in which all patients who survived to age 56 had developed ESRD [ 6 ]. The upper age ranges at CKD stages 4 and 5 were 79 and 74 years, for males and females, respectively. The six males in this subpopulation had been diagnosed at the mean age of 64.5 years (range 50–78 years). This indicates that a diagnosis of Fabry disease should be considered even at these outlying ages in both sexes and that the clinical spectrum of Fabry disease is more variable than was previously thought.
The distribution by CKD stages in females confirms and expands on previous observations [ 5 ]. The female individuals that reached advanced CKD (and eventually ESRD) did so at the same mean age as males and at a mean age that was lower as compared to the mean age of the cohort of females with CKD stage 3. This may simply reflect some sort of ascertainment bias in our cross-sectional study. Alternatively, some females apparently reach ESRD, while another subgroup of female Fabry patients may not progress from moderately advanced CKD to ESRD.
There were several males and females with distinctly elevated (e.g. >125 ml/min/1.73 m 2 ) eGFR. Because of the insensitivity of the MDRD equations with SCr measurements below 0.7 mg/dl [ 14 ], more direct measurements of the actual GFR will be required to better define this population of males and females who appear to have ‘hyperfiltration’ [ 19,20 ]. The association between hyperfiltration, proteinuria and progression of kidney disease is well described in diabetic nephropathy [ 21 ]. Hyperfiltration has also been previously described in Fabry disease [ 22 ], and other inherited disorders with kidney involvement [ 23–27 ].
Proteinuria was most frequently mild to moderate, between 300 and 3500 mg/24 h. Overt proteinuria >300 mg/ 24 h was found in 62% of males and 33% of females. The prevalence of proteinuria and its magnitude increased with decreasing renal function in both sexes. It is generally accepted that in most forms of proteinuric kidney disease, overt proteinuria, per se , is a specific risk of progression of kidney disease [ 28 ]. Overt proteinuria (even if eGFR initially is normal) has recently been described as an important risk factor for progression in the agalsidase beta (Fabrazyme®) phase III extension and phase IV studies [ 13 , 29 ], as well as in an agalsidase alfa (Replagal®) follow-up study [ 30 ]. A recent study [ 31 ] has shown that anti-proteinuric therapy with angiotensin-converting enzyme inhibitors and/or angiotensin receptor blockers can stop progression of the eGFR in Fabry patients treated with Fabrazyme at 1 mg/kg body weight every 2 weeks, but these findings need to be confirmed with a larger prospective study [ 32 ].
The overall prevalence of overt proteinuria in females was similar to that found in two smaller series [ 4,5 ]. Deegan et al . [ 5 ] concluded that proteinuria was not associated with a more rapid decline in GFR in females as no correlation between age and GFR was seen in 38 female patients with proteinuria. This conclusion appears to be at odds with our findings that suggest increases in both the prevalence and magnitude of proteinuria in females if renal function decreases. This issue has important therapeutic and prognostic implications and should be addressed with prospective follow-up studies.
Our study also provides new information on the prevalence of nephrotic range proteinuria in Fabry disease. Nephrotic range proteinuria was observed in a small cohort (2–3%) of males or females with CKD stage 1 or 2. The prevalence of nephrotic range proteinuria increased steeply in more advanced CKD stages. Our data confirm earlier observations that nephrotic range proteinuria may predate the decrease of GFR in males [ 6 ] and perhaps also in females, but suggest that, more often, it is associated with advanced CKD.
We are not aware of previous studies reporting albuminuria in the range of 30 to 300 mg/24 h in a cohort of Fabry patients. Albuminuria was found in a significant proportion of patients with early CKD and proteinuria <300 mg/24 h, both in males and females, and also in patients with eGFR > 90 ml/min/1.73 m 2 . Thus, in both sexes, albuminuria may be an even more sensitive marker of renal injury than proteinuria. We hypothesize that albuminuria may precede overt proteinuria, but additional longitudinal studies should define the prognostic value of albuminuria in patients with Fabry disease.
