-
PDF
- Split View
-
Views
-
Cite
Cite
Naoya Nishimura, Yasuko Shiomichi, Satoshi Takeuchi, Shun Akamine, Reiko Yoneda, Seiji Yoshizawa, IgA vasculitis following COVID-19 vaccination, Modern Rheumatology Case Reports, Volume 7, Issue 1, January 2023, Pages 122–126, https://doi.org/10.1093/mrcr/rxac014
Close - Share Icon Share
ABSTRACT
Immunoglobulin A (IgA) vasculitis is generally triggered by infectious causes, but it has also been reported after immunisation with various vaccines. Herein, we report two cases of IgA vasculitis after receiving the first or second dose of the Pfizer-BioNTech BNT16B2b2 mRNA vaccine. Two men, aged 22 and 30 years, developed palpable purpura on the extremities and arthritis. One patient also complained of fever and gastrointestinal symptoms. Laboratory findings revealed mild leucocytosis and slightly elevated C-reactive protein levels, although the platelet count and coagulation profile were within normal levels in both cases. Proteinuria and microhaematuria were seen in one patient. Skin biopsies were performed in both patients and revealed leucocytoclastic vasculitis. The deposits of IgA and C3 were shown in immunofluorescence studies in one patient. Both patients were diagnosed with IgA vasculitis and treated with prednisolone, and their symptoms resolved within 1 week after initiation of treatment. The coronavirus disease 2019 mRNA vaccine could trigger IgA vasculitis; however, a coincidence cannot be ruled out.
Introduction
Immunoglobulin A (IgA) vasculitis, previously also known as Henoch–Schönlein purpura, is an immune-mediated systemic small-vessel vasculitis characterised by cutaneous palpable purpura, arthritis, abdominal pain, and renal diseases. It occurs primarily in childhood and is in most cases self-limited. Although adult cases of IgA vasculitis are less common, adults have significantly worse renal outcomes than children do. The exact aetiology of IgA vasculitis is still unknown but seems to be related to genetic, environmental, and infectious causes. One-half of IgA vasculitis cases are preceded by upper respiratory tract infections, which possibly explains the association between IgA vasculitis and infection. In addition, cases of IgA vasculitis have been reported following immunisation with various vaccines, suggesting that vaccines could also trigger IgA vasculitis. Herein, we report two cases of IgA vasculitis following the COVID-19 mRNA vaccination.
Case presentation
Case 1
A 30-year-old Japanese man was admitted to our hospital with a rash on the extremities, fever, abdominal pain, and arthralgia. Five days before admission, he received a second dose of the Pfizer-BioNTech BNT16B2b2 mRNA vaccine. He complained of mild fatigue and myalgia after the first and second vaccination doses, but these symptoms were resolved within a few days. The patient was not taking any new medications and showed no signs of infection, including COVID-19, before admission. Physical examination revealed palpable purpura on the extremities and trunk (Figure 1), fever, abdominal pain, and swelling of the left knee. Laboratory findings indicated a white blood cell count of 11,200/µl (normal, <8600/µl), with neutrophils accounting for 90%, C-reactive protein (CRP) of 1.18 mg/dl (normal, <0.15 mg/dl), and IgA of 307 mg/dl (normal, 93–393 mg/dl). Microhaematuria of 30–49/high-power field and mild proteinuria of 0.20 g/g of creatinine were also observed. Haemoglobin, platelet count, liver enzymes, renal function, and coagulation tests were within normal limits. Antinuclear antibody was positive with a low titre, and myeloperoxidase antineutrophil cytoplasmic antibody and proteinase-3 antineutrophil cytoplasmic antibody were negative. Skin biopsy of the lesion on the left forearm revealed erosion in the epidermis with neutrophilic infiltration, extravasation of red blood cells, nuclear debris in the perivascular area, and fibrin deposits, all of which were consistent with leucocytoclastic vasculitis (Figure 2). No definite deposits of IgA and only slight deposits of C3 of the vessel walls were evident in immunofluorescence studies of the second skin site (Figure 3). His clinical presentation was consistent with the European League Against Rheumatism/Paediatric Rheumatology International Trials Organisation/Paediatric Rheumatology European Society classification criteria [1, 2], and the diagnosis of IgA vasculitis was made. He was treated with intravenous prednisolone (PSL) (60 mg) daily for 3 days, followed by oral PSL (40 mg) daily. His symptoms improved within 1 week after treatment began, except for haematuria and proteinuria. Although abnormalities in urinalysis persisted, they improved after 1 month. PSL was gradually tapered, as there was no exacerbation of IgA vasculitis.