There is very little information about the prevalence of hypertension and the level of blood pressure control in Fabry disease. Hypertension was not considered an important feature of the disease in the earliest systematic descriptions of Fabry disease [ 33 ] but, more recently, hypertension was found in 30% of male Fabry patients [ 6 ]. This contrasts to a 65–75% and 80–95% prevalence of hypertension among individuals enrolled in the MDRD study with eGFR 60–90 and <60 ml/min/1.73 m 2 , respectively [ 34 ]. The prevalence of hypertension could not be accurately assessed in the current cross-sectional analysis since information on the use of anti-hypertensive medications was not deemed to be complete. In CKD patients, target blood pressure for reduction of cardiovascular risk and progression of CKD should be <130/80 mmHg [ 17,18 ]. Although a significant proportion of Fabry patients have a higher blood pressure than recommended in patients with CKD, mean values for Fabry patients are, with the exception of stage 4/5 patients, within those limits. Figure 3 demonstrates a correlation between proteinuria and sBP in both male and female Fabry patients. Therefore, it is particularly worrisome that 51% of males under age 40 with eGFR ≥ 60 ml/min do not have adequate blood pressure control. If these patients have overt proteinuria, then they should be treated with anti-proteinuric agents to control their blood pressure and 24-h urine protein excretion to a target of 500 mg/day [ 31 ].
Participation in the registry is voluntary and analysis li- mitations are intrinsic to the design of registry studies. Thus, only a subset of the total patient population in the Fabry Registry has eGFR data, which may suggest some selection bias. Furthermore, not all patients with eGFR data had proteinuria and blood pressure measurements. However, the patient distribution among the different CKD stages is similar for those with only eGFR data and for those with proteinuria or blood pressure data. This suggests that patients with proteinuria or blood pressure data are representative of the whole cohort. Physicians were encouraged to also focus on enrolment of asymptomatic females and, in fact, more females were analysed than males. Nevertheless, it cannot be ascertained that all asymptomatic or mildly symptomatic females from most families were included in the registry and interpretation of results should be undertaken with the appropriate sensitivity to cohort bias. Despite these limitations, this study provides important information on the female population, in particular, less severely affected females, as the proportion of females with eGFR > 60 ml/ min is greater than previously reported [ 35 ]. In addition, this is a cross-sectional analysis and longitudinal data are needed. Longitudinal data may be used for a genotype–phenotype correlation analysis; however, such an analysis is marred by the high number of Fabry-causing mutations and the low prevalence of each of the alleles [ 1 ]. The recent description of the structure of the human α-galactosidase A glycoprotein allows for studying the effects of mutations on the three-dimensional structure and facilitates genotype–phenotype analysis [ 36 ].
In conclusion, albuminuria and proteinuria are common elements of a progressive Fabry nephropathy that may reach nephrotic range. However, the absence of proteinuria does not rule out Fabry disease in CKD. Albuminuria may be a more sensitive marker of Fabry kidney involvement, although its predictive value as a marker for risk of progression of CKD should be further studied. There is a significant percentage of patients whose blood pressure is above recommended values for patients with CKD. Adequate blood pressure control is recommended as proteinuria appears to be correlated with sBP in both sexes. This analysis, which for the first time includes more females than males, confirms that a significant proportion of females has moderate to severe CKD, and better defines the full spectrum of kidney involvement in Fabry disease.
We thank the Fabry patients along with their physicians and health care personnel whose clinical data as submitted to the Fabry Registry are the basis of this report. In particular, we would like to acknowledge J. Bultas, Czech Republic; D.P. Germain, France; M. Velinov, USA; G. Linthorst, the Netherlands; F. Breunig, Germany; R.C. Scott, USA; K. Sims, USA; J. Charrow, USA; P. Lee, UK; P. Fernhoff, USA; M. West, Canada; U. Feldt-Rasmussen, Denmark; S. Packman, USA; W. Wilcox, USA; R. Wüthrich, Switzerland; R. Hopkin, USA; J-O. Johansson, Sweden; P. Deegan, UK and D. Sillence, Australia. Furthermore, We wish to thank Marta Cizmarik, MS (Senior Biostatistician, BioMedical Operations, Genzyme Corporation) for statistical services. A.O. was supported by the Programa de Intensificación de la Actividad Investigadora in the Sistema Nacional de Salud of the Instituto de Salud Carlos III and the Agencia “Pedro Laín Entralgo” of the CAM, FIS-RETICS REDinREN RD 06/0016, and Comunidad de Madrid S-BIO 0283/2006 FRACM.
Conflict of interest statement. Consultancies: D.G. Warnock (Genzyme Corp.) Honoraria: S. Waldek (Genzyme Corp.), D.G. Warnock (Genzyme Corp.); Grants received: S. Waldek (Genzyme Corp., Amicus Therapeutics), D.G. Warnock (Genzyme Corp.), J. P. Oliveira (Genzyme Corp.), A. Qrtiz (Genzyme Corp.).





Comments