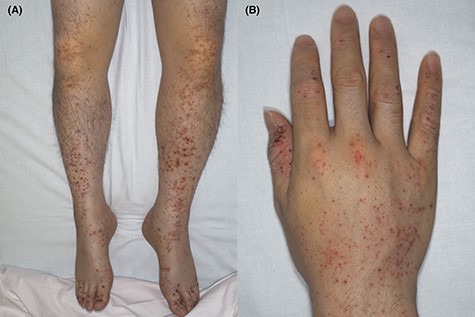
Palpable purpura on the lower limbs (A) and right hand (B) (Case 1).
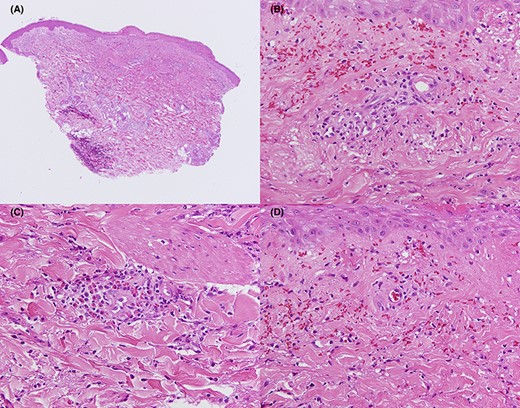
Histopathological study of the patient’s right forearm (Case 1). Erosions are seen on some parts of the epidermis (A). Inflammatory infiltration of neutrophils and lymphocytes, extravasation of red blood cells, and nuclear debris (B) and inflammatory infiltration of eosinophils (C) in the perivascular area. Fibrinoid necrosis is also noted (D). (A) Haematoxylin and eosin staining, ×40. (B–D) Haematoxylin and eosin staining, ×400.
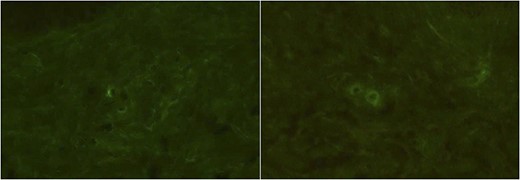
Immunofluorescence studies of the patient’s right forearm (Case 1). No definite deposits of IgA (A) and only slight deposits of C3 (B) of the vessel walls were observed.
Case 2
A 22-year-old Japanese man was admitted to our hospital with a rash on the lower limbs and arthralgia. He had a history of recurring tonsillitis. He received the first dose of the Pfizer-BioNTech BNT16B2b2 vaccine 6 days before onset without any symptoms after the vaccination. He was not taking any new medications but complained of discomfort in the throat before onset. He visited a dermatologist for a skin rash on the lower limbs and was prescribed medications, but the rash was spread and the pain of the left knee also appeared, then he was visited our hospital. Physical examination revealed palpable purpura on the lower limbs (Figure 4), swelling of the left knee, and only mild swelling of the tonsils. He did not complain of fever or gastrointestinal symptoms. Laboratory findings indicated a white blood cell count of 10,000/µl (normal, <8600/µl), with neutrophils accounting for 81%, and CRP of 0.27 mg/dl (normal, <0.15 mg/dl). IgA was not measured. Haemoglobin, platelet count, liver enzymes, renal function, urinalysis, and coagulation tests were within normal limits. Anti-streptolysin O antibody was 72 IU/ml (normal, <239 IU/ml) and anti-streptokinase antibody was ×320 (normal, <×2560). Skin biopsy of the lesion on the right lower leg revealed neutrophilic infiltration, extravasation of red blood cells, nuclear debris in the perivascular area, and fibrin deposits, all of which were consistent with leucoocytoclastic vasculitis (Figure 5). Moreover, immunofluorescence studies of the second skin site revealed the deposits of C3 and the slight deposits of IgA of the vessel walls (Figure 6). He was diagnosed with IgA vasculitis and treated with oral PSL 30 mg daily. His symptoms improved within 1 week after treatment. PSL was gradually tapered, as there was no exacerbation of IgA vasculitis.
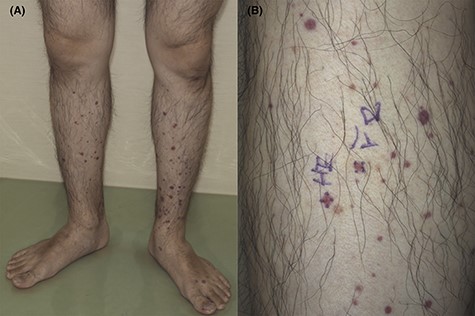
Palpable purpura on the lower limbs (A, B) (Case 2). The skin biopsy was taken from the purpura of the right lower leg (B).
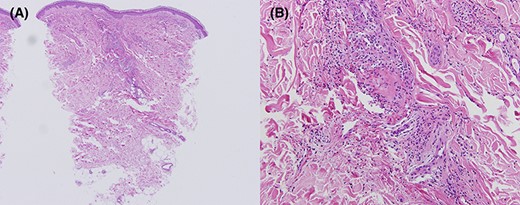
Histopathological study of the patient’s right lower leg (Case 2). Inflammatory infiltration of neutrophils, extravasation of red blood cells, nuclear debris, and fibrinoid necrosis are seen in the perivascular area (A, B). (A) Haematoxylin and eosin staining, ×40. (B) Haematoxylin and eosin staining, ×400.
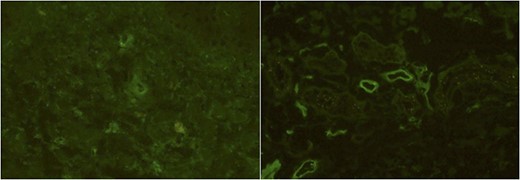
Immunofluorescence studies of the patient’s right lower leg (Case 2). The slight deposits of IgA (A) and the deposits of C3 (B) of the vessel walls were observed.
Discussion
Vaccines and autoimmune responses are closely related. The vaccine aims to induce a host immune response to antigens and to elicit a memory T-cell response over time. However, vaccines are also able to elicit immune responses toward the autoimmune reaction. Adjuvants are often administered with vaccines to enhance their immunogenicity by triggering innate and adaptive immune responses. However, the adjuvants also can induce different conditions: the so-called autoimmune/inflammatory syndrome induced by adjuvants [3]. The mRNA vaccines such as Pfizer-BioNTech BNT16B2b2 or Moderna mRNA-1273 have been first approved against COVID-19. The mechanism of the mRNA vaccine is that mRNA is used as a template in dendritic cells to produce proteins, and some of the produced proteins are presented to lymphocytes to trigger the immune response. Although the mRNA vaccines do not require an adjuvant, mRNA itself also can stimulate the innate immune response and promote immune induction via pattern-recognition receptors such as Toll-like receptor (TLR) 3, TLR7, or retinoid-inducible gene I [4].
Although the exact aetiology of IgA vasculitis remains unclear, infectious, genetic, and environmental causes have been suggested. As for the infectious causes, one-half of IgA vasculitis cases are preceded by upper respiratory tract infection, suggesting a correlation between respiratory pathogens and IgA vasculitis [5]. An abnormal inflammatory process derived from immune reactions to various antigens is thought to contribute to the pathogenesis of IgA vasculitis. Interactions between leucocytes and vascular endothelial cells induce the development of IgA vasculitis. Endothelial cell damage, perivascular leucocyte infiltrates, cytokines, and chemokines are the important factors of this process [6, 7]. In addition, the vascular deposition of the IgA1-containing immune complex plays a pathogenic role [8]. Complement activation, cellular damage, and IgA deposition suggest that IgA vasculitis is an IgA-mediated dysregulation of the immune responses to the antigen. The binding and activation of complement cause IgA to cross-react with endothelial cells and damage the cells. In IgA vasculitis, the dysregulated immune responses induced by these processes result in inflammation. Various inflammatory cytokines such as tumour necrosis factor, interleukin (IL)-6, and IL-8 are involved. In addition, various TLRs, including TLR3 and TLR7, were found to be upregulated in IgA vasculitis [9, 10]. These receptors are mainly expressed on immune cells, including dendritic cells and macrophages, which suggest the involvement of innate immune responses. Inflammation triggered by the adjuvant has similarities with the inflammation caused by infection, which could trigger IgA vasculitis.
Moreover, other possible mechanisms are also suggested. The first is molecular mimicry between self-peptides and viral spike protein. mRNA encoding the spike protein of SARS-CoV-2 is encapsulated in lipid nanoparticles in the COVID-19 mRNA vaccine. The anti-spike protein IgA antibody was significantly increased after the Moderna mRNA-1273 vaccination [11]. This report suggests the interaction between SARS-CoV-2 spike protein and pre-existing autoreactive IgA antibodies. The second is delayed-type hypersensitivity reaction. The cases of IgA nephropathy flare following both COVID19 mRNA vaccinations have been described [12–15]. These patients were not previously infected with SAES-CoV-2, suggesting the involvement of cell-mediated immune responses.
Although previous studies found no causal association between vaccination and subsequent development of IgA vasculitis [16], some cases of IgA vasculitis following the immunisation with the various vaccines have been reported [17–19]. No cases of IgA vasculitis were reported in Pfizer-BioNTech BNT16B2b2 [20] or Moderna mRNA-1273 [21] vaccine trials; there have been reports of cases presented [22] or relapsed [11] following the COVID-19 mRNA vaccine. Taken together, these reports suggest that the COVID-19 mRNA vaccine has the potential to induce IgA vasculitis, although it is a rare adverse event.
Although IgA deposition in the epidermis is observed in the majority of cases of IgA vasculitis, there were no deposits of IgA and only slight deposits of C3 in immunofluorescence studies in Case 1. It has been reported that a subset of IgA vasculitis cases exists in which vascular IgA deposits in the epidermis appear to be negative. The deposition of IgA in the skin site was shown in 80% and C3 in only 20% of adult cases of IgA vasculitis [23]. Furthermore, another study showed that the direct immunofluorescence positivity drops to ∼15% if the biopsy is performed >7 days after onset [24]. In our case, the skin biopsy was performed after treatment with PSL began, so we cannot deny the possibility of a false negative. Moreover, another possible hypothesis is also suggested. As we mentioned above, the vascular deposition of the IgA1-containing immune complex plays a pathogenic role in IgA vasculitis. Increased IgA production by the mucosal immune system in response to pathogenic antigens such as bacteria or viruses has been suggested as the mechanism for the development of IgA vasculitis. On the other hand, a possible mechanism suspected in the triggering of COVID-19 mRNA vaccine–induced IgA vasculitis is the activation of the innate immune system as we mentioned above. Indeed, the serum IgA level was not elevated in our case, suggesting that the direct innate immune cell activation could also contribute to the development of vasculitis. This could explain the difference between common IgA vasculitis and COVID-19 mRNA vaccine–induced IgA vasculitis. Renal biopsy was not performed in Case 1 because the patient did not show complication due to acute kidney injury. Because a previous study showed granular global mesangial deposits of IgA in the case of COVID-19 mRNA vaccine–induced IgA nephropathy [25], such changes could have been seen in our case if the renal biopsy had been performed. If the patient showed acute kidney injury, we should perform the renal biopsy to differentiate between rapidly progressive glomerulonephritis and other conditions. In Case 2, tonsillitis seems to trigger IgA vasculitis, but we cannot deny the possibility that the tonsillitis was also induced by the COVID-19 mRNA vaccination. Laboratory findings showed that CRP was only slightly elevated and anti-streptolysin O antibody and anti-streptokinase antibody were within normal limits, which were often elevated in bacterial tonsillitis. Moreover, tonsil organoids induced adaptive immune responses upon adenovirus vector–based COVID-19 vaccination ex vivo [26]. This report suggests that the spike protein induced by the COVID-19 vaccine could stimulate tonsils.
With the COVID-19 pandemic spreading worldwide, vaccination campaigns are proceeding rapidly. Physicians should be aware of this complication while continuing to encourage vaccination.
Conflict of interest
None declared.
Funding
None declared.
Patient consent
The patients in this case report provided written informed consent for its publication.
Ethical approval
Not applicable.



